Abstract
Rationale: Sphingosine kinases (SphKs) 1 and 2 regulate the synthesis of the bioactive sphingolipid sphingosine-1-phosphate (S1P), an important lipid mediator that promotes cell proliferation, migration, and angiogenesis.
Objectives: We aimed to examine whether SphKs and their product, S1P, play a role in the development of pulmonary arterial hypertension (PAH).
Methods: SphK1−/−, SphK2−/−, and S1P lyase heterozygous (Sgpl1+/−) mice, a pharmacologic SphK inhibitor (SKI2), and a S1P receptor 2 (S1PR2) antagonist (JTE013) were used in rodent models of hypoxia-mediated pulmonary hypertension (HPH). S1P levels in lung tissues from patients with PAH and pulmonary arteries (PAs) from rodent models of HPH were measured.
Measurements and Main Results: mRNA and protein levels of SphK1, but not SphK2, were significantly increased in the lungs and isolated PA smooth muscle cells (PASMCs) from patients with PAH, and in lungs of experimental rodent models of HPH. S1P levels were increased in lungs of patients with PAH and PAs from rodent models of HPH. Unlike SphK2−/− mice, SphK1−/− mice were protected against HPH, whereas Sgpl1+/− mice were more susceptible to HPH. Pharmacologic SphK1 and S1PR2 inhibition prevented the development of HPH in rodent models of HPH. Overexpression of SphK1 and stimulation with S1P potentially via ligation of S1PR2 promoted PASMC proliferation in vitro, whereas SphK1 deficiency inhibited PASMC proliferation.
Conclusions: The SphK1/S1P axis is a novel pathway in PAH that promotes PASMC proliferation, a major contributor to pulmonary vascular remodeling. Our results suggest that this pathway is a potential therapeutic target in PAH.
Keywords: S1P, sphingosine kinase 1, S1P receptor 2, pulmonary arterial hypertension, pulmonary vascular remodeling
At a Glance Commentary
Scientific Knowledge on the Subject
The role of the SphK1/S1P signaling axis in pulmonary vascular remodeling is unknown.
What This Study Adds to the Field
Our studies provide strong evidence that the SphK1/S1P signaling axis is a novel and therapeutic target for pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is a severe and progressive disease that results in increased pulmonary vascular resistance (PVR), right heart failure, and ultimately death. Sustained pulmonary vasoconstriction, excessive pulmonary vascular remodeling, and thrombosis in situ are the three major causes of elevated PVR in patients with PAH (1). Pulmonary vascular remodeling is characterized in part by significant medial and intimal hypertrophy caused by enhanced proliferation of pulmonary artery smooth muscle cells (PASMCs) and their resistance to apoptosis (1). Most of the current therapies in PAH target pulmonary vasoconstriction, and there is a need to develop new targets to combat pulmonary vascular remodeling.
A conserved oncogenic enzyme, sphingosine kinase 1 (SphK1), has been shown to play an active role in angiogenesis, lymphangiogenesis, development of bronchopulmonary dysplasia, lung fibrosis, apoptosis resistance, and proliferation and survival of tumor cells (2–4). Overexpression of SphK1 stimulates proliferation of NIH 3T3 fibroblasts, intestinal epithelial cells, and colon and breast cancer cell lines, whereas inhibition of SphK1 can suppress glioma cell proliferation under hypoxia (5). Therefore, SphK1 was reported to promote tumor progression, invasion, and metastasis in gastric, prostate, colon, breast, and human non–small cell lung cancers (6, 7). The SphK family includes SphK1 and SphK2 (8). SphK1 is the predominant form in the lung, kidney, blood, and spleen, whereas the SphK2 isoform is predominantly expressed in the brain, liver, and heart (8). SphKs catalyze the phosphorylation of sphingosine to generate sphingosine-1-phosphate (S1P), a potent bioactive sphingolipid mediator and a known regulator of cell proliferation, differentiation, motility, Ca2+ mobilization (9), and endothelial barrier regulation (10). S1P can be irreversibly degraded by S1P lyase (Sgpl1) to phosphoethanolamine and hexadecenal. S1P acts on a family of five specific high-affinity G-protein–coupled receptors (S1P receptors [S1PR] 1–5) to initiate intracellular events (11). In PASMCs, the predominant S1PR are S1PR2 and S1PR3 (12). In this study, we have tested the hypothesis that SphK1-derived S1P contributes to pulmonary vascular remodeling in PAH by promoting PASMC proliferation. Some of these data have been reported in abstract form (13–16).
Methods
Reagents, Pharmacologic Inhibitors, and Antibodies
The information for reagents, pharmacologic inhibitors, and antibodies is provided in the Methods section of the online supplement.
Human Lung Tissues and Human PASMCs
Approval for the use of human lung tissues and cells was granted by the UIC Institutional Review Board. Deidentified human explanted peripheral lung tissues used in this study were from four control subjects (two unsuitable organ donors and two chronic obstructive pulmonary disease patients without pulmonary hypertension) and patients with idiopathic PAH (IPAH; four patients diagnosed on the basis of National Institutes of Health PAH Registry). Human PASMCs (hPASMCs) were isolated from six donors not suitable for lung transplantation and six patients with IPAH. hPASMC from explanted lungs of normal donors and patients with IPAH were provided by the Pulmonary Hypertension Breakthrough Initiative and were isolated as previously described (17). The clinical information of these 12 hPASMC samples is listed in Table E1 in the online supplement. In addition, a primary hPASMC cell line from Lonza (CC-2581) was used for cell transfection and proliferation assays. Cells were cultured at 37°C in Medium-199 supplemented with 10% fetal bovine serum and studied at passages 5 to 8.
Cell Proliferation Assays
Cell proliferation was determined using either a 5-bromo-2’-deoxyuridine (BrdU) incorporation assay or cell counting. BrdU assays (QIA58; Calbiochem, San Diego, CA) were performed in a 96-well format according to manufacturer’s instructions, using starting cell densities of 4,000 cells per well. For cell counting, cells were trypsinized and resuspended after experimental procedures; densities were counted with a TC10 automated cell counter (Bio-Rad, Hercules, CA).
RNA Isolation and Real-Time Polymerase Chain Reaction
Total RNA from lung tissues or PASMCs was isolated using the RNeasy Plus Mini Kit (Cat.#74134; Qiagen, Valencia, CA). Two micrograms of purified RNA was reverse transcribed to single-stranded cDNA using the Taqman RNA reverse transcription kit (Cat.#N8080234; Applied Biosystems, Inc., Foster City, CA). Real-time polymerase chain reaction (PCR) was performed on an Applied Biosystems 7900HT machine. Specific Taqman quantitative real-time PCR assays were ordered from Applied Biosystems (specific assay IDs available on request). The relative mRNA expression levels were normalized to the expression of a housekeeping gene, glucose-6-phosphate dehydrogenase, and determined by calculating the ΔΔCt value, as detailed in the manufacturer’s guidelines.
Construction of Wild-Type and Dominant-Negative (G82D) SphK1 Plasmids
A PCR product from a mammalian SphK1 cDNA was digested and cloned into the pEGFP-C1 vector for transfection. The SphK1 dominant-negative mutant (G82D) was generated by site-directed mutagenesis with the QuickChange mutagenesis kit (Stratagene, La Jolla, CA). The SphK1 (G82D) mutant has been shown to block SphK1 activity and function (18, 19). All constructs were confirmed by DNA sequencing.
Transfection of Plasmid DNA and Small Interfering RNA in hPASMCs
hPASMCs were transfected with SphK1 wild-type (WT) and G82D dominant-negative plasmids, or with human S1PR2 in pCMV6-XL4 vector (Cat.#SC117485; OriGene Technologies, Rockville, MD) using Xfect Transfection reagent (Cat.#631318; Clontech, Mountain View, CA). Scrambled small interfering RNA (siRNA) (D-001810-02) or S1PR2 siRNA (J-005208-06) were purchased from Dharmacon (Thermo Fisher Scientific, Lafayette, CO) and transfected into primary hPASMCs using DharmaFECT 1 transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Animal Models of Pulmonary Hypertension and Hemodynamic Measurements
All experiments were approved by the Ethics and Animal Care Committee of the University of Illinois at Chicago. In the rodent models of hypoxia-mediated pulmonary hypertension (HPH), male mice (8-wk old SphK1-deficient [SphK1−/−] mice in C57BL/6 background, SphK2-deficient [SphK2−/−] mice in C57BL/6 background, S1P lyase heterozygous [Sgpl1+/−] mice in 129SV background, and their WT siblings) or male Sprague-Dawley rats (190–200 g) were exposed to hypoxia (10% O2) in a ventilated chamber for 3.5–6 weeks. Right ventricular systolic pressure (RVSP) was determined by right heart catheterization using a Millar pressure transducer catheter. A weight ratio of the right ventricle divided by the sum of left ventricle and septum (RV/[LV + S]) was measured to determine the extent of right ventricular hypertrophy (RVH). Pulmonary artery remodeling was assessed using Aperio ImageScope software (version 11) after lungs were stained with hematoxylin and eosin. A minimum of 10 microscopic fields were examined for each slide. Approximately 20 muscular arteries with diameters 50–100 μm or less than 50 μm per lung section were outlined and measured. Vessel remodeling was calculated as ([external vessel area – internal vessel area]/external vessel area), as previously described (20).
S1P Measurement
S1P levels from lung tissues (humans, mice, and rats) and pulmonary arteries isolated from mice and rats were measured using a method described previously (3). Briefly, lipids were extracted from lung tissues and pulmonary arteries by a modified Bligh and Dyer procedure with the use of 0.1 N HCl for phase separation. C17-S1P (40 pmol), used as an internal standard, was added during the initial step of lipid extraction. S1P content was determined by liquid chromatography/tandem mass spectrometry with electrospray ionization using API 5500 QTRAP mass spectrometer equipped with turbo-V ion source and normalized to total phospholipid content in the sample measured as described previously (3, 21).
Statistical Analysis
Statistical analysis of experimental data was performed using GraphPad Prism 5.1 (GraphPad Software, Inc., La Jolla, CA). Results are expressed as mean ± SEM from at least three experiments. Student t test and analysis of variance were used to compare two and three groups, respectively. P less than 0.05 was considered statistically significant.
Results
SphK1 and S1P Are Significantly Increased in the Lungs from Patients with PAH and in Experimental Rodent Pulmonary Hypertension Models
Protein levels of SphK1, but not SphK2, were significantly elevated in the lungs (Figures 1A, 1B, 1D, and 1E) and PASMCs (Figures 2A–2E) of patients with PAH when compared with control subjects. In addition, levels of S1P, the bioactive product of SphK, were significantly higher in the lungs of patients with PAH when compared with control subjects (Figure 1C).
Figure 1.
Sphingosine kinase 1 (SphK1) and sphingosine-1-phosphate (S1P) are increased in the lungs of patients with pulmonary arterial hypertension (PAH). (A and B) Representative Western blotting images and β-actin–normalized quantification of protein demonstrate that SphK1 expression is significantly increased in lungs from patients with PAH when compared with control subjects. (C) C18-S1P levels are increased in the lungs of patients with PAH. (D and E) Representative Western blotting images and β-actin–normalized quantification of protein demonstrate that SphK2 expression is not increased in lungs from patients with PAH when compared with control subjects. Results are expressed as mean ± SEM; n = 4 per group. *P < 0.05 versus control.
Figure 2.
Protein expression and mRNA levels of sphingosine kinase 1 (SphK1) are increased in pulmonary artery smooth muscle cells (PASMCs) from patients with pulmonary arterial hypertension (PAH). (A–C) Representative Western blotting image and β-actin–normalized quantification of protein demonstrate that SphK1 expression is significantly increased in PASMCs from patients with PAH when compared with control subjects, whereas no changes in SphK2 expression are demonstrated. (D and E) A similar pattern was demonstrated for SphK1 and SphK2 mRNA levels in PASMCs isolated from control subjects and patients with PAH (n = 6 per group). **P < 0.01; ***P < 0.001 versus control.
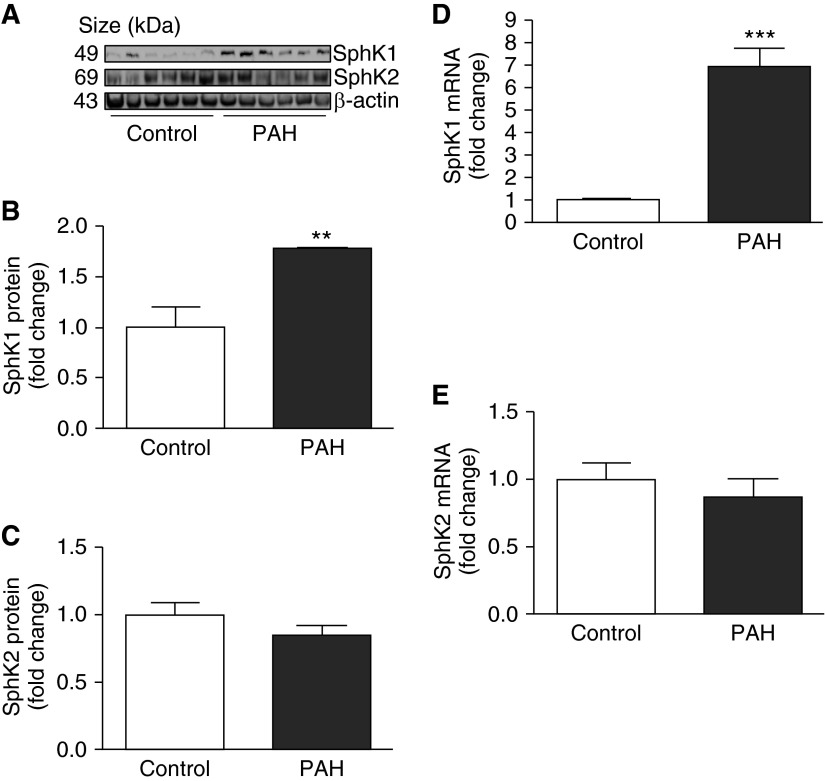
A similar pattern was found in experimental rodent models of HPH (Figure 3). The protein expression of SphK1, but not SphK2, was significantly elevated in the lungs of mice and rats exposed to hypoxia (10% O2) for 4 weeks (Figures 3A–3C and 3E–3G, respectively). Additionally, C18-S1P levels were significantly increased in pulmonary arteries isolated from the hypoxia-exposed mice (Figure 3D) and rats (Figure 3H). We did not observe significant differences in levels of ceramide, sphingosine, and their dihydro components in human lung tissues and pulmonary arteries from rodent models of HPH, either between control subjects and patients with PAH or between normoxia and hypoxia groups in rodent models of HPH (see Figures E1–E3). In aggregate, these results suggest that the SphK1 and S1P signaling axis may play a role in the development of pulmonary hypertension.
Figure 3.
Sphingosine kinase 1 (SphK1) and sphingosine-1-phosphate (S1P) levels are significantly increased in lungs and pulmonary arteries of rodent models of hypoxia-mediated pulmonary hypertension. (A–C) Representative Western blotting images and β-actin–normalized quantification of protein demonstrate that SphK1 expression is significantly increased in mouse lungs after 4-week hypoxia exposure, whereas no changes in SphK2 expression are demonstrated. (D) When compared with normoxic control animals, C18-S1P levels are significantly increased in the pulmonary arteries of mice exposed to 4-week hypoxia. (E–G) Representative Western blotting images and β-actin–normalized quantification of protein demonstrate that SphK1 expression is significantly increased in rat lungs after 4-week hypoxia exposure, whereas no changes in SphK2 expression are demonstrated. (H) When compared with normoxic control animals, C18-S1P levels are significantly increased in the pulmonary arteries of rats exposed to 4-week hypoxia (n = 5 per group). *P < 0.05; **P < 0.01; ***P < 0.001 versus normoxia.
SphK1-Deficient Mice Are Protected from HPH
To examine the effect of SphK1 on the development of pulmonary hypertension, SphK1-deficient (SphK1−/−) mice and their WT siblings in C57BL/6 background were exposed to hypoxia for 4 weeks. Compared with WT siblings, SphK1−/− mice exhibited lower RVSP, decreased RVH (assessed by RV/[LV + S] ratio), and less severe pulmonary vascular remodeling in response to hypoxia (Figure 4). In contrast, SphK2 knockout (SphK2−/− mice) had no significant effect on the development of HPH (see Figure E4).
Figure 4.

Sphingosine kinase 1 (SphK1)-deficient mice are protected against hypoxia-mediated pulmonary hypertension. When compared with wild-type (WT) control animals, SphK1−/− mice exposed to hypoxia developed (A) less severe elevations in right ventricular systolic pressure (RVSP), (B) less right ventricular hypertrophy (RV/[LV + S]), and lower increases in ratios of wall area to total vessel area of pulmonary arteries less than 50 μm (C) and 50–100 μm in diameter (D). (E) Representative pulmonary artery images in the lung sections of control and SphK1-deficient mice exposed to normoxia or hypoxia. Bar size: 20 μm. Results are expressed as mean ± SEM; n = 10 per group. *P < 0.05; **P < 0.01 versus hypoxia WT group.
Sgpl1+/− Mice Are More Susceptible to HPH
To test whether Sgpl1 deficiency, resulting in increased levels of S1P, could contribute to the development of HPH, we examined Sgpl1+/− (heterozygous) mice, because deletion of both alleles of the Sgpl1 gene in mice results in vascular development defects and mortality within 4–6 weeks of birth (22). The Sgpl1+/− mice and their WT siblings in 129SV background were exposed to hypoxia. Consistent with previous results in normoxic animals (23), Sgpl1+/− mice exhibited increased C18-S1P levels in lung tissues under normoxia or after 4–6 weeks hypoxia exposure, compared with WT siblings (see Figure E5). After 4-week hypoxia exposure, we did not find any significant differences between Sgpl1+/− mice and their WT siblings in terms of RVSP and RVH (data not shown). However, after 6-week hypoxia exposure, Sgpl1+/− mice exhibited significantly higher RVSP, RVH, and more severe pulmonary vascular remodeling, compared with their WT siblings (Figure 5). Although the size of the effect is not large, likely related to the fact that these are heterozygous mice bred on a background strain that is more resistance to HPH, these data demonstrate that deficiency of Sgpl1 contributes to the development of HPH.
Figure 5.

Sphingosine-1-phosphate lyase heterozygous (Sgpl1+/−) mice are more susceptible to hypoxia-mediated pulmonary hypertension. When compared with wild-type (WT) control mice, Sgpl1+/− mice exposed to hypoxia (6 wks) developed (A) more severe elevations in right ventricular systolic pressure (RVSP), (B) right ventricular hypertrophy (RV/[LV + S]), and more severe increases in ratios of wall area to total vessel area of pulmonary arteries less than 50 μm (C) and 50–100 μm in diameter (D). (E) Representative pulmonary artery images in the lung sections of control and Sgpl1+/− mice exposed to normoxia or hypoxia. Bar size: 20 μm. Results are expressed as mean ± SEM; n = 10 per group. *P < 0.05; **P < 0.01; ***P < 0.001 versus hypoxia WT group.
SphK Inhibition Prevents HPH in Rats
To further examine the potential role of SphKs in PH, we evaluated the effect of SKI2, an inhibitor of both SphK1 and SphK2, in rats exposed to hypoxia. When compared with vehicle-treated rats, treatment with SKI2 (10 mg/kg body weight, intraperitoneally, once every other day [3] for 3.5 wk) prevented the development of HPH, as assessed by RVSP, RVH, and pulmonary vascular remodeling (Figure 6). Taken together with the results from the hypoxia studies in SphK1−/− and Sgpl1+/− mice, these data suggest that S1P plays a critical role in the development of HPH.
Figure 6.
Sphingosine kinase (SphK) inhibition via SKI2 prevents hypoxia-mediated pulmonary hypertension in rats. (A) Changes in right ventricular systolic pressure (RVSP). (B) Changes in RV/(LV + S). (C) Changes in ratios of wall area to total vessel area of pulmonary arteries less than 50 μm in diameter in the lung sections of control and SKI2-treated groups after normoxia or hypoxia exposure. (D) Changes in ratios of wall area to total vessel area of pulmonary arteries 50–100 μm in diameter in the lung sections of control and SKI2-treated groups after normoxia or hypoxia exposure. (E) Representative pulmonary artery images in the lung sections of control and SKI2-treated groups after normoxia or hypoxia exposure. Bar size: 20 μm. Results are expressed as mean ± SEM; n = 6 per group. *P < 0.05; **P < 0.01 versus normoxia without SKI2 group.
SphK1 and Its Product, S1P, Promote PASMC Proliferation via Ligation of S1PR2
In cultured hPASMCs, overexpression of SphK1 by transfection with a plasmid containing WT SphK1 significantly promoted cell proliferation, whereas expression of a dominant-negative SphK1 (G82D, a mutant that blocks SphK1 activity and functions [19]) inhibited cell proliferation, as demonstrated by cell counting and a BrdU incorporation assay (Figure 7). A similar pattern was observed in vivo, where PASMCs from SphK1−/− mice exhibited lower proliferation in response to chronic hypoxia, compared with WT control mice (see Figure E6). In addition, treatment with S1P promoted PASMC proliferation in a dose-dependent manner (Figure 8A).
Figure 7.
Transfection of wild-type (WT) sphingosine kinase 1 (SphK1) plasmid in human pulmonary artery smooth muscle cells promotes cell proliferation, whereas transfection of dominant-negative (G82D) SphK1 plasmid inhibits cell proliferation. Human pulmonary artery smooth muscle cells were plated and transfected with either empty plasmid, WT SphK1 plasmid, or G82D dominant-negative SphK1 plasmid. (A) Representative photomicrograph of cells in culture 2 days after transfection. Cell proliferation was measured by cell counting (B) and 5-bromo-2’-deoxyuridine (BrdU) incorporation assay (C). Bar size: 100 μm. Data are expressed as mean ± SEM (n = 4). *P < 0.05; ***P < 0.001 versus control group. #P < 0.05; ###P < 0.001 versus transfection with WT SphK1 group.
Figure 8.

Sphingosine-1-phosphate (S1P) stimulates human pulmonary artery smooth muscle cells (PASMCs) proliferation and silencing S1PR2 attenuates PASMC proliferation. (A) S1P stimulates human PASMC proliferation in a dose-dependent manner. PDGF = platelet-derived growth factor. (B and C) Representative Western blotting images and β-actin–normalized quantification of protein levels demonstrate that S1PR2 expression is significantly increased in PASMCs from patients with pulmonary arterial hypertension (PAH) when compared with control subjects. (D–F) 5-Bromo-2’-deoxyuridine (BrdU) incorporation assays show that S1PR 2 antagonism or silencing attenuate basal and S1P-mediated PASMC proliferation. A human PASMC cell line from Lonza (CC-2581) was used in this experiment. (E) Representative Western blotting images show S1PR2 silencing effect, β-actin was used for normalization. S1P concentration: 100 nM. Results are expressed as mean ± SEM; n = 6 per group. *P < 0.05; **P < 0.01 versus control (or Ctrl, A and C) or no S1P+ JTE013 group (D) or scrambled siRNA without S1P group (F); ***P < 0.001 versus control (no S1P no JTE013, D); ###P < 0.001 versus no JTE013 but with S1P group (D) or scrambled siRNA with S1P group (F).
To examine whether S1P-mediated PASMC proliferation is dependent on S1PR ligation, we first examined the expression of S1PR1, S1PR2, and S1PR3 in hPASMCs. We confirmed previous reports (data not shown) that S1PR2 and S1PR3 are the predominant S1PR in PASMCs (24). We then compared S1PR2 and S1PR3 expression in PASMCs from control subjects and patients with IPAH. Compared with control subjects, S1PR2 expression was significantly increased in PASMCs isolated from patients with IPAH (Figures 8B and 8C), whereas no differences were found in S1PR3 expression (see Figure E7). Given the increased expression of S1PR2 in PASMCs from patients with IPAH, we investigated whether the proproliferative effects of S1P were mediated via ligation of S1PR2. As shown in Figures 8D–8F, silencing of S1PR2 expression via siRNA or S1PR2 antagonism with JTE013 decreased basal and S1P-mediated PASMC proliferation. Finally, we examined whether S1P-mediated PASMC proliferation is dependent on ERK activation. As shown in Figure E8A, an inhibitor of ERK phosphorylation (PD98059, 10 μM) attenuated S1P-mediated PASMC proliferation. Inhibition of S1PR2 by JTE013 significantly attenuated S1P-mediated ERK phosphorylation (see Figure E8B).
We also examined whether S1P affects some key signaling pathways involved in pulmonary hypertension, including bone morphogenetic protein receptor 2, transforming growth factor-β, platelet-derived growth factor (PDGF), and signal transducer and activator of transcription (STAT) 3. We did not observe any effect of S1P on bone morphogenetic protein receptor 2, transforming growth factor-β, or PDGF signaling pathways, but found that S1P induced a time-dependent nuclear translocation of phosphorylated STAT3 (see Figure E8C). Additionally, silencing of S1PR2 expression via siRNA or S1PR2 antagonism with JTE013 attenuated S1P-mediated STAT3 phosphorylation (see Figures E8D and E8E).
S1PR2 Inhibition Prevents HPH in Mice
To examine the potential role of S1PR2 inhibition in pulmonary hypertension, we evaluated the effect of JTE013 in mice exposed to hypoxia. When compared with vehicle-treated mice, treatment with JTE013 (8 mg/kg body weight, intraperitoneally, once every other day starting Day 1 for 3.5 wk or starting at Day 14 after hypoxia followed for 2 more weeks of hypoxia exposure) prevented and reversed the development of HPH as assessed by RVSP, RVH, and pulmonary vascular remodeling (Figure 9). These data suggest that S1PR2 plays an important role in the development of HPH.
Figure 9.

Sphingosine-1-phosphate receptor 2 antagonism via JTE013 prevents and reverses hypoxia-mediated pulmonary hypertension in mice. JTE treatment (8 mg/kg body weight, intraperitoneally, once every other day) started Day 1 for 3.5 weeks in the prevention experiments or started at Day 14 after hypoxia followed for 2 more weeks of hypoxia exposure in the reversal experiments. (A) Changes in right ventricular systolic pressure (RVSP). (B) Changes in RV/(LV + S). (C) Changes in ratios of wall area to total vessel area of pulmonary arteries less than 50 μm in diameter in the lung sections of control and JTE013-treated groups after normoxia or hypoxia exposure. (D) Changes in ratios of wall area to total vessel area of pulmonary arteries 50–100 μm in diameter in the lung sections of control and JTE013-treated groups after normoxia or hypoxia exposure. (E) Representative pulmonary artery images in the lung sections of control and JTE013-treated groups after normoxia or hypoxia exposure. Bar size: 20 μm. Results are expressed as mean ± SEM; n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001 versus normoxia without JTE013 group.
Discussion
PAH is a fatal lung disease for which novel therapies are desperately needed. The present study provides evidence suggesting that the SphK1/S1P signaling axis is a novel and therapeutic target for PAH. Specifically, we show that SphK1 and S1P are up-regulated in patients with PAH and in experimental PH, and that SphK1 genetic deficiency or pharmacologic inhibition protects against the development of experimental PH. A decrease in S1P catabolism by Sgpl1 was also shown to promote the development of experimental PH. In addition, our in vitro studies suggest that S1P promotes PASMC proliferation in an ERK-dependent manner via ligation of S1PR2. This is supported by our in vivo data, demonstrating that S1PR2 inhibition by JTE013 attenuates HPH in mice.
There are a significant number of studies delineating the effects of S1P in pulmonary vascular tone. Relatively high concentrations of S1P cause contraction of isolated rat (25) and porcine (26) pulmonary arteries. Szczepaniak and colleagues (27) showed that S1P (10 μM) increased PVR by 36% in an isolated mouse lung model. Their data also demonstrated that S1P-induced vasoconstriction was reduced by 64% by concomitant administration of the Rho-kinase inhibitor Y27632 (10 μM), suggesting that the S1P response is at least in part dependent on the Rho-kinase signal transduction pathway. Similarly, the S1P response was attenuated by more than 50% after S1PR2 antagonism (JTE013; 10 μM) and in S1PR2-deficient mice (27). Interestingly, in a recent report (28) using isolated perfused lungs from normoxic rats, infusion of S1P caused sustained vasoconstriction, which was not reduced by pretreatment with a dual S1PR1 and S1PR3 antagonist (VPC23019) and a S1PR2 antagonist (JTE013). Furthermore, S1PR4 agonists (phytosphingosine-1-phospate and VPC23153) were shown to elicit dose-dependent vasoconstriction in normoxic and hypertensive lungs from chronically hypoxic rats (28). S1P also activates store-operated calcium entry via S1PR-mediated and non–receptor-mediated pathways in mouse vascular smooth muscle cells (29). This effect was greater in proliferative than in contractile vascular smooth muscle cells and correlated with increases in STIM1, Orai1, S1PR1, and S1PR3 mRNA levels.
It is well established that uncontrolled PASMC proliferation and resistance to apoptosis are major components of the pathobiology of PAH (1). Our results demonstrate that S1P promotes PASMC proliferation in a dose-dependent manner, suggesting that in addition to its effects on pulmonary vascular tone, the SphK1/S1P signaling pathway plays a significant role in the pulmonary vascular remodeling associated with PAH. These novel data are consistent with the observation that S1P promotes proliferation of human satellite cells (24), human intestinal epithelial cells (30), quiescent Swiss 3T3 fibroblast (31), NIH 3T3 fibroblast (4), rat aortic smooth muscle cells (32), and endothelial cells (33). In addition, S1P regulates the expression of proapoptotic and antiapoptotic proteins. Exogenous S1P increases the expression of the antiapoptotic mediators BCL2 and MCL1, down-regulates the proapoptotic proteins BAD and BAX, and blocks the translocation of BAX to the mitochondria (34), a process required for mitochondrial cytochrome C release and caspase activation.
The demonstrated increase in S1P levels in lung tissues from patients with PAH and rodent pulmonary arteries exposed to chronic hypoxia was accompanied by up-regulation of SphK1 protein and mRNA levels in these tissues. In addition, expression of a dominant-negative SphK1 inhibited PASMC proliferation. These data support the notion that SphK1 could promote PASMC proliferation in an autocrine manner. This model has been described as “inside-out” signaling where intracellular S1P, produced by SphK, is released via transporter proteins, such as the ABC transporter family (35) or Spns2 (36), into the extracellular space close to S1PR, potentially allowing for efficient ligation of S1P. SphK1-derived S1P could also exert paracrine effects on cell proliferation not only in PASMCs but on other cells, such as pulmonary artery endothelial cells. For example, we have previously shown that in lung endothelial cells, exogenous S1P is a preferred source for the intracellular S1P and that intracellular S1P generation is essential for S1P-induced motility in these cells (37, 38). Additionally, SphK1 overexpression has been shown to stimulate intestinal epithelial cell proliferation through increased c-Myc translation (30). c-Myc, a protooncogene, encodes a nuclear transcription factor involved in the regulation of cell proliferation, differentiation, and apoptosis (39). SphK1 overexpression promotes checkpoint kinase 2 activity and enhances HuR phosphorylation, which allows increased translation of c-Myc mRNA through HuR binding at the 3′-UTR (30). Another study demonstrated that SphK1 promotes tumor cell proliferation by regulating the focal adhesion kinase activity and adhesion molecules (40).
There are several lines of evidence that suggest potential mechanisms for the up-regulation of SphK1 in patients with IPAH and in our rodent models of HPH. Hypoxia-induced up-regulation of hypoxia-inducible factor, growth factors, and inflammatory pathways are well established mediators of PAH pathobiology. The SphK1 promoter has two hypoxia-inducible factor responsive elements (hypoxia-inducible factor 1α and hypoxia-inducible factor 2α) that can affect the transcriptional regulation of SphK1 (41). Specifically, it has been demonstrated that hypoxia increases SphK1 and SphK2 expression in proliferating hPASMCs (42). In addition, Ader and coworkers (43) showed that under hypoxic conditions, SphK1 activity is stimulated and could regulate hypoxia-inducible factor 1α accumulation via phosphorylation of Akt/glycogen synthase kinase-3β, and by reactive oxygen species. There are significant interactions between SphK1/S1P pathways and growth factors, such as PDGF and vascular endothelial growth factor, including receptor transactivation (44). These interactions have been associated with PDGF-stimulated airway smooth muscle cell (45) and fibroblast migration (46), PDGF-mediated expression of SphK1 in human coronary artery smooth muscle cells (47), as well as SphK1/S1P-dependent promotion of vascular endothelial growth factor–induced Ras-ERK1-ERK2 signaling in endothelial cells (48, 49). Emerging data suggest that SphK1 expression is induced by inflammatory stimuli and cytokines, such as LPS (50) and IL-1 (51). We also speculate that functional single nucleotide polymorphisms in SphK1 could affect SphK1 function and influence PAH susceptibility.
Our data suggest that S1P promotes PASMC proliferation in an ERK-dependent manner via ligation of S1PR2. It is known that S1PR ligation activates Rho kinase, PI3K, and Akt, and also stimulates ERK phosphorylation via a Gαi-dependent pathway and increases cytosolic [Ca2+] (9). Here we show that the expression of S1PR2 is increased in PASMCs from patients with PAH and that silencing of S1PR2 inhibits PASMC proliferation. S1PR2 inhibition also attenuated S1P-mediated ERK phosphorylation. In addition, S1PR2 inhibition via JTE013 attenuated HPH in mice. Interestingly, ligation of S1PR2 has been shown to prevent systemic vascular SMC migration (52) and proliferation, and to promote differentiation (53). There are, however, no data on the role of S1PR2 in the migration and proliferation of PASMCs, and these effects may be cell specific. Hashimoto and coworkers showed that S1P alone does not influence human lung fibroblast migration but it potentiates fibroblast chemotaxis through S1PR2 (54). In muscle repair, S1P enhances satellite cell proliferation via S1PR2-dependent inhibition of RAC1 and repression of cell cycle inhibitors p21 and p27 (55). Cotransfection of S1PR2 and 3 in Jurkat and hepatoma cells leads to S1P-mediated cell proliferation and inhibition of apoptosis via activation of the ERK/MAPK pathway (56). Finally, our data suggest that S1P promotes STAT3 phosphorylation via S1PR2. STAT3 activation has been shown to be involved in the prosurvival and proliferative pulmonary vascular phenotype associated with PAH (57–60). The potential mechanisms underlining the S1P/SphK1/S1PR2 signaling axis in PAH is shown in Figure 10.
Figure 10.
Potential mechanisms underlining the sphingosine-1-phosphate (S1P)/sphingosine kinase 1 (SphK1)/S1P receptor (S1PR) 2 signaling axis in pulmonary arterial hypertension (PAH). Hypoxia and other potential stimuli, such as growth factors and inflammatory mediators, up-regulate SphK1 expression, increasing S1P production in pulmonary artery smooth muscle cells (PASMCs). S1P ligates S1PR2 on the PASMC surface, leading to the phosphorylation of both ERK and signal transducer and activator of transcription (STAT) 3, both known to induce cell proliferation. Abnormal PASMC proliferation leads to pulmonary vascular remodeling, thereby increasing pulmonary vascular resistance (PVR), which results in PAH.
In summary, we have demonstrated that the SphK1/S1P signaling axis is a novel pathway in PAH that promotes pulmonary vascular remodeling by enhancing PASMC proliferation. Most importantly, our results suggest that SphK1, S1P, and S1PR2 are novel potential therapeutic targets for PAH that should be explored in future preclinical and clinical studies.
Acknowledgments
Acknowledgment
The authors thank Yangbasai Dong, Abrehim Fahs, Hector Quijada, Jaime Torres, and Vamsi Reddy for their technical support.
Footnotes
Supported in part by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL111656 and K23HL098454, R.F.M.; P01HL98050, V.N.; R01HL066012 and R01HL115014, J.X.-J.Y.); University of Illinois at Chicago Area of Excellence Award (R.F.M.); and Pulmonary Hypertension Association Proof-of-Concept Research Grant (J.C.). Research reported in this publication was supported by the Office of The Director of the National Institutes of Health under Award Number S10OD010660 for the LC/MS equipment.
Author Contributions: Conception and design, R.F.M., V.N., J.G.N.G., J.C., H.T., J.R.S., and L.M.-V. Analysis and interpretation, J.C., H.T., J.R.S., L.M.-V., K.M.S., T.C., I.G., P.V.U., L.W., S.S., L.S.H., E.B., V.N., and R.F.M. Drafting the manuscript for important intellectual content, J.C., H.T., J.R.S., G.Z., J.U.R., E.B., J.X.-J.Y., V.N., and R.F.M.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201401-0121OC on September 2, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 2.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, Ma W, Noth I, Ma SF, Pendyala S, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J. 2013;27:1749–1760. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Li W, Sun S, Yu S, Zhang M, Zou F. Inhibition of sphingosine kinase 1 suppresses proliferation of glioma cells under hypoxia by attenuating activity of extracellular signal-regulated kinase. Cell Prolif. 2012;45:167–175. doi: 10.1111/j.1365-2184.2012.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 9.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 10.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argraves KM, Wilkerson BA, Argraves WS. Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J Biol Chem. 2010;1:291–297. doi: 10.4331/wjbc.v1.i10.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birker-Robaczewska M, Studer R, Haenig B, Menyhart K, Hofmann S, Nayler O. bFGF induces S1P1 receptor expression and functionality in human pulmonary artery smooth muscle cells. J Cell Biochem. 2008;105:1139–1145. doi: 10.1002/jcb.21918. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Moreno-Vinasco L, Shioura KM, Sammani S, Mohan V, Huang L, Pendyala S, Yuan J, Garcia JG, Natarajan V, et al. Sphingosine kinase 1 deficiency protects rodents from chronic hypoxia-mediated pulmonary hypertension [abstract] Am J Respir Crit Care Med. 2012;185:A3440. [Google Scholar]
- 14.Chen J, Sysol JR, Chen T, Tang H, Berdyshev E, Gorshkova I, Zhou G, Dong Y, Fahs A, Raj JU, et al. Sphingosine kinase 1 is upregulated in pulmonary artery smooth muscle cells (PASMCs) from patients with pulmonary arterial hypertension and modulates human pasmc proliferation [abstract] Am J Respir Crit Care Med. 2013;187:A1766. [Google Scholar]
- 15.Chen J, Tang H, Sysol JR, Sridhar A, Yuan J, Natarajan V. S1P lyase deficiency in mice enhances susceptibility to hypoxia-mediated pulmonary hypertension [abstract] Am J Respir Crit Care Med. 2014;189:A3298. [Google Scholar]
- 16.Sysol JR, Chen J, Tang H, Reddy V, Yuan J, Natarajan V. Sphingosine-1-phosphate receptor 2 is elevated in patients with pulmonary arterial hypertension and regulates S1P-induced pulmonary artery smooth muscle cell proliferation [abstract] Am J Respir Crit Care Med. 2014;189:A4776. [Google Scholar]
- 17.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L789–L799. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitson SM, Moretti PA, Zebol JR, Xia P, Gamble JR, Vadas MA, D’Andrea RJ, Wattenberg BW. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. J Biol Chem. 2000;275:33945–33950. doi: 10.1074/jbc.M006176200. [DOI] [PubMed] [Google Scholar]
- 19.Di A, Kawamura T, Gao XP, Tang H, Berdyshev E, Vogel SM, Zhao YY, Sharma T, Bachmaier K, Xu J, et al. A novel function of sphingosine kinase 1 suppression of JNK activity in preventing inflammation and injury. J Biol Chem. 2010;285:15848–15857. doi: 10.1074/jbc.M109.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KK, Caldwell RW, Su Y. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J Clin Invest. 2011;121:4548–4566. doi: 10.1172/JCI57734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaskovsky VE, Kostetsky EY, Vasendin IM. A universal reagent for phospholipid analysis. J Chromatogr A. 1975;114:129–141. doi: 10.1016/s0021-9673(00)85249-8. [DOI] [PubMed] [Google Scholar]
- 22.Kihara A, Ikeda M, Kariya Y, Lee EY, Lee YM, Igarashi Y. Sphingosine-1-phosphate lyase is involved in the differentiation of F9 embryonal carcinoma cells to primitive endoderm. J Biol Chem. 2003;278:14578–14585. doi: 10.1074/jbc.M211416200. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, Su Y, Usatyuk PV, Pendyala S, Oskouian B, et al. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am J Respir Cell Mol Biol. 2011;45:426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823:439–450. doi: 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Thomas GD, Snetkov VA, Patel R, Leach RM, Aaronson PI, Ward JP. Sphingosylphosphorylcholine-induced vasoconstriction of pulmonary artery: activation of non-store-operated Ca2+ entry. Cardiovasc Res. 2005;68:56–64. doi: 10.1016/j.cardiores.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao SH, Constable PD, Smith GW, Haschek WM. Effects of exogenous sphinganine, sphingosine, and sphingosine-1-phosphate on relaxation and contraction of porcine thoracic aortic and pulmonary arterial rings. Toxicol Sci. 2005;86:194–199. doi: 10.1093/toxsci/kfi167. [DOI] [PubMed] [Google Scholar]
- 27.Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am J Physiol Lung Cell Mol Physiol. 2010;299:L137–L145. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF. S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circ. 2011;1:399–404. doi: 10.4103/2045-8932.87309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopson KP, Truelove J, Chun J, Wang Y, Waeber C. S1P activates store-operated calcium entry via receptor- and non-receptor-mediated pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;300:C919–C926. doi: 10.1152/ajpcell.00350.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang P, Smith AD, Li R, Rao JN, Liu L, Donahue JM, Wang JY, Turner DJ. Sphingosine kinase 1 overexpression stimulates intestinal epithelial cell proliferation through increased c-Myc translation. Am J Physiol Cell Physiol. 2013;304:C1187–C1197. doi: 10.1152/ajpcell.00271.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J. 2000;348:71–76. [PMC free article] [PubMed] [Google Scholar]
- 34.Avery K, Avery S, Shepherd J, Heath PR, Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17:1195–1205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 35.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, Pyne NJ, Pyne S, Sabbadini RA, Garcia JG, Natarajan V. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS ONE. 2011;6:e16571. doi: 10.1371/journal.pone.0016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001;414:768–773. doi: 10.1038/414768a. [DOI] [PubMed] [Google Scholar]
- 40.Liu SQ, Su YJ, Qin MB, Mao YB, Huang JA, Tang GD. Sphingosine kinase 1 promotes tumor progression and confers malignancy phenotypes of colon cancer by regulating the focal adhesion kinase pathway and adhesion molecules. Int J Oncol. 2013;42:617–626. doi: 10.3892/ijo.2012.1733. [DOI] [PubMed] [Google Scholar]
- 41.Schwalm S, Döll F, Römer I, Bubnova S, Pfeilschifter J, Huwiler A. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun. 2008;368:1020–1025. doi: 10.1016/j.bbrc.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad M, Long JS, Pyne NJ, Pyne S. The effect of hypoxia on lipid phosphate receptor and sphingosine kinase expression and mitogen-activated protein kinase signaling in human pulmonary smooth muscle cells. Prostaglandins Other Lipid Mediat. 2006;79:278–286. doi: 10.1016/j.prostaglandins.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res. 2008;68:8635–8642. doi: 10.1158/0008-5472.CAN-08-0917. [DOI] [PubMed] [Google Scholar]
- 44.Schuchardt M, Tölle M, Prüfer J, van der Giet M. Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system. Br J Pharmacol. 2011;163:1140–1162. doi: 10.1111/j.1476-5381.2011.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters C, Sambi B, Kong KC, Thompson D, Pitson SM, Pyne S, Pyne NJ. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J Biol Chem. 2003;278:6282–6290. doi: 10.1074/jbc.M208560200. [DOI] [PubMed] [Google Scholar]
- 46.Long JS, Natarajan V, Tigyi G, Pyne S, Pyne NJ. The functional PDGFbeta receptor-S1P1 receptor signaling complex is involved in regulating migration of mouse embryonic fibroblasts in response to platelet derived growth factor. Prostaglandins Other Lipid Mediat. 2006;80:74–80. doi: 10.1016/j.prostaglandins.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Francy JM, Nag A, Conroy EJ, Hengst JA, Yun JK. Sphingosine kinase 1 expression is regulated by signaling through PI3K, AKT2, and mtor in human coronary artery smooth muscle cells. Biochim Biophys Acta. 2007;1769:253–265. doi: 10.1016/j.bbaexp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Wu W, Shu X, Hovsepyan H, Mosteller RD, Broek D. VEGF receptor expression and signaling in human bladder tumors. Oncogene. 2003;22:3361–3370. doi: 10.1038/sj.onc.1206285. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi K, Ishizaki T, Ayada T, Sugiyama Y, Wakabayashi Y, Sekiya T, Nakagawa R, Yoshimura A. Sprouty4 deficiency potentiates Ras-independent angiogenic signals and tumor growth. Cancer Sci. 2009;100:1648–1654. doi: 10.1111/j.1349-7006.2009.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2008;85:107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paugh BS, Bryan L, Paugh SW, Wilczynska KM, Alvarez SM, Singh SK, Kapitonov D, Rokita H, Wright S, Griswold-Prenner I, et al. Interleukin-1 regulates the expression of sphingosine kinase 1 in glioblastoma cells. J Biol Chem. 2009;284:3408–3417. doi: 10.1074/jbc.M807170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takashima S, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, Takata S, Kaneko S, Takuwa Y. G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc Res. 2008;79:689–697. doi: 10.1093/cvr/cvn118. [DOI] [PubMed] [Google Scholar]
- 53.Grabski AD, Shimizu T, Deou J, Mahoney WM, Jr, Reidy MA, Daum G. Sphingosine-1-phosphate receptor-2 regulates expression of smooth muscle alpha-actin after arterial injury. Arterioscler Thromb Vasc Biol. 2009;29:1644–1650. doi: 10.1161/ATVBAHA.109.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto M, Wang X, Mao L, Kobayashi T, Kawasaki S, Mori N, Toews ML, Kim HJ, Cerutis DR, Liu X, et al. Sphingosine 1-phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptor. Am J Respir Cell Mol Biol. 2008;39:356–363. doi: 10.1165/rcmb.2006-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, Fyrst H, Zhang M, Proia RL, Hoffman EP, Saba JD. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE. 2012;7:e37218. doi: 10.1371/journal.pone.0037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem. 2000;275:288–296. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 57.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 58.Paulin R, Meloche J, Bonnet S. STAT3 signaling in pulmonary arterial hypertension. JAK-STAT. 2012;1:223–233. doi: 10.4161/jkst.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Côté J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu W, Erzurum SC. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol. 2011;1:357–372. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]