Abstract
Rationale: HIV-infected persons on antiretroviral therapy (ART) remain at higher risk of pulmonary tuberculosis (TB) than HIV-uninfected individuals. This increased susceptibility may be caused by impairment of alveolar macrophage (AM) function and/or mycobacteria-specific alveolar CD4+ T-cell responses observed in HIV-infected ART-naive adults.
Objectives: To determine whether ART was associated with improvement in both AM function, assessed by phagosomal proteolysis, and alveolar CD4+ T-cell responses to Mycobacterium in HIV-infected individuals.
Methods: Peripheral blood was drawn and bronchoalveolar lavage (BAL) performed on healthy, 35 HIV-uninfected, 25 HIV-infected ART-naive, and 50 HIV-infected ART-treated asymptomatic adults. Phagosomal proteolysis of AM was assessed with fluorogenic beads. Mycobacteria-specific CD4+ T-cell responses were measured by intracellular cytokine staining.
Measurements and Main Results: HIV-infected adults on ART exhibited lower plasma HIV viral load and higher blood CD4+ T-cell count than ART-naive adults. AM proteolysis and total mycobacteria-specific Th1 CD4+ T-cell responses in individuals on ART for greater than or equal to 4 years were similar to HIV-uninfected control subjects but those on ART for less than 4 years had impaired responses. Total influenza-specific alveolar Th1 CD4+ T-cell responses were intact in all individuals receiving ART. In contrast, BAL and blood mycobacteria-specific polyfunctional CD4+ T-cell responses were impaired in adults on ART irrespective of duration.
Conclusions: AM and mycobacteria-specific alveolar CD4+ T-cell responses in HIV-infected adults on ART for less than 4 years are impaired and may partly explain the high risk of TB in HIV-infected individuals on ART. Strategies to augment ART to improve lung immune cell function and reduce the high incidence of TB in HIV-infected adults who initiate ART should be investigated.
Keywords: HIV, T cells, macrophages, Mycobacterium tuberculosis, ART
At a Glance Commentary
Scientific Knowledge on the Subject
The use of potent antiretroviral therapy (ART) has led to a dramatic fall in the burden of tuberculosis (TB) among HIV-infected individuals, but the incidence of TB still remains higher in HIV-infected adults receiving ART compared with HIV-uninfected individuals.
What This Study Adds to the Field
We show that HIV-infected adults on ART have impaired alveolar macrophage function and mycobacteria-specific alveolar CD4+ T-cell responses, most markedly during early ART treatment. These findings underscore the need for strategies to augment ART to improve lung immune cell function and reduce the high incidence of TB. The development and use of subunit vaccines that promote repopulation of the lung with crucial mycobacteria-specific polyfunctional CD4+ T-cell subsets and the use of prophylactic TB chemotherapy in HIV-infected adults, especially during the early years of ART when they are most vulnerable, should be explored.
Pulmonary tuberculosis (TB) causes high morbidity and mortality in HIV-infected individuals (1). Unlike other opportunistic infections, the risk of developing active TB is greatly increased in persons even before significant depletion of CD4+ T cells in peripheral blood (2). In high transmission regions, most TB cases in HIV-individuals result from recent Mycobacterium tuberculosis (Mtb) infection indicating that in these individuals the lung is more permissive for the establishment or progression of primary infections (3). Recent studies have reported that alveolar macrophage (AM) proteolytic function and mycobacterial antigen-specific Th1 CD4+ T-cell responses in the lung are impaired in asymptomatic HIV-infected, antiretroviral therapy (ART)–naive persons (4–6). These findings indicate that HIV alters both innate and adaptive immunity in the lung to render HIV-infected individuals incapable of mounting adequate pulmonary immune responses to control Mtb.
Potent ART inhibits HIV replication to allow immune recovery. Several previous studies have profiled the kinetics of peripheral blood CD4+ T-cell count as a surrogate marker of immune recovery during ART. These studies reported a rapid increase in CD4+ T cells during the first few months of treatment, followed by a more gradual increase during the subsequent months or years before reaching a plateau after approximately 4 years of ART (7, 8). ART-associated immune recovery reduces morbidity and mortality caused by HIV-related opportunistic infections (9). For example, a 65% reduction in the incidence of TB in HIV-infected adults on ART irrespective of the CD4+ T-cell count has been reported previously (10). However, despite the benefits of ART, TB incidence rates in HIV-infected individuals who received ART for more than 3 years remain 5- to 10-fold higher than HIV-uninfected persons (11).
The development of strategies to reduce the burden of TB in HIV-infected individuals treated with ART requires a better understanding of the host immune factors that underlie their persistent risk of TB. To this end, we investigated the association between use of ART and the restoration of immune cell function in the lung. We conducted a prospective cross-sectional study in healthy, HIV-1–uninfected and asymptomatic HIV-1–infected adults to determine whether ART was associated with improvement in both AM function, assessed by phagosomal proteolysis, and alveolar CD4+ T-cell responses to Mycobacterium in HIV-infected individuals.
Methods
Subjects
The study was conducted at the Queen Elizabeth Central Hospital, a large teaching hospital in Blantyre, Malawi. Participants were recruited from the hospital’s voluntary counseling and testing and ART clinics. They were healthy, asymptomatic adults (≥18 yr) comprising HIV-1–uninfected and HIV-1–infected volunteers with no clinical evidence of active disease, willing to undergo bronchoscopy and bronchoalveolar lavage (BAL) for research purposes. HIV testing was performed on whole blood using two commercial point-of-care rapid HIV test kits, Determine HIV 1/2 kit (Abbott Diagnostic Division, Abbott Park, IL) and Unigold HIV 1/2 kit (Trinity Biotech Inc., Bray, Ireland). A participant was considered HIV-uninfected if the test was negative by both kits or HIV-infected if the test was positive by both kits. If Determine and Unigold results were discordant, a third rapid test using Bioline HIV 1/2 kit (Standard Diagnostics Inc., Gyeonggi-do, Republic of Korea) was performed to resolve the discordance.
HIV-1–infected participants were divided into three subgroups based on the time on ART at the time of recruitment: (1) ART-naive, (2) on ART for less than 4 years, and (3) on ART for greater than or equal to 4 years. First-line ART consisted of stavudine, lamivudine, and nevirapine, or tenofovir, lamivudine, and efavirenz (following a change in the national guidelines in 2011). Exclusion criteria for the study were current or past history of smoking, use of immunosuppressive drugs, severe anemia (hemoglobin < 8 g/dl), and known or suspected pregnancy. Peripheral blood CD4+ T-cell counts were measured in all participants, and HIV plasma viral load measurements were performed in HIV-infected participants only. The research =ethics committee of the Malawi College of Medicine approved the study and all participants provided written-informed consent.
Collection and Processing of BAL and Blood Samples
BAL samples were collected at bronchoscopy and processed as previously described (5, 12). AM were isolated from BAL cells by adherence to plastic (6) and adherent cells were used for assessment of AM phagosomal proteolysis; nonadherent BAL cells, which were predominantly lymphocytes, were used to assess intracellular cytokine production by alveolar CD4+ T cells. Peripheral blood samples were also collected from participants. Plasma samples were used for measurements of HIV viral load, whereas peripheral blood mononucleated cells were used to assess intracellular cytokine production by blood CD4+ T cells. Because of limitations in cell numbers, not all the assays were performed on cells obtained from every participant.
Measurement of AM Phagosomal Proteolysis
Phagosomal proteolysis in AM was measured using a flow cytometry–based reporter bead assay as described previously (6, 13). The readout for the assay was Activity Index, which was calculated by first determining the ratio of median fluorescence intensity of the reporter over calibration flour at 10-minute and 240-minute time points, and then dividing the ratio at 240 minutes by ratio at 10 minutes.
Intracellular Cytokine Staining
Antigen-specific CD4+ T-cell responses were measured as previously described (5). In brief, peripheral blood mononucleated cells (1 × 106 cells per well) and nonadherent BAL cells (0.5 × 106 cells per well) suspended in 200 μl of complete media were cultured in 96-well plates and stimulated with purified protein derivative (10 μg/ml; Statens Serum Institut, Copenhagen) and influenza vaccine (0.45 μg/ml; Sanofi Pasteur MSD, United Kingdom). Cells were stained with Violet Viability dye (LIVE/DEAD Fixable Dead Cell Stain kit, Invitrogen, United Kingdom), surface markers (anti-CD3 phycoerythrin [PE]-Cy5, anti-CD4 allophycocyanin [APC]-H7, and anti-CD8 PE-Cy7 antibodies; BD Bioscience, United Kingdom), and intracellular markers (anti–IFN-γ APC, anti–tumor necrosis factor [TNF]-α Alexa Fluor 488, and anti-IL17 PE antibodies; BD Bioscience). Approximately 50,000 events were acquired in the CD4+ gate using a CyAn ADP 9 color flow cytometer (Beckman Coulter). Flow cytometry data were analyzed using FlowJo software (TreeStar).
Statistical Analyses
Statistical analyses and graphical presentation were performed using GraphPad Prism 5 (GraphPad Software) or PESTLE 1.7 and SPICE 5.3 (both NIAID). The programs PESTLE and SPICE were kindly provided by Mario Roederer, Vaccine Research Center, NIAID, National Institutes of Health. Flow cytometry data were log transformed and analyzed using one-way analysis of variance and Student t test. SPICE histograms were analyzed using Student t test. Results are given as geometric mean with confidence intervals (CI), except SPICE histograms, which are given as mean and SEM. Differences were considered statistically significant when P less than 0.05.
Results
Participant Characteristics
We recruited 35 HIV-uninfected and 75 HIV-infected adults of whom 25 were ART-naive, 28 had received ART for less than 4 years, and 22 had received ART for greater than or equal to 4 years. All study participants had received bacillus Calmette-Guérin vaccination during childhood (purified protein derivative skin testing or γ-IFN release assays were not performed). Their characteristics are summarized in Table 1.
Table 1.
Demographics of Study Participants
| HIV-uninfected (n = 35) | HIV-infected ART-Naive (n = 25) | HIV-infected ART < 4 yr (n = 28) | HIV-infected ART ≥ 4 yr (22) | |
|---|---|---|---|---|
| Age, yr, median (range) | 30 (18–45) | 29 (20–44) | 32 (20–50) | 37 (21–46) |
| Sex (M:F) | 24:11 | 9:16 | 8:20 | 7:15 |
| Blood CD4 count, cells/μl, median (IQR) | 799 (642–942) | 395 (249–558) | 450 (331–640) | 445 (286–604) |
| Alveolar macrophage count, 106 cells/100 ml, median (IQR) | 12.4 (10.4–19.1) | 15.1 (10.0–21.8) | 17.2 (10.0–28.3) | 22.0 (15.2–27.3) |
| BAL lymphocyte count, 106 cells/100 ml, median (IQR) | 5.8 (3.6–11.7) | 8.7 (4.9–17.5) | 10.0 (5.6–16.4) | 7.2 (4.8–16.5) |
| BAL CD4 count, 106 cells/100 ml, median (IQR) | 3.0 (1.5–6.2) | 2.5 (1.7–5.1) | 2.7 (1.5–5.3) | 2.6 (1.6–4.2) |
| BAL CD8 count, 106 cells/100 ml, median (IQR) | 1.8 (0.9–4.3) | 3.5 (1.2–4.8) | 5.3 (2.0–7.0) | 3.2 (2.2–8.0) |
| Plasma viral load, copies/ml, median (IQR) | N/A | 28,131 (618–262,032) | 0 (0–149) | 0 (0–75) |
| Years on ART, mean (range) | N/A | N/A | 1.6 (0.3–3.9) | 6.8 (4.9–8.9) |
| Stavudine/lamivudine/nevirapine | N/A | N/A | 16 | 22 |
| Tenofovir/lamivudine/efavirenz | N/A | N/A | 12 | 0 |
Definition of abbreviations: ART = antiretroviral therapy; BAL = bronchoalveoalar lavage; IQR = interquartile range; N/A = not applicable.
AM Phagosomal Proteolysis Function Is Impaired in HIV-infected Adults on ART during the Early Years of Treatment
The digestion of particulate matter and microbes by proteolysis following phagocytosis is required for both microbicidal activity and antigen presentation (13, 14). Recently, we reported that AM phagosomal proteolysis function is impaired in asymptomatic HIV-infected ART-naive adults (6). To determine whether use of ART is associated with improvement in this functional defect, we used the flow cytometry–based reporter bead assay to measure phagosomal proteolysis in AM from HIV-uninfected and HIV-infected ART-naive adults and those on ART for less than or greater than or equal to 4 years. We found that AM proteolysis was lower in HIV-infected ART-naive adults (geometric mean log activity index, 0.09 [CI, 0.07–0.12] vs. 0.16 [CI, 0.13–0.19]; P = 0.004) and HIV-infected individuals who had been on ART less than 4 years (geometric mean log activity index, 0.09 [CI, 0.06–0.14] vs. 0.16 [CI, 0.13–0.19]; P = 0.05) compared with HIV-uninfected participants (Figure 1). However, there was no difference in AM proteolysis between HIV-infected individuals who had been on ART for greater than or equal to 4 years and HIV-uninfected participants (geometric mean log activity index, 0.17 [CI, 0.14–0.21] vs. 0.16 [CI, 0.13–0.19]; P = 0.39) (Figure 1). These findings suggest that in asymptomatic HIV-infected individuals on ART, AM phagosomal proteolysis function is impaired during the early years of treatment.
Figure 1.
Impaired alveolar macrophage proteolysis function in HIV-infected adults on antiretroviral therapy (ART) for less than 4 years. Adherent alveolar macrophages were incubated with DQ-BSA reporter beads for 10 minutes or 240 minutes to measure phagosomal bulk proteolysis. The cells were acquired on a flow cytometer and the activity index was determined. Data were log transformed. Gray bars represent geometric mean and black horizontal lines represent confidence intervals. Data were analyzed using one-way analysis of variance and Student t test (HIV+ ART naive, n = 18; HIV+ ART < 4 yr, n = 15; HIV+ ART ≥ 4 yr, n = 16; HIV−, n = 19).
Low Frequency of Total Mycobacteria-Specific Cytokine-Producing Alveolar Th1 CD4+ T Cells in HIV-infected Adults on ART during the Early Years of Treatment
Next, we investigated Th1 and Th17 mycobacteria- and influenza-specific CD4+ T-cell responses in BAL and peripheral blood using an intracellular cytokine-staining assay. Th1 CD4+ T cells were defined by their production of IFN-γ and/or TNF, whereas Th17 cells were defined by their production of IL-17. A representative flow cytometry dot plot of BAL CD4+ T-cell responses from an HIV-uninfected adult is shown in Figure 2.
Figure 2.

Representative flow cytometry dot plot from an HIV-uninfected adult showing multiple subsets of antigen-specific CD4+ T cells in bronchoalveolar lavage (BAL). Nonadherent BAL cells and peripheral blood mononuclear cells were stimulated overnight with purified protein derivative or influenza vaccine for 18 hours and CD4+ T-cell responses were measured by intracellular cytokine staining. The dot plots were obtained by gating on singlets, lymphocytes, live cells, CD3+ cells, CD4+ cells, and combination of three cytokines. They show the frequency (percentage) of IFN-γ–, tumor necrosis factor (TNF)-α–, and/or IL-17–producing CD4+ T cells in BAL, in an unstimulated negative control and cells stimulated with PMA/ionomycin (positive control), mycobacteria, and influenza virus antigens. PMA = phorbol myristate acetate.
We found that the frequencies of total mycobacteria- and influenza-specific cytokine-producing alveolar Th1 CD4+ T cells were lower in HIV-infected ART-naive adults compared with HIV-uninfected adults (mycobacteria, 0.5% [0.2–1.3] vs. 1.7% [1.0–2.8], P = 0.01; influenza, 0.2% [0.1–0.6] vs. 0.9% [0.4–1.6], P = 0.02) (Figures 3A and 3B). In HIV-infected participants receiving ART, the frequency of total mycobacteria-specific cytokine-producing alveolar Th1 CD4+ T cells was lower in those on ART for less than 4 years compared with HIV-uninfected individuals (0.5% [0.2–1.3] vs. 1.7% [1.0–2.8]; P = 0.02), but was not different between individuals on ART for greater than or equal to 4 years and HIV-uninfected adults (1.6% [0.7–3.9] vs. 1.7% [1.0–2.8]; P = 0.90) (Figure 3A).
Figure 3.

Impaired total mycobacteria-specific alveolar Th1 CD4+ T-cell responses in HIV-infected adults on antiretroviral therapy (ART) for less than 4 years. Nonadherent bronchoalveoalar lavage (BAL) cells were stimulated overnight with purified protein derivative and influenza vaccine. Cells were analyzed by flow cytometry for IFN-γ and tumor necrosis factor production by the CD4+ T-cell population. (A and C) Mycobacterium-specific responses in BAL and peripheral blood mononuclear cells (PBMCs). (B and D) Influenza-specific responses in BAL and PBMCs. The data were log transformed and are shown as the total frequency of antigen-specific Th1 IFN-γ– and tumor necrosis factor–producing CD4+ T cells. The horizontal bars represent geometric mean and confidence interval. Data were analyzed using one-way analysis of variance and Student t test (HIV−, n = 32; HIV+ ART naive, n = 25; HIV+ ART < 4 yr, n = 28; HIV+ ART ≥ 4 yr, n = 22).
In contrast, there was no difference in the frequency of total influenza-specific cytokine-producing alveolar Th1 CD4+ T cells between HIV-infected participants on ART and HIV-uninfected individuals (ART < 4 yr, 1.1% [0.7–1.8] vs. 0.9% [0.4–1.6], P = 0.58; ART ≥ 4 yr, 0.9% [0.7–1.4] vs. 0.9% [0.4–1.6], P = 0.83) (Figure 3B). There was no significant difference in the frequencies of mycobacteria- and influenza-specific cytokine-producing alveolar Th17 CD4+ T cells between HIV-uninfected and HIV-infected ART-naive adults (mycobacteria, 0.10% [0.05–0.17] vs. 0.08% [0.03 vs. 0.19], P = 0.89; influenza, 0.05% [0.02–0.09] vs. 0.02% [0.01–0.04], P = 0.14) or between HIV-uninfected adults and HIV-infected participants on ART (mycobacteria: ART < 4 yr, 0.10% [0.05–0.17] vs. 0.16% [0.07–0.39], P = 0.30; ART ≥ 4 yr, 0.10% [0.05–0.17] vs. 0.16% [0.05–0.54], P = 0.24) (influenza: ART < 4 yr, 0.05% [0.02–0.09] vs. 0.08% [0.04–0.18], P = 0.23; ART ≥ 4 yr, 0.05% [0.02–0.09] vs. 0.08% [0.03–0.23], P = 0.26) (see Figures E1A and E1B in the online supplement).
In blood, we found no difference in the frequencies of total Th1 and Th17 mycobacteria- and influenza-specific cytokine-producing CD4+ T cells between HIV-uninfected and HIV-infected adults in the three subgroups (all P > 0.05) (Figures 3C and 3D; see Figures E1C and E1D). Furthermore, the frequencies of total antigen-specific Th1 CD4+ T cells in BAL and peripheral blood did not correlate with total CD4+ T-cell counts in BAL and blood, respectively (see Figure E2). Our data suggest that HIV-associated depletion of mycobacteria- and influenza-specific Th1 CD4+ T cells differs between the lung and peripheral blood compartments. They also suggest that in asymptomatic HIV-infected adults on ART, the frequency of total mycobacteria-specific cytokine-producing alveolar Th1 CD4+ T cells is low during the early years of ART, which is in contrast to the normal frequency of total influenza-specific cytokine-producing alveolar Th1 CD4+ T cells seen in individuals on ART.
Low Mycobacteria-Specific Polyfunctional Alveolar and Blood Th1 CD4+ T-Cell Response in HIV-infected Adults on ART
The previous analysis compared the total Th1 and Th17 populations of mycobacteria- and influenza-specific cytokine-producing alveolar and blood CD4+ T cells between HIV-infected and HIV-uninfected adults but did not determine changes in individual antigen-specific CD4+ T-cell subsets. To assess the impact of HIV infection on antigen-specific cytokine-producing CD4+ T-cell subsets, we used the intracellular cytokine-staining assay to determine the relative contribution of mycobacteria- and influenza-specific CD4+ T-cell subsets in the total responses, identified by the ability to produce IFN-γ, TNF, and IL-17 in different combinations. The analysis allowed identification of seven different subsets: (1) IFN-γ+TNF+IL17+, (2) IFN-γ+TNF+IL17−, (3) IFN-γ+TNF−IL17+, (4) IFN-γ−TNF+IL17+, (5) IFN-γ+TNF−IL17−, (6) IFN-γ−TNF+IL17−, and (7) IFN-γ−TNF−IL17+ cells.
First, we determined the dominant mycobacteria- and influenza-specific cytokine-producing CD4+ T-cell subsets in BAL and blood from HIV-uninfected adults. In BAL, we found that IFN-γ+TNF+ double-producing (DP; red), IFN-γ+ single-producing (SP; pink), and TNF+ SP (purple) cells were the dominant mycobacteria- and influenza-specific CD4+ T-cell subsets (mycobacteria, IFN-γ+TNF+ DP 36%, IFN+ SP 22%, and TNF+ SP 24%; influenza, IFN-γ+TNF+ DP 20%, IFN+ SP 48%, and TNF+ SP 18%) (Figures 4A and 4B). In blood, IFN-γ+TNF+ DP and TNF+ SP cells were the dominant mycobacteria- and influenza-specific CD4+ T-cell subsets (mycobacteria, IFN-γ+TNF+ DP 50% and TNF+ SP 22%; influenza, IFN-γ+TNF+ DP 44% and TNF+ SP 38%) (Figures 5A and 5B). These data show that cells that simultaneously produce IFN-γ and TNF (IFN-γ+TNF+ DP) form an integral part of the mycobacteria- and influenza-specific CD4+ T-cell responses in BAL and peripheral blood in HIV-uninfected adults.
Figure 4.
Impaired polyfunctional mycobacteria-specific alveolar CD4+ T cells in HIV-infected adults on antiretroviral therapy (ART). Nonadherent bronchoalveoalar lavage (BAL) cells were stimulated overnight with (A) purified protein derivative and (B) influenza vaccine. Cells were analyzed by flow cytometry for IFN-γ, tumor necrosis factor (TNF), and IL-17 production by the CD4+ T-cell population. Data are shown as relative distribution of IFN-γ–, TNF–, and IL-17–producing CD4+ T-cell subsets within the total response. Bar charts represent the mean and SEM of the contribution of the indicated subset (x axis) toward the total antigen-specific CD4+ T-cell response against the indicated participant groups (color coded as shown). P values were computed using Student t test (denoted as “+“ if P < 0.05), comparing each group against HIV-uninfected adults. Each pie chart represents the mean distribution across subjects of different antigen-specific cytokine-producing CD4+ T-cell subsets (color coded as shown) within the total response in a particular group. (C) Relative distribution of polyfunctional IFN-γ– and TNF-producing CD4+ T cells within the total response. (HIV−, n = 32; HIV+ ART naive, n = 25; HIV+ ART < 4 yr, n = 24; HIV+ ART ≥ 4 yr, n = 15).
Figure 5.
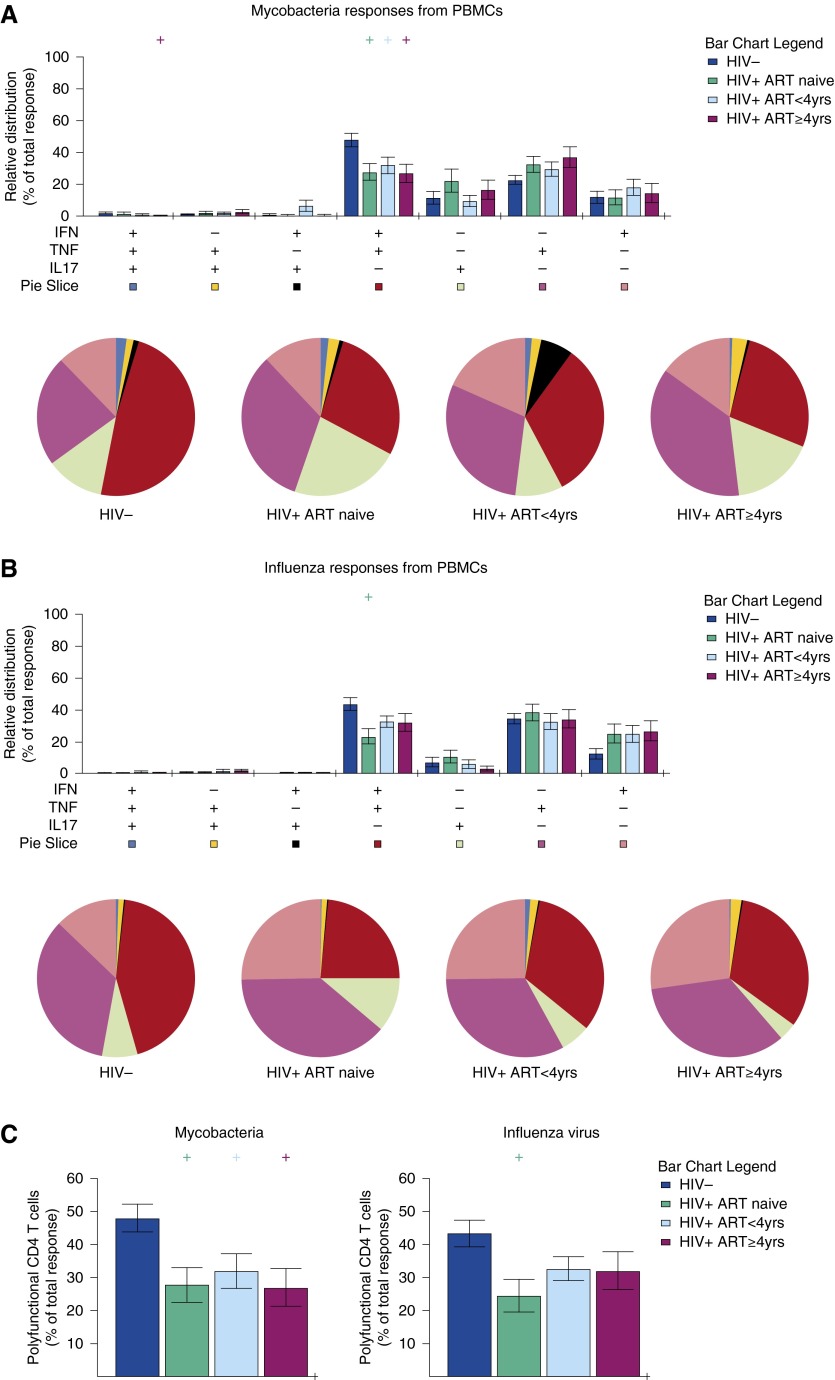
Impaired polyfunctional mycobacteria-specific blood CD4+ T cells in HIV-infected adults on antiretroviral therapy (ART). Peripheral blood mononuclear cells (PBMCs) were stimulated overnight with (A) purified protein derivative and (B) influenza vaccine. Cells were analyzed by flow cytometry for IFN-γ, tumor necrosis factor (TNF), and IL-17 production by the CD4+ T-cell population. Data are shown as relative distribution of IFN-γ–, TNF–, and IL-17–producing CD4+ T-cell subsets within the total response. Bar charts represent the mean and SEM of the contribution of the indicated subset (x axis) toward the total antigen-specific CD4+ T-cell response against the indicated participant groups (color coded as shown). P values were computed using Student t test (denoted as “+ “ if P < 0.05), comparing each group against HIV-uninfected adults. Each pie chart represents the mean distribution across subjects of different antigen-specific cytokine-producing CD4+ T-cell subsets (color coded as shown) within the total response in a particular group. (C) Relative distribution of polyfunctional IFN-γ– and TNF-producing CD4+ T cells within the total response. (HIV−, n = 32; HIV+ ART naive, n = 25; HIV+ ART < 4 yr, n = 28; HIV+ ART ≥ 4 yr, n = 22).
Next, we compared the relative contribution of mycobacteria- and influenza-specific cytokine-producing CD4+ T-cell subsets within the total responses between HIV-infected and HIV-uninfected adults. We found that the relative contribution of mycobacteria-specific IFN-γ+TNF+ DP CD4+ T cells in the total response was lower in HIV-infected ART-naive compared with HIV-uninfected adults, both in BAL (20% vs. 36%; P = 0.017) and blood (26% vs. 50%; P = 0.006) (Figures 4C and 5C). The relative contribution of influenza-specific IFN-γ+TNF+ DP CD4+ T cells in the total response was lower in HIV-infected ART-naive compared with HIV-uninfected adults, both in BAL (8% vs. 20%; P = 0.018) and blood (26% vs. 44%; P = 0.006) (Figures 4C and 5C). These findings suggest that mycobacteria- and influenza-specific polyfunctional CD4+ T-cell responses are impaired in the lung and peripheral blood after HIV infection.
Following the observation that mycobacteria- and influenza-specific polyfunctional CD4+ T-cell responses are impaired in HIV-infected ART-naive adults, we investigated whether they are also impaired in individuals on ART. We found that the relative contribution of mycobacteria-specific IFN-γ+TNF+ DP CD4+ T cells in the total response was lower in HIV-infected individuals on ART compared with HIV-uninfected adults, both in BAL (<4 yr ART, 20% vs. 36%, P = 0.017; ≥4 yr ART, 23% vs. 36%, P = 0.046) and blood (<4 yr ART, 30% vs. 50%, P = 0.023; ≥4 yr ART, 26% vs. 50%, P = 0.005) (Figures 4C and 5C). However, the relative contribution of influenza-specific alveolar IFN-γ+TNF+ DP CD4+ T cells in the total response was lower in BAL in HIV-infected individuals on ART compared with HIV-uninfected adults (<4 yr ART, 10% vs. 20%, P = 0.045; ≥4 yr ART, 4% vs. 10%, P = 0.001), but was normalized in peripheral blood in both ART groups (all P > 0.05) (Figures 4C and 5C). These data suggest that asymptomatic HIV-infected adults on ART have impaired mycobacteria-specific polyfunctional CD4+ T-cell responses in BAL and peripheral blood irrespective of duration of treatment. They also suggest differences in antigen-specific polyfunctional CD4+ T-cell responses between pathogens, which in the case of influenza-specific polyfunctional CD4+ T-cell responses seems compartmentalized.
Discussion
HIV impairs pulmonary innate and antigen-specific adaptive immune responses leading to increased risk to lower respiratory tract infections (LRTI). Although morbidity and mortality caused by LRTI has fallen dramatically among HIV-infected adults on ART (7–10), they still remain at higher risk of TB than their HIV-uninfected counterparts (11). In this study, we investigated whether impaired pulmonary innate and antigen-specific adaptive immune responses persist in HIV-infected adults on ART to explain, at least in part, their increased susceptibility to pulmonary TB. Although our findings do not directly demonstrate causality, they are consistent with a delay in the reversal of impaired pulmonary innate immune responses and an incomplete reconstitution of mycobacteria-specific CD4+ T-cell responses.
We have shown that compared with HIV-uninfected adults, AM phagosomal proteolysis was not impaired in HIV-infected adults who had been on ART for greater than or equal to 4 years but was impaired in those on ART for less than 4 years. The temporal association between recovery of AM proteolysis and duration of ART is consistent with previous studies that profiled the kinetics of peripheral blood CD4+ T-cell count recovery during ART (7, 8). AM are key effectors of innate immunity in the lung (15) and constitute over 90% of immune cells in BAL from healthy HIV-uninfected adults (16). AM also serve as APCs (17), although they are less efficient than pulmonary dendritic cells (18). In the alveolar space, primary CD4+ T-cell immune responses are initiated by dendritic cells, whereas secondary responses are initiated by both dendritic cells and AM (17, 18). Costimulation is essential for initiation of primary CD4+ T-cell responses (19). AM do not initiate these responses due to defective costimulation via the CD28 pathway because they do not express B7-1 or B7-2 antigens (20). Secondary CD4+ T-cell responses, however, are less dependent on accessory costimulatory signals (19); consequently, AM are able to initiate these responses (17). We speculate that impaired AM proteolysis affects antigen processing and presentation (21), resulting in suboptimal initiation of antigen-specific alveolar CD4+ T-cell responses. Impaired AM innate immune functions may, therefore, leave HIV-infected individual on ART vulnerable to LRTI.
Antigen-specific CD4+ T-cell responses are important in defense against common respiratory pathogens including Mtb (22–24) and influenza virus (25). Consistent with our previous observations (5) and those of others (4), we found low frequencies of total mycobacteria- and influenza-specific cytokine-producing Th1 CD4+ T cells in BAL but not peripheral blood in asymptomatic HIV-infected adults. However, the frequencies of total antigen-specific Th1 CD4+ T cells were normalized in individuals on ART, with influenza-specific CD4+ T cells normalizing earlier than mycobacteria-specific CD4+ T cells. Together, these data suggest that impaired respiratory pathogen-specific CD4+ T-cell responses are compartmentalized in the lung in HIV-infected adults, and normalization of the total Th1 mycobacteria-specific alveolar CD4+ T cells requires longer duration on ART.
Although the frequency of total mycobacteria- and influenza-specific cytokine-producing Th1 CD4+ T cells in BAL was normal in HIV-infected adults who had been on ART for greater than or equal to 4 years, the proportion of some CD4+ T-cell subsets within the total population were altered. Specifically, the proportion of mycobacteria- and influenza-specific IFN+TNF+ DP CD4+ T cells were lower than those observed in HIV-uninfected adults. IFN-γ and TNF play important roles in defense against Mtb (1, 9, 22) and influenza virus infections (26, 27). Our findings suggest preferential depletion of these antigen-specific polyfunctional CD4+ T cells by HIV and incomplete recovery during long-term ART. Polyfunctional CD4+ T cells correlate with control of viral pathogens in humans (28, 29) and protection against disease progression in murine models of bacterial pathogens (30), including Mtb (31). Given the importance of IFN-γ and TNF in host defense against Mtb and influenza virus, it is plausible that incomplete restoration of mycobacteria- and influenza-specific IFN-γ and TNF-producing alveolar CD4+ T-cell responses early during ART would put the host at increased risk of developing active TB (11) and influenza-associated pathology (32). In support of this hypothesis, the highest mortality caused by HIV-related LRTI occurs during the early years following initiation of ART (33–35).
We also explored the impact of HIV infection and ART on antigen-specific cytokine-producing Th17 CD4+ T cells in BAL and blood. Previous studies reported lower mycobacteria-specific cytokine-producing peripheral blood Th17 CD4+ T cells in TB patients compared with persons with latent TB infection. Furthermore, patients with severe TB had significantly lower Th17 response than those with mild disease (36). However, there are limited data on IL-17 in the alveolar compartment because previous studies were hampered by difficulties in detecting alveolar Th17 cells because of their very low frequencies (37) and low concentrations of IL-17 in diluted BAL fluid (38). Consistent with the findings of Semple and coworkers (39), we detected low frequencies of mycobacteria-specific cytokine-producing Th17 CD4+ T cells in BAL and found no significant difference between healthy HIV-uninfected and asymptomatic HIV-infected adults, irrespective of their ART duration status. These findings suggest that susceptibility to TB in HIV-infected adults on ART may not be caused by depletion of mycobacteria-specific alveolar Th17 CD4+ T cells.
The cross-sectional nature of this study constitutes a limitation. Ideally, a longitudinal study sampling at different time points following initiation of ART would have allowed more detailed comparisons at the level of individual participants. However, given the invasive nature and the associated risk of bronchoscopy, especially in HIV-infected persons, a longitudinal study was judged not to be justifiable. Furthermore, the alveolar lymphocyte counts from HIV-uninfected individuals were higher than has been reported previously (4, 5, 40). Household indoor air pollution is highly prevalent in Malawi (41) and it is possible that this group of individuals had ongoing alveolar inflammation. Nonetheless, the abnormal alveolar lymphocyte cell count did not correlate with frequency of mycobacteria- and influenza-specific alveolar Th1 CD4+ T cells, and does not alter the main findings and conclusion of the study.
In conclusion, we have shown that asymptomatic HIV-infected adults on ART have impaired AM phagosomal proteolysis and low frequencies of total mycobacteria-specific cytokine-producing alveolar Th1 CD4+ T cells, most marked during the first few years of treatment. These defects are not present in those who receive ART for greater than or equal to 4 years. In contrast, mycobacteria antigen-specific polyfunctional alveolar and blood CD4+ T-cell responses are impaired in HIV-infected adults on ART irrespective of the duration of treatment. Persistence of these immune defects particularly early during ART may in part explain the high susceptibility to pulmonary TB among HIV-infected individuals on ART.
These findings have important public health implications for developing strategies to reduce the high burden of TB in patients initiating ART. Use of TB preventive chemoprophylaxis during the first 4 years of ART has recently been shown to be a possible intervention strategy in a clinical trial (42). Although the trial did not address the underlying reasons for the high TB incidence, it showed that isoniazid prophylaxis reduced the incidence of TB when used in combination with ART. To the best of our knowledge, our study is the first to show that HIV-infected adults on ART still have impaired anti-TB adaptive immune responses in the lung. Alternatively, the development of subunit vaccines that promote repopulation of the lung with crucial mycobacteria-specific polyfunctional CD4+ T-cell subsets ought to be considered. Irrespective of the strategy, this study identifies early ART as a period of particular vulnerability in the treatment of HIV infections.
Acknowledgments
Acknowledgment
The authors thank all study participants, Mrs. Kunkeyani, Mrs. Kanyandula, and staff of Malawi-Liverpool-Wellcome Trust Clinical Research Programme and Queen Elizabeth Central Hospital for their support and cooperation during the study.
Footnotes
Supported by the National Commission for Science and Technology-HRCSI (Malawi) through grant number RG-J/2009/KJ/0003 awarded to K.C.J., the Wellcome Trust through an Intermediate Clinical Fellowship number 088,696/Z/09/Z awarded to H.C.M., and the National Institutes of Health through grant numbers HL100928 and AI095519 awarded to D.G.R. R.S.H. is supported by a Strategic Award for the MLW Clinical Research Program from the Wellcome Trust.
Author Contributions: Conception and design, K.C.J., H.C.M., S.B.G., R.S.H., D.G.R., R.D.M., A.M.K., and T.J.A. Analysis and interpretation, K.C.J., H.C.M., D.G.R., R.S.H., L.A., D.H.B., and S.B.G. Drafting the manuscript for important intellectual content, K.C.J., H.C.M., D.G.R., R.S.H., and S.B.G. Final approval, K.C.J., H.C.M., S.B.G., R.S.H., D.G.R., R.D.M., A.M.K., T.J.A., L.A., and D.H.B.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201405-0864OC on September 16, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, Chimzizi R, Harrington M, Maher D, Williams BG, De Cock KM. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–1919. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 3.Houben RM, Crampin AC, Ndhlovu R, Sonnenberg P, Godfrey-Faussett P, Haas WH, Engelmann G, Lombard CJ, Wilkinson D, Bruchfeld J, et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int J Tuberc Lung Dis. 2011;15:24–31. [PubMed] [Google Scholar]
- 4.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med. 2009;180:1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jambo KC, Sepako E, Fullerton DG, Mzinza D, Glennie S, Wright AK, Heyderman RS, Gordon SB. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 2011;66:375–382. doi: 10.1136/thx.2010.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, Losso M, Lazzarin A, Fatkenheuer G, Lundgren JD EuroSIDA study group. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–413. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 8.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 9.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 10.Suthar AB, Lawn SD, del Amo J, Getahun H, Dye C, Sculier D, Sterling TR, Chaisson RE, Williams BG, Harries AD, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 12.Gordon SB, Malamba R, Mthunthama N, Jarman ER, Jambo K, Jere K, Zijlstra EE, Molyneux ME, Dennis J, French N. Inhaled delivery of 23-valent pneumococcal polysaccharide vaccine does not result in enhanced pulmonary mucosal immunoglobulin responses. Vaccine. 2008;26:5400–5406. doi: 10.1016/j.vaccine.2008.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol. 2009;9:594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6:413–420. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 15.Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Riches DWH, Fenton MJ.Monocytes, macrophages and dendritic cells of the lung Mason RJ, Broaddus VC, Murray JF, Nadel JA.Murray and Nadel's textbook of respiratory medicine, online versionPhiladephia, PA: Elsevier; 2009. [Google Scholar]
- 17.Kugathasan K, Roediger EK, Small CL, McCormick S, Yang P, Xing Z. CD11c+ antigen presenting cells from the alveolar space, lung parenchyma and spleen differ in their phenotype and capabilities to activate naïve and antigen-primed T cells. BMC Immunol. 2008;9:48. doi: 10.1186/1471-2172-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt PG. Regulation of antigen-presenting cell function(s) in lung and airway tissues. Eur Respir J. 1993;6:120–129. [PubMed] [Google Scholar]
- 19.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 20.Chelen CJ, Fang Y, Freeman GJ, Secrist H, Marshall JD, Hwang PT, Frankel LR, DeKruyff RH, Umetsu DT. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Invest. 1995;95:1415–1421. doi: 10.1172/JCI117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musson JA, Walker N, Flick-Smith H, Williamson ED, Robinson JH. Differential processing of CD4 T-cell epitopes from the protective antigen of Bacillus anthracis. J Biol Chem. 2003;278:52425–52431. doi: 10.1074/jbc.M309034200. [DOI] [PubMed] [Google Scholar]
- 22.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun. 2010;78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahey T, Sheth S, Matee M, Arbeit R, Horsburgh CR, Mtei L, Mackenzie T, Bakari M, Vuola JM, Pallangyo K, et al. Interferon γ responses to mycobacterial antigens protect against subsequent HIV-associated tuberculosis. J Infect Dis. 2010;202:1265–1272. doi: 10.1086/656332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allie N, Grivennikov SI, Keeton R, Hsu NJ, Bourigault ML, Court N, Fremond C, Yeremeev V, Shebzukhov Y, Ryffel B, et al. Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection. Sci Rep. 2013;3:1809. doi: 10.1038/srep01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 26.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76:1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciuffreda D, Comte D, Cavassini M, Giostra E, Bühler L, Perruchoud M, Heim MH, Battegay M, Genné D, Mulhaupt B, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 29.Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, Lennox J, Amara RR. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007;81:12071–12076. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 31.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34:304–307. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, Pellegrin JL, Katlama C, Dabis F, Leport C Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO8 APROCO-COPILOTE Study Group; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO3 AQUITAINE Study Group. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72–77. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 34.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, Dabis F, Pascoe M, Egger M International Epidemiological Databases to Evaluate AIDS (IeDEA) Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, Liu H, Zhou B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 37.Perreau M, Rozot V, Welles HC, Belluti-Enders F, Vigano S, Maillard M, Dorta G, Mazza-Stalder J, Bart PA, Roger T, et al. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4+ T cells in active disease. Eur J Immunol. 2013;43:939–948. doi: 10.1002/eji.201243090. [DOI] [PubMed] [Google Scholar]
- 38.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semple PL, Binder AB, Davids M, Maredza A, van Zyl-Smit RN, Dheda K. Regulatory T cells attenuate mycobacterial stasis in alveolar and blood-derived macrophages from patients with tuberculosis. Am J Respir Crit Care Med. 2013;187:1249–1258. doi: 10.1164/rccm.201210-1934OC. [DOI] [PubMed] [Google Scholar]
- 40.Schwander S, Dheda K. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. Am J Respir Crit Care Med. 2011;183:696–707. doi: 10.1164/rccm.201006-0963PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fullerton DG, Suseno A, Semple S, Kalambo F, Malamba R, White S, Jack S, Calverley PM, Gordon SB. Wood smoke exposure, poverty and impaired lung function in Malawian adults. Int J Tuberc Lung Dis. 2011;15:391–398. [PubMed] [Google Scholar]
- 42.Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, Wilkinson KA, Goliath R, Mathee S, Goemaere E, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]