Abstract
Background
The purpose of this study was to determine factors that impact recurrence and long-term survival of head and neck adenoid cystic carcinoma (ACC).
Methods
We conducted a retrospective review of 87 patients with head and neck ACC who were evaluated between 1992 and 2009. Staining for Ki-67, p53, α-estrogen receptor (αER), and progesterone receptor (PR) was performed.
Results
Forty men (46%) and 47 women (54%) were included in this study. Median follow-up for patients was 98 months. Five-year recurrence-free and overall survival (OS) rates were 56% and 81%, respectively. Ki-67 and p53 expression was observed in 5 (6%) and 2 (2%) patients, respectively. αER and PR were all negative. The most important determinants of disease-free survival (DFS) were perineural invasion (PNI; p = .001) and female sex (p = .027). Disease site (major vs minor salivary gland) was the only predictor of worse OS on multivariate analysis.
Conclusion
Perineural invasion, female sex, and disease site were the most consistent predictors of poor outcome in head and neck ACC.
Keywords: adenoid cystic carcinomas, head and neck, prognostic factors, disease-free survival, overall survival
INTRODUCTION
Adenoid cystic carcinoma (ACC) is the fourth most common malignant epithelial salivary gland neoplasm, accounting for approximately 10% of all salivary carcinomas. 1 The 5-year survival rates for ACC are relatively high and lymph node metastases are rare. However, despite aggressive treatment, the disease is difficult to manage because of extensive local tissue infiltration and perineural spread, with 5-year recurrence rates of 35% to 50%.2-4
There is much discordance among the literature regarding which clinicopathological factors significantly affect outcomes of head and neck ACC. Previously identified variables include perineural invasion (PNI), positive margin status at surgery, cervical lymph node metastasis, solid histology subtype,2 site of origin,5,6 high Ki-67 expression,3,7 and sex.8,9
In this study, the authors retrospectively reviewed cases of ACC treated at a major tertiary care center, the Ohio State University Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (James Cancer Hospital) in Columbus, Ohio, and compared clinicopathological features, treatment modalities, patterns of failure, and outcomes among patients with ACC originating from various head and neck sites. Immunohistochemical staining for Ki-67, p53, α-estrogen receptor (αER), and progesterone receptor (PR) was performed and correlated with the clinical parameters. The purpose of this study was to determine patient-related factors that impact disease-free survival (DFS) and overall survival (OS) of ACC of the head and neck among patients who underwent definitive treatment in the modern surgical era.
MATERIALS AND METHODS
Patient characteristics and tumor definition
The James Cancer Hospital Institutional Review Board approved the study (protocol no. 2009C0094). The study was exempt from written informed consent because of its retrospective nature. From 1992 to 2009, patients with the diagnosis of ACC of the head and neck were identified from the James Cancer Hospital Tumor Registry. Data concerning patient characteristics, clinical and pathologic tumor characteristics, and treatment modalities were obtained through a retrospective review of the medical records. All patients with a diagnosis of ACC who had tumor pathology reviewed at our institution were included in the study. Patients with evidence of metastatic disease at presentation or incomplete medical records were excluded from the study. Follow-up data were obtained until May 2012.
Major salivary gland tumors were classified according to the TNM classification of malignant tumors of the International Union Against Cancer.10 Minor salivary gland tumors were classified according to the staging system for the site of origin.11 All cases were validated after re-review by a head and neck pathologist to confirm the diagnosis of ACC. Tumors were subcategorized into 1 of 4 subtypes (cribriform, tubular, trabecular, or solid) when the predominant architectural subtype represented at least 70% of the specimen.12
Tissue microarray construction and immunohistochemistry
Representative tumor areas were selected and marked on hematoxylin-eosin–stained slides for the construction of microarrays. Duplicate cylindrical cores with a diameter of 1.5 mm were taken from tissue formalin-fixed paraffin-embedded tumor blocks using a tissue microarrayer and were assembled in a tissue microarray format. Serial 4-μm sections were used for immunohistochemical (IHC) staining.
IHC stains were performed using commercially available antibodies, steam-induced epitope retrieval, and DAKO Envision detection systems (DAKO, Carpentaria, CA) on formalin-fixed paraffin-embedded sections. The antibodies included PR (clone 1A6, dilution 1:800; DAKO), αER (clone ID5, dilution 1:600; DAKO), p53 (clone DO-7, dilution 1:50; DAKO), and Ki-67 (clone MIB-1, dilution 1:150; DAKO).
The expression of PR and αER was assessed semiquantitatively for the percent of tumor cells staining (0%; <5%; 5% to <25%; 25% to <50%; 50% to <75%; and ≥75%) and intensity (0, negative; 1+, weak staining/trace; 2+, moderate staining; and 3+, strong staining). Only nuclear staining was considered positive. Criteria set forth by Luo et al13 and Motta Rda et al14 for p53 and Ki-67 proliferative index were used to classify the IHC staining results as positive when staining was ≥10% and negative when staining was <10%. The final expression of each marker was the average of the 2 microarray cores for each sample.
Statistical analysis
OS was defined as the time from the date of initial definitive treatment to the date of death. Patients who were alive at the date of last observation were censored for survival analysis. DFS was defined as the time from the date of the initial definitive treatment to the date of disease recurrence. Patients without recurrence at the data of last observation were censored for disease control analysis. Survival curves were estimated using the method of Kaplan–Meier.15 Because of the retrospective nature of the data collection, we were unable to determine diseasespecific survival in the patient cohort. The following predictors were analyzed as prognostic variables: age, older than 50 years; history of alcohol and/or tobacco use; radiation therapy and/or chemotherapy; sex; overall stage; PNI; lymphovascular invasion; resection margins; histological subtype; site of primary tumor; and p53, Ki-67, αER, and PR expression. Patients were subcategorized according to these variables and the curves were compared by the logrank tests for the univariate analysis. Multivariable Cox regression models were fit to the OS and DFS, respectively, using all the variables with p < .2 in the univariate analysis.16 Variables with p > .05 were removed sequentially from the Cox regression model. The associations between categorical variables were assessed by chi-square test or Fisher exact test, whichever was appropriate.
RESULTS
Clinicopathological features
Eighty-seven patients met the inclusion criteria of the study (Table 1). This included 40 men (46%) and 47 women (54%) with a median age of 51 years (range, 21–83 years). Eighty-five patients (98%) had symptoms on presentation. Fifty-eight patients (67%) presented with a head and neck mass; 19 patients (22%) presented with neurologic dysfunction, such as facial pain, numbness, or weakness; and the remaining patients (n = 16; 18%) presented with a range of symptoms that included headache, congestion, vision changes, ear pain, hearing loss, eye tearing, hoarseness, and nasal regurgitation.
TABLE 1.
Characteristics of patients with adenoid cystic carcinoma.
| Patient characteristics | No. of patients (%) |
|---|---|
| No. of patients | 87 |
| Sex | |
| Male | 40 (46) |
| Female | 47 (54) |
| Median age, y | 51 (range, 21–83) |
| <50 | 43 (49) |
| >50 | 44 (51) |
| Tobacco use, yes | 49 (56) |
| Alcohol use, yes | 24 (28) |
| Primary site | |
| Parotid gland | 19 (22) |
| Sublingual/submandibular gland | 18 (21) |
| Oral cavity/oropharynx salivary glands | 15 (17) |
| Sinonasal cavity salivary glands | 27 (31) |
| Other | 8 (9) |
| T Classification | |
| T1 | 26 (30) |
| T2 | 21 (24) |
| T3 | 17 (20) |
| T4 | 23 (26) |
| N Classification | |
| N0 | 79 (91) |
| N1+ | 8 (9) |
| Overall stage | |
| I | 24 (28) |
| II | 22 (25) |
| III | 16 (18) |
| IV | 25 (29) |
| Histologic subtype | |
| Cribriform | 47 (54) |
| Tubular | 22 (25) |
| Trabecular | 7 (8) |
| Solid | 10 (11) |
| Symptoms at presentation, yes | 86 (98) |
| Mass | 58 (67) |
| Weakness/numbness/pain | 19 (22) |
| Other | 16 (18) |
| Primary treatment modality | |
| Surgery only | 39 (45) |
| Surgery and adjuvant XRT | 45 (52) |
| Surgery and adjuvant chemotherapy/XRT | 4 (5) |
| Recurrence, yes | 53 (61) |
| Median time to recurrence, mo | 59 (range, 1–240) |
| Alive at last follow-up | 52 (60) |
| Median follow-up, mo | 98 (range, 1–431) |
Abbreviation: XRT, external beam radiation therapy.
Thirty-seven patients (43%) presented with major salivary gland (parotid, sublingual, or submandibular gland) and 58 patients (57%) presented with minor salivary gland involvement. At presentation, T classification distribution was as follows: T1, 30% (n = 26); T2, 24% (n = 21); T3, 20% (n = 17); and T4, 26% (n = 23). Eight patients (9%) were found to have lymph node involvement at presentation.
Histopathology and immunohistochemistry
On pathological analysis, 19 patients (22%) had lymphovascular invasion and 52 patients (60%) had PNI. Forty-two patients (48%) had negative margins. The cribriform pattern was the predominant histological subtype identified in 47 patients (54%), followed by the tubular pattern in 22 patients (25%), solid pattern in 10 patients (11%), and trabecular pattern in 7 patients (8%; Table 1).
The p53 and Ki-67 staining was positive in 2 patients (2%) and 5 patients (6%), respectively. None of the tumor samples showed positive expression for either αER or PR.
Treatment modalities and outcomes
All patients were treated with primary surgery. Neck dissection was performed in 28 patients (32%). Neck dissection was reserved for those patients with clinical examination or imaging suspicious for lymph node involvement. Forty-nine patients (57%) received adjuvant radiation therapy after surgical resection. The average radiation dose delivered to the primary site was 5600 cGy (range, 3000–6840 cGy). Twelve patients (14%) received concomitant chemoradiation therapy. The median follow-up was 98 months (range, 3–432 months).
The 5-year DFS and OS rates were 56% and 81%, respectively. Thirty-four patients (39%) experienced local recurrence, 12 patients (14%) had regional recurrence at the level of the neck, and 20 patients (23%) had distant recurrence. Fourteen patients (16%) had ACC recurrence at more than 1 site. Of the patients with distant metastases, 19 had metastases to the lungs and 3 had bone metastases. The most commonly involved bone was the femur.
Prognostic factors
Univariate analysis revealed significantly worse DFS with the presence of PNI and positive resection margins (Table 2). Of the 35 patients without PNI, 18 patients (51%) had disease recurrence at an estimated median of 132 months (95% confidence interval [CI], 96–208) versus recurrence at 60 months (95% CI, 43–76) in the 34 patients (65%) with PNI (p = .002). A positive resection margin after primary resection also conferred significantly poorer DFS with an estimated median recurrence at 60 months (95% CI, 41–73) versus 108 months (95% CI, 96–204) in patients with negative resection margins (p = .02). Presence of PNI (p = .001) and sex (p = .027) carried a statistically significant impact on DFS on multivariate analysis (Table 3, Figure 1).
TABLE 2.
Univariate analysis assessed by log-rank tests.
| Variable | DFS
|
OS
|
||
|---|---|---|---|---|
| Median, mo | p value | Median, mo | p value | |
| Age at diagnosis, y | .09 | .44 | ||
| ≤50 | 60 | 204 | ||
| >50 | 99 | 178 | ||
| Sex | .07 | .73 | ||
| Female | 60 | 237 | ||
| Male | 99 | 195 | ||
| Tobacco use | .47 | .68 | ||
| No | 96 | 195 | ||
| Yes | 61 | 237 | ||
| Alcohol use | .48 | .84 | ||
| No | 76 | 195 | ||
| Yes | 61 | 207 | ||
| Histologic subtype | .71 | .52 | ||
| Cribriform | 96 | 204 | ||
| Tubular | 72 | 313 | ||
| Trabecular | 144 | 125 | ||
| Solid | 41 | 380 | ||
| Stage | .25 | .01 | ||
| I | 108 | 207 | ||
| II | 96 | N/A | ||
| III | 72 | 178 | ||
| IV | 48 | 91 | ||
| Site | .22 | .002 | ||
| Major salivary gland | 61 | 237 | ||
| Sinonasal cavity | 59 | 105 | ||
| Oral cavity | 204 | 313 | ||
| PNI | .002 | .13 | ||
| No | 132 | 204 | ||
| Yes | 60 | 178 | ||
| Lymphovascular invasion | .31 | .02 | ||
| No | 96 | 207 | ||
| Yes | 59 | 66 | ||
| Margin status | .02 | .28 | ||
| Negative | 108 | 195 | ||
| Positive | 60 | 237 | ||
| Adjuvant radiation therapy | .61 | .45 | ||
| No | 96 | 313 | ||
| Yes | 73 | 178 | ||
| Chemotherapy | .33 | .86 | ||
| No | 96 | 195 | ||
| Yes | 41 | 204 | ||
| Ki-67 | .21 | .09 | ||
| Negative | 76 | 204 | ||
| Positive | 41 | 105 | ||
Abbreviations: DFS, disease-free survival; OS, overall survival; N/A, not applicable; PNI, perineural invasion.
The p values in boldface are statistically significant.
TABLE 3.
Multivariate analysis assessed by the Cox regression model with backward selection using all the variables with p < .2 from the univariate analysis.
| DFS
|
OS
|
||
|---|---|---|---|
| Variable | p value | Variable | p value |
| PNI | .001 | Site | .022 |
| Sex | .027 | Stage | .123 |
| Age | .150 | Lymphovascular invasion | .138 |
| Surgical margins | .970 | Ki-67 expression | .176 |
| PNI | .851 | ||
Abbreviations: DFS, disease-free survival; OS, overall survival; PNI, perineural invasion.
FIGURE 1.
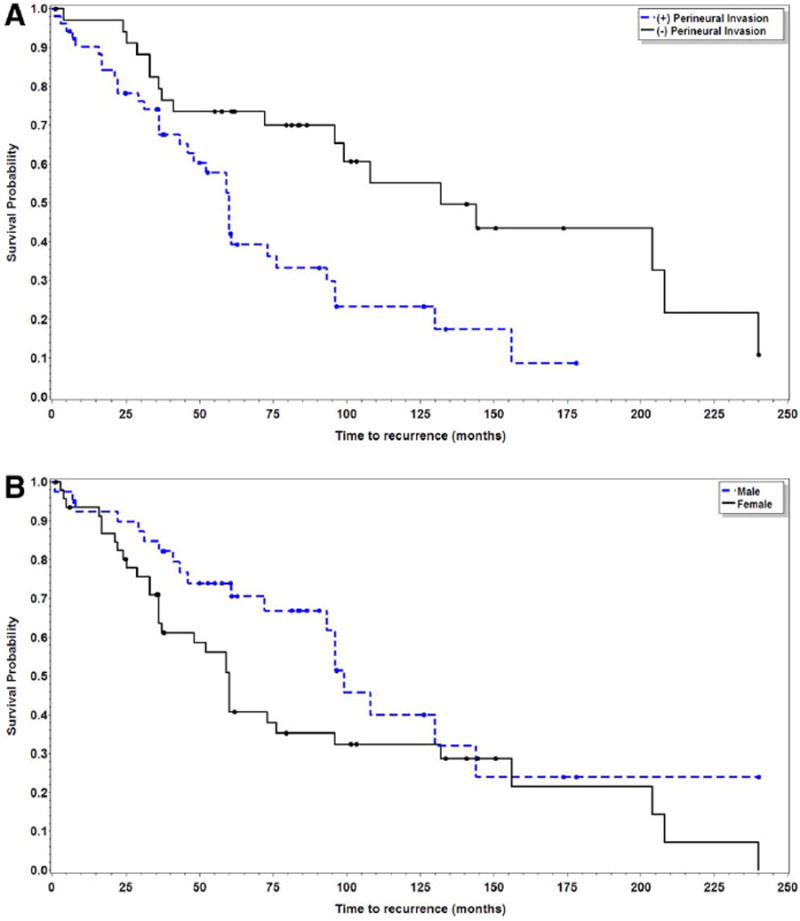
Kaplan–Meier plots for disease recurrence in patients with and without perineural invasion (p < .01) (A) and sex (p = .03) (B). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
On univariate analysis, OS was negatively impacted by the presence of lymphovascular invasion, site of primary tumor, and stage (Table 2). The estimated median OS was 207 months (95% CI, 173–380) in patients without lymphovascular invasion versus 66 months (95% CI, 31–NA) in those with lymphovascular invasion (p = .02). Twelve patients (32%) with major salivary gland disease were deceased at the conclusion of the study for an estimated median OS of 237 months (95% CI, 195–NA), and 23 patients (46%) with minor salivary gland disease were deceased at the conclusion of the study for an estimated median OS of 128 months (95% CI, 86–313; p = .01). Stage of disease was a poor prognostic factor for OS (p = .01) with median survival of 207 months (95% CI, 134–380) and 91 months (95% CI, 25–NA) for stage I and IV disease, respectively. On multivariate analysis, the site of the primary tumor was the only factor that conferred a worse prognosis in terms of OS (Table 3, Figure 2).
FIGURE 2.
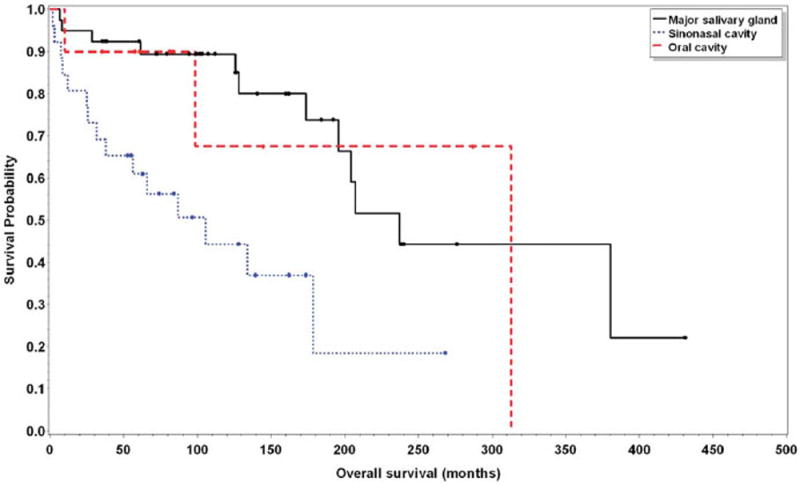
Kaplan–Meier plot for overall survival in patients with major salivary gland, oral cavity, and sinonasal cavity sites (p = .02). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
ACC accounts for 10% of malignant salivary gland neoplasms.1 The tumor is predominantly biologically low grade and is known for its chronic, indolent nature; however, in the long-term, it has a significant ability to metastasize. Precedent literature on the clinicopathologic predictors of prognosis in head and neck ACC are conflicting. In this study, we found that the prognosis of head and neck ACC is worse with the presence of PNI, female sex, and disease site.
Extensive PNI in patients with ACC has previously been identified as an important prognostic factor for OS and DFS.2,5,17,18 This study supports previous data that identifies PNI as a poor prognostic factor of DFS (p < .01). We did not identify a correlation between PNI and OS (p = .13). The lack of significant impact on OS in these patients may be secondary to the indolent nature of ACC with frequent recurrences but overall good longterm survival. It may be that these patients’ are succumbing to diseases unrelated to their cancer resulting in the lack of correlation between prognostic factors for disease recurrence and OS.
Advanced stage has been reported as a significant prognostic factor in multiple previous studies.5,8,18 In our study, advanced stage had a negative impact on OS (p < .01) but not on DFS (p = .25) on univariate analysis alone. We found significantly higher rates of PNI (81% vs 41%; p < .01) and positive postsurgical margins (69% vs 37%; p < .01) in more advanced disease.
We found the minor salivary glands of the sinonasal cavity as the most common site of ACC in the head and neck, followed by tumors of the parotid, submandibular, and sublingual glands. The minor salivary glands of the oral cavity, oropharynx, and larynx were least common. The primary site of involvement of ACC has previously been reported as a prognostic factor, with minor salivary gland ACC having a worse prognosis when compared to major salivary gland ACC.5,9,18,19 We compared the OS between sinonasal (estimated median=105; 95% CI, 37– NA), oral cavity (estimated median OS=313; 95% CI, 25–313), and major salivary gland (estimated median OS 237, 95% CI, 195–NA) sites of ACC, and found significantly worse survival in tumors originating in the sinonasal cavity (p = .002). Pitman et al19 also reported that patients with ACC of the sinonasal cavity were at high risk of recurrence and speculated that this might be due to higher stage at presentation, higher incidence of PNI, and higher rates of positive margins in these patients. This is, in part, because of the challenge of resecting tumors arising in the sinonasal cavity. In our study, the site of disease correlated only with overall disease stage in which minor salivary gland involvement was more likely to present with advanced-stage disease (stage III or IV) when compared to major salivary gland ACC (53% vs 39%, respectively; p = .13).
In contrast to head and neck squamous cell carcinoma, lymphatic spread to the neck plays a minor role in the spread of ACC.3 In our study, only 8 patients showed regional nodal metastases at diagnosis, and 12 patients developed regional recurrence. Despite low rates of lymphatic spread, we found lymphovascular invasion to be correlated with a worse outcome in terms of OS (p = .02) on univariate analysis.
The majority of patients (52%; n = 45) in our cohort were found to have positive surgical margins on histopathology. The high rate of positive margin status in ACC is thought to be related to high rates of skip lesions, PNI, and perivascular invasion, and is supported by previous studies reporting higher rates of positive margins for ACC when compared to other head and neck malignancies. 2,20 The prognostic value of negative surgical margins, however, remains controversial in the literature. Most authors describe better local control rates after complete resection.5,6 We were able to confirm this in our study with positive surgical margins being highly correlated with disease recurrence (p < .01) but it did not correlate with OS (p = .13) on univariate analysis. This reaffirms the indolent nature of this disease including long-term survival despite high recurrence rates.
Nascimento et al18 previously reported that older age significantly correlated with worse DFS and OS. In our study, age >50 approached significance on both univariate and multivariate analysis (p = .09 and p = .15, respectively) for correlating with decreased DFS. We found no statistical difference between patients under and over age 50 in the aggressiveness of surgical resection (p = .06), rates of adjuvant therapy (p = .18), rates of minor salivary gland involvement (p = .28), or stage of disease (p = .53) that would explain this difference. Interestingly, patients aged >50 had lower rates of positive surgical margins (42% vs 63%; p = .06).
The literature on the impact of sex in the clinical outcome of head and neck ACC is mixed. The largest epidemiologic data on ACC in the head and neck seems to suggest that women have better prognosis compared with men.21 However, in another study, female patients were noted to have shorter DFS.9 The findings from our study parallel the latter, with female sex approaching statistical significance as a prognostic factor for ACC recurrence (p = .07). It has been suggested that there may be a hormonal influence accounting for the different biological behaviors of ACC among the sexes.22 Reports of αER and PR expression in salivary gland ACC range from 0% to 100%.23-25 Possible explanations for this variation include clone variability and different methods of antigen retrieval. Our study revealed the absence of αER or PR expression in all the samples tested and paralleled those of others.23,24 These results suggest that estrogen and progesterone play a limited role, if any, in salivary gland ACC tumorigenesis.
Ki-67 is a marker of cellular proliferation. It is present during all active phases of the cell cycle, but is absent in resting cells. Higher Ki-67 expression has previously been correlated with poorer DFS and the solid subtype. 7,19 In our study, positive Ki-67 expression was noted in only 5 patients (6%). Ki-67 expression was not found to be significant on either multivariate or univariate analysis, however, these results should be viewed cautiously because of the small number of patients analyzed. P53 is the most common multifunctional gene aberration identified in human malignancy. Its expression in salivary gland ACC has been reported in 17% to 55% of cases.12,26 In our study, positive p53 expression was noted in 2 cases (2%). The low levels of p53 expression may be related to sample size and the use of stricter grading criteria that were set forth by Luo et al13 and Motta Rda et al14 in which positive expression was defined as staining ≥10% of the tumor nuclei. The low levels of significant p53 expression in this study suggest that p53 mutation is not a common occurrence in biologically lowgrade ACC.
The histologic subtype of ACC has been suggested to be predictive of its biologic behavior, with the solid subtype being the most clinically aggressive.5,8,18 Our study does not indicate worse prognosis for the solid ACC subtype nor did it correlate with a higher proliferate index. This may, however, be due to the low sample number with only 10 cases in the cohort identified with the solid pattern.
The current standard of care for head and neck ACC is complete surgical resection.2,4 That is also the current practice at our institution with all patients included in this cohort receiving primary curative surgery. However, because of the high rates of recurrence of ACC, the literature supports the use of adjuvant radiation therapy to reduce recurrence rates.3,4,6,17,18 The indications for postoperative radiation therapy vary from study to study. Miglianico et al4 recommends postoperative radiation when resection margins are positive, whereas other authors recommend postoperative radiation for all patients with ACC, independently of other prognostic factors.1 In our study, there was no impact on DFS and OS with the addition of adjuvant radiation therapy (p = .61 and p = .45, respectively). In addition, there was no individual effect on local (p = .30), regional (p = .53), and distant (p = .83) disease recurrence. At our institution, we found that radiation therapy was used more in patients with advanced stage disease (p < .01), PNI (p = .01), and positive resection margins (p < .01), however, subgroup analysis of these higher risk groups similarly revealed no impact on DFS and OS with the addition of postoperative radiation therapy.
We recognize several inherent limitations to this study. This is a retrospective analysis of a single institution lending to potential bias. Our patient population was fairly homogenous in terms of the extent of surgical resection and use of adjuvant radiation therapy, and, therefore, the study results may not necessarily be generalized to all treatment contexts. Nonetheless, this study does represent the largest cohort of ACC cases treated in the modern surgical era from a single institution and does highlight pertinent clinicopathologic parameters that impact disease outcome.
In summary, we present clinical and pathological predictors for head and neck ACC through a retrospective chart review at a single tertiary care, multidisciplinary center. The most important predictors of DFS and OS were PNI, sex, and disease site. Several other factors, including advanced stage, lymphovascular invasion, and surgical margin status, also suggested poorer disease prognosis, but did not reach statistical significance on multivariate analysis. The addition of radiation therapy confers no recurrence or survival advantage. Therefore, surgery should remain the mainstay of therapy for head and neck ACC. The use of postoperative radiation therapy should be limited as it may increase patient morbidity.
Footnotes
This work was presented in part at the Annual American Head and Neck Society Meeting, Orlando, Florida, April 10–11, 2013.
References
- 1.Kim KH, Sung MW, Chung PS, Rhee CS, Park CI, Kim WH. Adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1994;120:721–726. doi: 10.1001/archotol.1994.01880310027006. [DOI] [PubMed] [Google Scholar]
- 2.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–152. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. Management of adenoid cystic carcinoma of minor salivary glands. J Oral Maxillofac Surg. 2006;64:1114–1120. doi: 10.1016/j.joms.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Miglianico L, Eschwege F, Marandas P, Wibault P. Cervico-facial adenoid cystic carcinoma: study of 102 cases. Influence of radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13:673–678. doi: 10.1016/0360-3016(87)90284-7. [DOI] [PubMed] [Google Scholar]
- 5.Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 6.Prokopakis EP, Snyderman CH, Hanna EY, Carrau RL, Johnson JT, D’Amico F. Risk factors for local recurrence of adenoid cystic carcinoma: the role of postoperative radiation therapy. Am J Otolaryngol. 1999;20:281–286. doi: 10.1016/s0196-0709(99)90028-5. [DOI] [PubMed] [Google Scholar]
- 7.Norberg–Spaak L, Dardick I, Ledin T. Adenoid cystic carcinoma: use of cell proliferation. BCL-2 expression, histologic grade, and clinical stage as predictors of clinical outcome. Head Neck. 2000;22:489–497. doi: 10.1002/1097-0347(200008)22:5<489::aid-hed8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. Int J Oral Maxillofac Surg. 1997;26:435–439. doi: 10.1016/s0901-5027(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 9.Oplatek A, Ozer E, Agrawal A, Bapna S, Schuller DE. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope. 2010;120:65–70. doi: 10.1002/lary.20684. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM-classification of Malignant Tumours (UICC International Union Against Cancer) 7. New Jersey, Chichester, Oxford: Wiley–Blackwell; 2009. [Google Scholar]
- 11.Brown JS. Prognostic factors in oral, oropharyngeal and salivary gland cancer. In: Booth PW, Schendel SA, Hausamen JE, editors. Maxillofacial Surgery. Vol. 1. Edinburgh, London, New York: Churchill Livingstone; 1999. pp. 291–308. [Google Scholar]
- 12.Barrera JE, Shroyer KR, Said S, et al. Estrogen and progesterone receptor and p53 gene expression in adenoid cystic cancer. Head Neck Pathol. 2008;2:13–18. doi: 10.1007/s12105-007-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo SD, Su CY, Chuang HC, Huang CC, Chen CM, Chien CY. Estrogen receptor overexpression in malignant minor salivary gland tumors of the sinonasal tract. Otolaryngol Head Neck Surg. 2009;141:108–113. doi: 10.1016/j.otohns.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Motta Rda R, Zettler CG, Cambruzzi E, Jotz GP, Berni RB. Ki-67 and p53 correlation prognostic value in squamous cell carcinomas of the oral cavity and tongue. Braz J Otorhinolaryngol. 2009;75:544–549. doi: 10.1016/S1808-8694(15)30494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Hosmer D, Lemeshow S. Applied survival analysis: regression modeling of time to event data. New York: John Wiley & Sons; 1991. p. 159. [Google Scholar]
- 17.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–626. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento AG, Amaral AL, Prado LA, Kligerman J, Silveira TR. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312–319. doi: 10.1002/1097-0142(19860115)57:2<312::aid-cncr2820570220>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Pitman KT, Prokopakis EP, Aydogan B, et al. The role of skull base surgery for the treatment of adenoid cystic carcinoma of the sinonasal tract. Head Neck. 1999;21:402–407. doi: 10.1002/(sici)1097-0347(199908)21:5<402::aid-hed4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Whear NM, Addy JM. Adenoid cystic carcinoma of the sublingual gland— an unusual presentation. Br J Oral Maxillofac Surg. 1993;31:113–116. doi: 10.1016/0266-4356(93)90173-t. [DOI] [PubMed] [Google Scholar]
- 21.Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: incidence and survival trends based on 1973–2007 Surveillance, Epidemiology, and End Results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408. [DOI] [PubMed] [Google Scholar]
- 22.Elkin AD, Jacobs CD. Tamoxifen for salivary gland adenoid cystic carcinoma: report of two cases. J Cancer Res Clin Oncol. 2008;134:1151–1153. doi: 10.1007/s00432-008-0377-3. [DOI] [PubMed] [Google Scholar]
- 23.Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am J Clin Pathol. 2003;119:801–806. doi: 10.1309/RVTP-1G0Q-727W-JUQD. [DOI] [PubMed] [Google Scholar]
- 24.Pires FR, da Cruz Perez DE, de Almeida OP, Kowalski LP. Estrogen receptor expression in salivary gland mucoepidermoid carcinoma and adenoid cystic carcinoma. Pathol Oncol Res. 2004;10:166–168. doi: 10.1007/BF03033746. [DOI] [PubMed] [Google Scholar]
- 25.Jeannon JP, Soames JV, Bell H, Wilson JA. Immunohistochemical detection of oestrogen and progesterone receptors in salivary tumours. Clin Otolaryngol Allied Sci. 1999;24:52–54. doi: 10.1046/j.1365-2273.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- 26.Kiyoshima T, Shima K, Kobayashi I, et al. Expression of p53 tumor suppressor gene in adenoid cystic and mucoepidermoid carcinomas of the salivary glands. Oral Oncol. 2001;37:315–322. doi: 10.1016/s1368-8375(00)00083-x. [DOI] [PubMed] [Google Scholar]


