Abstract
Rationale: Obesity and underweight are contraindications to lung transplantation based on their associations with mortality in studies performed before implementation of the lung allocation score (LAS)–based organ allocation system in the United States
Objectives: To determine the associations of body mass index (BMI) and plasma leptin levels with survival after lung transplantation.
Methods: We used multivariable-adjusted regression models to examine associations between BMI and 1-year mortality in 9,073 adults who underwent lung transplantation in the United States between May 2005 and June 2011, and plasma leptin and mortality in 599 Lung Transplant Outcomes Group study participants. We measured body fat and skeletal muscle mass using whole-body dual X-ray absorptiometry in 142 adult lung transplant candidates.
Measurements and Main Results: Adjusted mortality rates were similar among normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9), and class I obese (BMI 30–34.9) transplant recipients. Underweight (BMI < 18.5) was associated with a 35% increased rate of death (95% confidence interval, 10–66%). Class II–III obesity (BMI ≥ 35 kg/m2) was associated with a nearly twofold increase in mortality (hazard ratio, 1.9; 95% confidence interval, 1.3–2.8). Higher leptin levels were associated with increased mortality after transplant surgery performed without cardiopulmonary bypass (P for interaction = 0.03). A BMI greater than or equal to 30 kg/m2 was 26% sensitive and 97% specific for total body fat–defined obesity.
Conclusions: A BMI of 30.0–34.9 kg/m2 is not associated with 1-year mortality after lung transplantation in the LAS era, perhaps because of its low sensitivity for obesity. The association between leptin and mortality suggests the need to validate alternative methods to measure obesity in candidates for lung transplantation. A BMI greater than or equal to 30 kg/m2 may no longer contraindicate lung transplantation.
Keywords: obesity, sarcopenia, adiposity, leptin, biomarker
At a Glance Commentary
Scientific Knowledge on the Subject
Obesity defined as a body mass index of 30 kg/m2 or greater is currently a contraindication to adult lung transplantation based on associations between obesity and both mortality and primary graft dysfunction after lung transplantation.
What This Study Adds to the Field
Higher mortality rates were not observed among adult lung transplant recipients with a body mass index between 30 and 35 kg/m2. Body mass index is a poor measure of both adipose tissue mass and muscle mass in adult lung transplant candidates. Circulating leptin levels, a biomarker of adipose tissue mass, were associated with increased mortality in some lung transplant recipients.
Lung transplantation is a potentially life-saving therapy for adults with advanced lung diseases. Mortality following lung transplantation, however, is high: 21% die within the first year and nearly 50% die within 5 years after transplantation (1). In light of this risk and the ethical obligation to best steward a scarce resource, the lung transplant community follows restrictive selection guidelines, excluding those at high risk of early postoperative death (2). These guidelines, however, have largely been informed by single-center experiences, unadjusted or incompletely adjusted registry analyses, and expert opinion (2).
Selection guidelines recommend that obesity, defined as a body mass index (BMI) of 30 kg/m2 or greater, be considered a relative contraindication to lung transplantation (2, 3). In practice, BMI thresholds for candidacy vary across centers, with many centers requiring a BMI below 30 kg/m2. Although not explicitly addressed by guidelines, some centers also consider underweight (BMI < 18.5 kg/m2) a contraindication to transplantation (4). These approaches stem from studies demonstrating associations between both obesity and underweight and increased mortality after transplantation (4, 5). These approaches are also driven by the association of obesity with an increased risk of primary graft dysfunction (PGD), a form of acute lung injury and a leading cause of death early after transplantation (6).
The use of BMI to determine transplant candidacy may be prone to error. BMI misclassifies 39% of healthy adults as nonobese when compared with methods that define obesity by adipose tissue mass, such as whole-body dual X-ray absorptiometry (DXA) (7–9). BMI is a relatively poor measure of adiposity and sarcopenia (low muscle mass), both inflammatory states (10, 11) associated with mortality (12, 13). Furthermore, circulating levels of leptin, a satiety hormone produced by adipose tissue (14, 15), correlate more strongly with total body adipose tissue mass than with BMI (16), and have been used to “correct” BMI to provide a more accurate measure of adiposity (7).
In this study, we examined the associations of BMI and circulating leptin levels with mortality in the modern era of lung transplantation. We also examined the operating characteristics of BMI for adiposity and sarcopenia in adult lung transplant candidates. Some of the results of these studies have been previously reported in the form of abstracts (17, 18).
Methods
The online supplement contains a full version of the Methods.
Data Sources, Participants, and Study Design
We used data from three sources (Figure 1). First, we performed a retrospective cohort study using US national data supplied by the United Network for Organ Sharing. We included adults (age ≥ 18 yr) undergoing lung transplantation between May 4, 2005 (initiation of the Lung Allocation Score [LAS], an urgency-based allocation system that prioritizes those in greatest need of transplantation) and June 14, 2011. Follow-up continued through December 14, 2012. We excluded recipients who underwent living donor, lobar, or multiorgan transplantation, or transplantation from a donor after circulatory determination of death.
Figure 1.
Study flow for (A) Organ Procurement and Transplantation Network, (B) Lung Transplant Body Composition, and (C) Lung Transplant Outcomes Group cohorts. DXA = dual X-ray absorptiometry.
Next, we performed a cross-sectional analysis of the Lung Transplant Body Composition (LTBC) study, an ongoing prospective cohort study investigating the impact of body composition on outcomes after transplantation. Candidates for lung transplantation age greater than or equal to 18 at the University of California, San Francisco (UCSF) and Columbia University Medical Center (CUMC) were eligible. We analyzed subjects recruited between March 17, 2011 and September 20, 2013.
Finally, we performed a secondary analysis of adults enrolled in the Lung Transplant Outcomes Group (LTOG) study who underwent lung transplantation between May 4, 2005 and December 29, 2010. Follow-up continued through December 29, 2011. LTOG is a multicenter prospective cohort study aimed at identifying risk factors for PGD (19). LTOG participants who underwent transplantation from a donor after circulatory determination of death, or multiorgan transplantation, or who did not have preoperative blood samples available (n = 405) were excluded.
Participants provide written informed consent for participation in the LTBC and LTOG studies. This study was approved by the UCSF and CUMC institutional review boards and by institutional review boards at each LTOG site (see the online supplement for a list of LTOG collaborators).
Measurements
Body composition
In the Organ Procurement and Transplantation Network (OPTN) cohort, the primary exposure variable was BMI, calculated as weight (kilograms) divided by height squared (meters). In both the OPTN and LTOG cohorts, weight and height were measured at each reporting transplant center. We analyzed BMI using World Health Organization–defined thresholds: underweight (BMI < 18.5), normal (BMI 18.5–24.9), overweight (BMI 25.0–29.9), obese class I (BMI 30.0–34.9), obese class II (BMI 35.0–39.9), and obese class III (BMI ≥ 40.0) (3, 20). Because of few subjects in class III (n = 9) we collapsed classes II and III into class II–III.
In the LTBC study, we conducted study visits at Clinical and Translational Science Award–funded clinical research centers at UCSF and CUMC. Weight was measured wearing light indoor clothing and no shoes. Height was measured with a wall-mounted stadiometer. Body composition was assessed with whole-body DXA (Lunar Prodigy DXA, GE Healthcare [Madison, WI] at UCSF; and Discovery W, Hologic Inc. [Bedford, MA] at CUMC) (21, 22). DXA measures fat, muscle, and bone mass compartments and total body mass. The appendicular skeletal muscle mass index (ASMI) was calculated as the sum of lean tissue mass of all four extremities divided by height squared (23). We defined sarcopenia as an ASMI of less than or equal to 5.45 kg/m2 for women and less than or equal to 7.26 kg/m2 for men (23). We defined obesity by DXA as percent body fat (% fat) greater than or equal to 30% for women and greater than or equal to 25% for men (24).
Leptin
In the LTOG cohort, we measured leptin, a measure of adiposity, in duplicate in citrated plasma stored at −80°C using commercially available ELISA kits (R&D Systems, Minneapolis, MN) (6). The intraassay coefficients of variation were less than 4.4% at CUMC and 5.4% at Vanderbilt University School of Medicine.
The primary outcome was survival during the first year after transplantation. For both the OPTN and LTOG cohorts, survival was calculated as the number of days from the date of transplantation until the date of death with right censoring at 365 days.
Analysis Approach
Using the OPTN cohort, we examined the linearity of the relationship between BMI and 1-year survival using a multivariable-adjusted generalized additive model (4, 6). Similarly, in the LTOG cohort, we used generalized additive models to examine the linearity and form of adjusted associations between leptin and 1-year survival. We used stratified multivariable Cox proportional hazards models to examine associations between BMI, leptin, and survival time. Covariates were selected based on plausible associations with obesity and/or mortality. In the LTBC cohort, we estimated Pearson correlation coefficients between BMI and DXA measures of adiposity and skeletal muscle mass and estimated agreement, kappa, sensitivity, specificity, and positive and negative predictive values. We estimated correlation coefficients between plasma leptin levels and both BMI and % fat in the 73 participants with available plasma. We evaluated the relationship between leptin and DXA-defined obesity by estimating sensitivity, specificity, positive and negative predictive values, and generating a receiver operating characteristic curve. Consistent with previous work (6), we identified a multiplicative interaction (P = 0.03) between plasma leptin and cardiopulmonary bypass (CPB) in the LTOG cohort; therefore, we stratified survival models by CPB status while controlling for factors potentially related to leptin levels (including BMI and sex) and/or survival.
Results
BMI and Survival after Lung Transplantation
During the OPTN cohort study period, 9,548 individuals underwent lung transplantation in the United States. Of these, 9,073 met study criteria (Figure 1A). The cohort was 58.7% male, median age was 58 years (interquartile range, 48–63), and median BMI was 25.0 kg/m2 (interquartile range, 21.1–28.4) (see Table E1 in the online supplement).
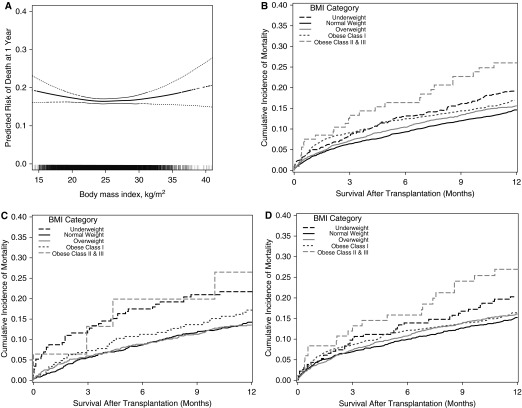
The 1-year unadjusted mortality rate per 100 person-years was 16.5 among those with a normal BMI, 20.1 among underweight, 19.0 among overweight, 20.5 among class I obese, and 33.2 among class II–III obese recipients (Table 1). The adjusted relationship between BMI and 1-year mortality had a shallow U-shape with a nadir near 25 kg/m2 (Figure 2A) (P value for nonlinearity = 0.02). Multivariable-adjusted predicted survival curves are shown in Figures 2B–2D and in Figure E1.
Table 1.
Associations between Preoperative Body Mass Index and 1-Year Mortality after Lung Transplantation
| Body Mass Index Category |
|||||
|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese |
||
| Class I | Class II–III | ||||
| No. |
900 |
3,645 |
3,151 |
1,270 |
107 |
| Years of follow-up, median (IQR) | 2.1 (1.1–3.9) | 2.3 (1.1–4.0) | 2.4 (1.0–4.0) | 2.1 (1.0–4.0) | 1.9 (0.6–3.5) |
| No. of decedents | 160 | 542 | 527 | 226 | 28 |
| Person-years | 795.0 | 3276.8 | 2774.3 | 1102.6 | 84.5 |
| 1-yr mortality rate per 100 person-years (95% CI) | 20.1 (17.2–23.5) | 16.5 (15.2–18.0) | 19.0 (17.4–20.7) | 20.5 (18.0–23.4) | 33.2 (22.9–48.0) |
| Hazard ratios (95% CI) | |||||
| Unadjusted | 1.21 (1.01–1.45) | 1 | 1.14 (1.01–1.29) | 1.23 (1.06–1.44) | 1.95 (1.33–2.85) |
| Model 1 | 1.37 (1.12–1.67) | 1 | 1.05 (0.93–1.20) | 1.07 (0.91–1.27) | 1.85 (1.26–2.70) |
| Model 2 | 1.34 (1.09–1.66) | 1 | 1.06 (0.93–1.21) | 1.08 (0.91–1.28) | 1.90 (1.30–2.77) |
| Model 3 | 1.35 (1.10–1.66) | 1 | 1.05 (0.92–1.20) | 1.08 (0.91–1.27) | 1.90 (1.29–2.78) |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range.
Data derived from the Organ Procurement and Transplantation Network cohort of 9,073 lung transplant recipients transplanted between May 4, 2005 and June 14, 2011.
Body mass index (BMI) categories: underweight (BMI < 18.50), normal (BMI 18.50–24.99), overweight (BMI 25.00–29.99), obese class I (BMI 30.00–34.99), obese class II–III (BMI ≥ 35.00).
Model 1: Adjusted for recipient factors: age, sex, height, previous cardiac surgery, lung allocation score, center,* diagnosis (chronic obstructive pulmonary disease includes α1-antitrypsin disease),* previous lung transplantation,* mechanical ventilation.*
Model 2: Model 1 + adjustment for donor factors: age, sex, height, BMI, arterial partial pressure of oxygen, alcohol use, smoking history, pulmonary infection, cause of death, diabetes,* donor-recipient height interaction, donor-recipient sex interaction.
Model 3: Model 2 + adjustment for procedural factors: ischemic time, transplant type.
Included in the model as stratification variable.
Figure 2.
(A) Continuous association between pretransplant body mass index (BMI) and the risk of death 1 year after lung transplantation, and predicted 1-year mortality curves by BMI category (B) overall and for subgroups with (C) chronic obstructive pulmonary disease and (D) interstitial lung disease. In A, the thick dotted line = smoothed regression line adjusted for age, diagnosis, lung allocation score, transplant type (single or bilateral lung), ischemic time, mechanical ventilation at the time of transplant, and transplant center. Thin dashed lines = 95% confidence intervals. The vertical hash marks in the rug plot at the bottom represent unique study participants. The P value for linearity is 0.02, supporting a nonlinear association between body mass index and risk of mortality at 1 year. The P value for the linear association is 0.80. The wide confidence intervals at the extremes of BMI are caused by fewer transplant recipients with these values. In B–D, predicted survival estimates with covariates listed in the footnote to Table 1 set to their mean values (Model 3). Body mass index categories: underweight (BMI < 18.5), normal (BMI 18.5–24.9), overweight (BMI 25.0–29.9), obese class I (BMI 30.0–34.9), obese class II–III (BMI ≥ 35.0).
Underweight was associated with a multivariable-adjusted 35% relative increase in the 1-year mortality rate (hazard ratio [HR], 1.35; 95% confidence interval [CI], 1.10–1.66) (Table 1). In contrast, there was no meaningful association between mortality and either overweight (HR, 1.05; 95% CI, 0.92–1.20) or class I obesity (HR, 1.08; 95% CI, 0.91–1.27) (Table 1). Notably, adjustment for recipient-related factors reduced the hazard ratio for class I obesity from 1.23 (95% CI, 1.06–1.44) to 1.07 (95% CI, 0.91–1.27), suggesting that recipient-related factors other than BMI account for some of the crude association between obesity and mortality. Class II–III obesity (BMI ≥ 35 kg/m2) was associated with a nearly twofold increased rate of death (HR, 1.90; 95% CI, 1.29–2.78). Analyses stratified on key clinical factors, including age, sex, height, LAS, diagnosis, diabetes, procedure type, hospitalization, and mechanical ventilation did not identify a subgroup in which class I obesity was a meaningful risk factor for early mortality (P values for each interaction > 0.10) (see Table E3). Sensitivity analyses did not substantively change the point estimates and CI ranges for the associations between mortality and BMI categories (see Table E2). Among 1-year survivors, we did not identify increased adjusted mortality rates during years 2 through 5 among underweight (HR, 1.09; 95% CI, 0.91–1.31), class I obese (HR, 1.15; 95% CI, 0.99–1.34), or class II–III obese recipients (HR, 1.55; 95% CI, 0.98–2.44) (see Table E4 and Figure E2).
Operating Characteristics of DXA Measures of Obesity and Sarcopenia
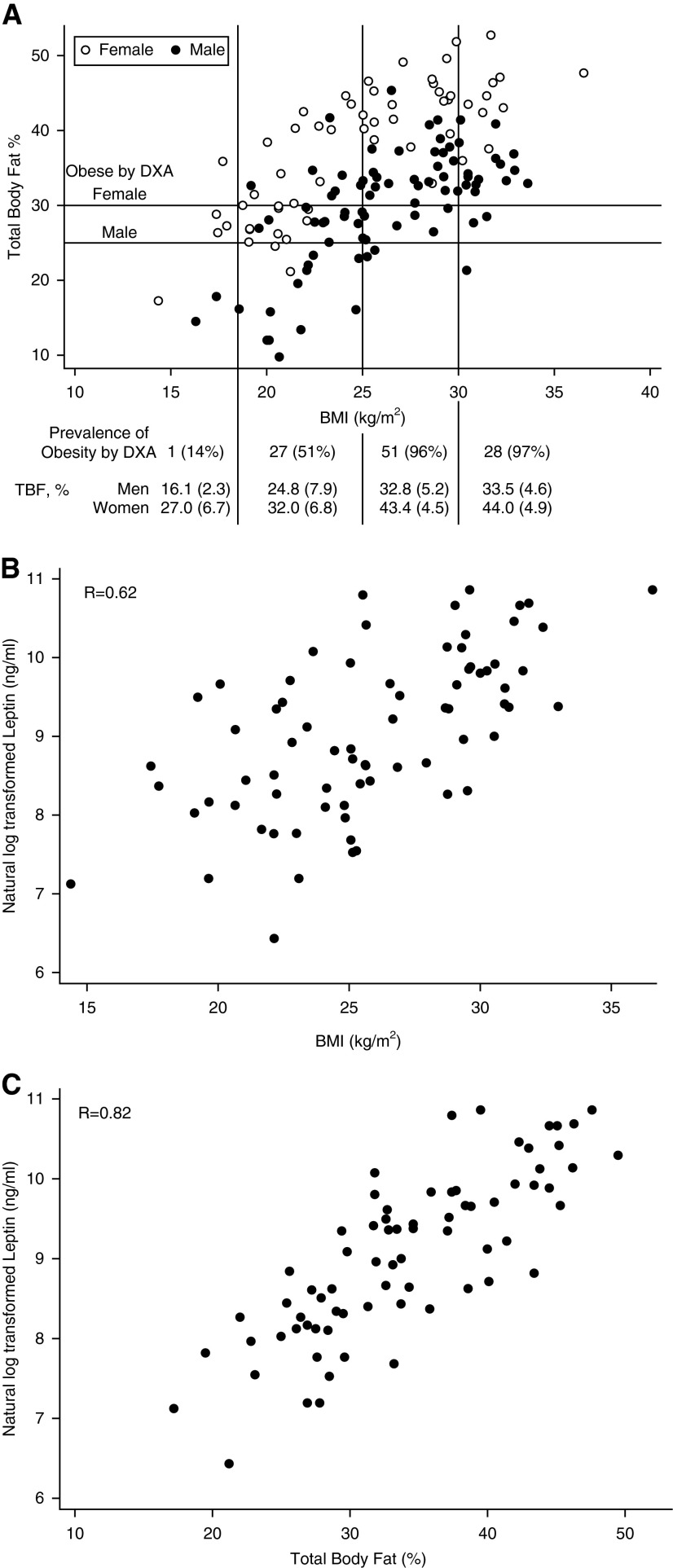
The LTBC cohort included 142 lung transplant candidates. Demographics were similar to the OPTN cohort (see Table E5). The mean (SD) % fat was 29.8% (7.6%) for men and 37.5% (8.6%) for women. The prevalence of obesity was 75% by DXA, but only 20% by BMI. Furthermore, 70% of those with a BMI less than 30 kg/m2 and 51% of those with a normal BMI were classified as obese by DXA. BMI only moderately correlated with % fat (r = 0.57; P < 0.001) (Figure 3A). Agreement between DXA and BMI for “obesity” was 44% (kappa, 0.13; 95% CI, 0.06–0.20). Notably, leptin levels correlated more strongly with % fat (r = 0.82) than with BMI (r = 0.62) (Figures 3B and 3C). The mean (SD) ASMI was 7.30 (1.11) kg/m2 for men and 5.69 (0.97) kg/m2 for women. A scatterplot of BMI and ASMI is shown in Figure E5. Although only 5% of the cohort was underweight by BMI, 46% were sarcopenic by DXA, with an overall agreement of 58% for underweight (kappa, 0.09; 95% CI, 0.00–0.26). Operating characteristics for selected BMI thresholds for DXA-defined obesity and sarcopenia are shown in Table 2 and selected leptin thresholds for DXA-defined obesity in Table E6.
Figure 3.
Scatterplots of (A) pretransplant body mass index (BMI) and percent body fat, (B) leptin and pretransplant BMI, and (C) leptin and pretransplant percent body fat in Lung Transplant Body Composition study participants. In A, vertical lines denote World Health Organization BMI categories: underweight (BMI < 18.5), normal (BMI 18.50–24.99), overweight (BMI 25.00–29.99), and obese (BMI ≥ 30.00). Horizontal lines are sex-specific thresholds for obesity defined by whole-body dual X-ray absorptiometry (DXA) (24). Table below: prevalence of obesity and mean (standard deviation) percent body fat within each BMI category. In B and C, correlation coefficients are (B) r = 0.62 for leptin and BMI, and (C) r = 0.82 for leptin and percent body fat measured by whole-body DXA. TBF = total body fat.
Table 2.
Operating Characteristics of Selected Body Mass Index Thresholds for Obesity and Sarcopenia Measured by Whole-Body Dual X-Ray Absorptiometry
| BMI Threshold | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value* (95% CI) | Negative Predictive Value* (95% CI) |
|---|---|---|---|---|
| Obesity |
|
|
|
|
| BMI > 30 kg/m2 | 26% (18–36%) | 97% (85–100%) | 97% (82–99%) | 30% (22–39%) |
| BMI > 25 kg/m2 | 74% (64–82%) | 91% (77–98%) | 96% (90–99%) | 53% (40–66%) |
| BMI > 22.5 kg/m2 | 89% (81–94%) | 83% (66–93%) | 94% (88–98%) | 71% (54–84%) |
| Sarcopenia | ||||
| BMI < 18.5 kg/m2 | 9% (3–19%) | 99% (93–100%) | 86% (42–98%) | 56% (48–65%) |
| BMI < 21 kg/m2 | 31% (20–43%) | 91% (82–96%) | 74% (54–89%) | 61% (51–70%) |
| BMI < 25 kg/m2 | 78% (67–88%) | 82% (71–90%) | 78% (67–88%) | 82% (71–90%) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval.
Data derived from the Lung Transplant Body Composition cohort of 142 lung transplant candidates.
Whole-body dual X-ray absorptiometry (DXA) measure of sarcopenia defined as an appendicular skeletal muscle mass index of less than or equal to 5.45 kg/m2 for women and less than or equal to 7.26 kg/m2 for men (23). Appendicular skeletal muscle mass index is calculated as the sum of lean tissue mass of all four extremities divided by height squared in meters. DXA measure of obesity defined as greater than or equal to 30% total body fat for women and greater than or equal to 25% total body fat for men (24).
DXA-defined obesity prevalence = 75%; sarcopenia prevalence = 46%.
Leptin and Survival after Lung Transplantation
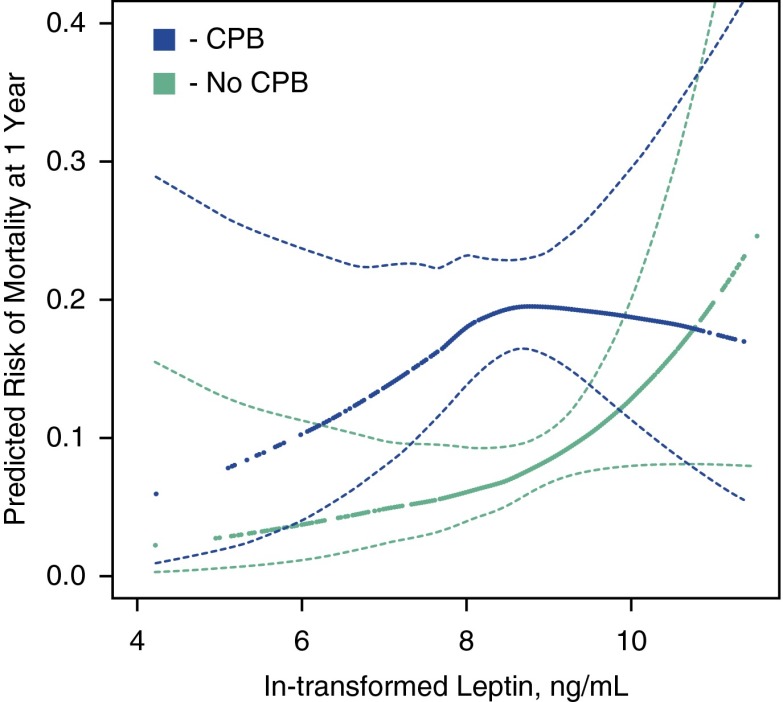
In the LTOG cohort, 1,032 subjects underwent lung transplantation during the study period. Of these, 599 had available plasma and met our criteria (Figure 1C; see Table E7); subjects with available plasma were more likely to have obstructive lung disease, less likely to have restrictive lung disease, and more likely to be receiving invasive mechanical ventilation than those without available plasma (see Table E8). In adjusted models, higher preoperative plasma leptin levels were associated with higher 1-year mortality rates among those who did not undergo CPB during transplant surgery, but not among those who underwent CPB (P for interaction = 0.03). Among those not undergoing CPB, each natural log unit increase in leptin was associated with an 83% increased 1-year mortality rate after adjustment for BMI, the LAS, and other factors (adjusted HR, 1.83; 95% CI, 1.04–3.22) (Figure 4; see Table E9). Visual inspection of the continuous association between leptin levels and mortality (Figure 4) suggests that the relationships may not be completely linear, yet statistical hypothesis testing did not confirm this (e.g., P = 0.15 for the non-CPB group). The wide CIs at the extremes of leptin levels reflect the uncertainty of the size and shape of the relationship.
Figure 4.
Continuous associations between pretransplant plasma leptin level and the predicted risk of death at 1 year after transplantation in the Lung Transplant Outcomes Group Cohort. Green = lung transplant recipients not exposed to cardiopulmonary bypass (CPB) during transplant surgery. Blue = lung transplant recipients exposed to CPB during transplant surgery. Thick dotted lines = smoothed regression lines in 599 adult lung transplant recipients adjusted for body mass index category, age, recipient sex, lung allocation score, diagnosis (chronic obstructive pulmonary disease includes α1-antitrypsin disease), donor sex, donor smoking history, ischemic time, transplant type (single vs. bilateral), mechanical ventilation, transplant year, and transplant center. Thin dashed lines = 95% confidence bands. Leptin levels were natural log transformed. CPB-stratified models are presented (P for interaction = 0.03). The P values for linearity are 0.15 for CPB users and 0.33 for nonusers, suggesting a lack of evidence that the associations are nonlinear. The P values for the association between leptin and mortality are 0.39 for CPB users and 0.03 for nonusers. These results suggest higher leptin levels are associated with an increased risk of death after lung transplantation among those who did not undergo CPB during transplant surgery.
Discussion
In this contemporary cohort of adults undergoing lung transplantation in the United States, we found that preoperative underweight, class II–III obesity, and (in a subgroup analysis) greater adiposity defined by higher plasma leptin levels were each independently associated with an increased rate of death during the first year after lung transplantation. In contrast to previous studies (4), however, overweight and class I obesity were not associated with early mortality. We also found BMI to be a poor measure of both obesity and skeletal muscle mass measured by whole-body DXA imaging in lung transplant candidates. Our finding is consistent with studies in healthy populations that demonstrate high prevalences of obesity and sarcopenia even among those with a normal BMI (7, 25). Taken together, our findings suggest that although adiposity is an important predictor of early mortality after transplantation, BMI is a poor measure of adiposity in adult lung transplant candidates.
Our study has immediate clinical relevance and could influence lung transplant candidate selection criteria. Relaxed BMI thresholds for candidacy might provide many adults with end-stage lung disease an opportunity to undergo lung transplantation. Although we were unable to exclude a small increase in mortality for class I obese transplant recipients, this effect is unlikely to be substantially greater than the effect of overweight in our study (5% increased risk) or the risks attributed to conditions prevalent at the time of lung transplantation, such as prior blood product transfusion (18% increased risk), hospitalization (70% increased risk), or use of mechanical ventilation (53% increased risk) (1). Thus, the importance currently accorded to BMI-defined obesity as a contraindication to lung transplantation may be out of proportion to its independent impact on mortality in this setting. At present, many centers prescribe highly restrictive diets to obese candidates to achieve a BMI less than 30 kg/m2 as a contingency for placement on the transplant waitlist. Caloric restriction and the time needed to achieve this BMI threshold may result in poorer nutrition, debilitation, and loss of muscle mass, and may allow for lung disease progression. The composite effect increases waiting list mortality and may actually increase the risk of perioperative morbidity and mortality (26–28).
The lack of a meaningful association between class I obesity and early mortality after transplantation in our study is discrepant with previous work in lung transplantation (4), but is consistent with a recent metaanalysis of nearly 3 million adults in the general population that isolated obesity-attributable mortality risk to a BMI greater than or equal to 35.0 kg/m2 and identified no increased risk for a BMI between 30.0 and 34.9 kg/m2 (29). Possible explanations for this discrepancy might include improved control of confounding (e.g., by transplant center and disease severity) or an evolution in the body composition of lung transplant candidates between the 1995 and 2003 cohort and the current cohort (4). Aging and chronic disease contribute to preferential loss of lean muscle and gain of fat (30–32). Referred to as sarcopenic obesity, this change in body composition is associated with increased mortality risk, even in those with a normal BMI (33). It has also been proposed as an explanation for the “obesity paradox” in cardiovascular disease (34). Adults undergoing transplantation today tend to be older and have more advanced lung disease, possibly contributing to a greater burden of adiposity and sarcopenia through decreased physical activity, increased resting energy expenditure, medication effects, and systemic inflammation (35–37). Our finding of a DXA-defined obesity prevalence of 51% among transplant candidates with a “normal” BMI suggests that any risk associated with adiposity might be distributed across the spectrum of BMI. Thus, the lack of an observed association between BMI greater than 30 kg/m2 and mortality in our study may reflect the poor operating characteristics of BMI for measuring adiposity rather than the lack of an effect of adiposity on mortality. Although a BMI between 30 and 35 kg/m2 may increase the technical difficulty of the transplant surgery, ischemic times, atelectasis, and wound dehiscence, these issues do not seem to increase mortality rates.
Our findings should not be interpreted to mean that obesity is unimportant when considering transplant candidacy. Adiposity is associated with inflammatory “adipokines” (including leptin) and other cytokines, such as IL-6 and monocyte chemotactic protein-1 (38, 39). These factors have been associated with PGD, a leading cause of early mortality after lung transplantation (40, 41). Our study extends the association between leptin and PGD to include mortality, at least among those who did not receive CPB during transplant surgery, consistent with previous work in the same cohort that identified CPB as an effect modifier of the associations of both obesity and leptin with PGD (6). We hypothesize that the proinflammatory state induced by CPB might overwhelm any potential inflammatory effect of adiposity on 1-year mortality after lung transplantation. Additional work is needed to further characterize the form and magnitude of the relationship between leptin levels and mortality and to determine whether thresholds can be identified that provide clinically meaningful information. Although we found that class I obesity is not associated with mortality even controlling for comorbidities, such as diabetes, obesity is associated with other unmeasured comorbid conditions, such as hypertension and obstructive sleep apnea among others. Additionally, we did not evaluate the impact of changes in body composition after lung transplantation and emergent obesity-related comorbidities. These factors may impact clinically relevant outcomes, such as longer-term survival and functioning, health care use, and health-related quality of life.
Preoperative underweight remains an important risk factor for 1-year mortality after lung transplantation. Underweight in lung transplant candidates likely subsumes multiple mechanistic pathways, one of which may be cachexia, an inflammatory “wasting syndrome” occasionally used interchangeably with sarcopenia (42). Cachexia is prevalent in advanced chronic diseases, especially of the lung, and is independently associated with mortality (43, 44); sarcopenia is associated with mortality in transplant populations (45, 46).
Overall, our findings suggest the relationship between body composition and mortality following lung transplantation is more complicated than can be accounted for by BMI alone. Disentangling this relationship will likely require more than one method of assessment. Both imaging modalities (e.g., DXA and quantitative computerized tomography) and biomarkers, such as leptin, quantify adiposity and sarcopenia better than BMI (7, 8, 23, 47). Just as cystatin C improved classification and outcome prediction for patients with chronic kidney disease (48), more direct body composition phenotyping through imaging and biomarkers or evaluation of related clinical phenotypes, such as frailty (49–51), may ultimately improve the risk prediction and candidate prioritization capabilities of BMI.
Our study has limitations. We could not exclude a small effect of class I obesity on mortality. Additionally, the observed mortality risk associated with class II–III obesity was informed by only 107 patients (1.1% of the cohort) most of whom had either chronic obstructive pulmonary disease or interstitial lung disease. This modest sample size tempers our ability to make robust conclusions or estimate obesity-related risk for other diagnoses. Compared with other BMI strata, these patients tended to have fibrotic lung disease, be hospitalized, and receive invasive mechanical ventilation, all known risk factors for early mortality (see Table E1) (1, 52). After adjustment for these factors, however, the risk of early mortality was only slightly attenuated. It is also likely that clinical and nonclinical factors not captured in the registry (or reflected in allocation scoring) influenced the decision to offer these patients lung transplantation. Although these unmeasured factors might have attenuated the mortality risk we observed, it is also possible that they explain the risk currently ascribed to obesity. Second, it is possible that height was measured with shoes on in the OPTN and LTOG cohorts. This possibility, however, would likely bias our results toward the null because it is unlikely to have occurred differentially by body weight. Third, misclassification and missingness of covariates limited our ability to control for confounding. Fourth, although we demonstrated BMI to have poor operating characteristics in measuring lean body mass and % fat, small numbers of subjects precluded an examination of the impact of DXA-defined obesity and sarcopenia on mortality. Fifth, our findings may not generalize to non–LAS-based organ allocation systems.
In conclusion, we did not identify an association between preoperative mild obesity (BMI 30–35 kg/m2) and early mortality after lung transplantation in the United States, perhaps because of the poor ability of BMI to identify adiposity. In contrast, higher preoperative plasma leptin levels, a marker of increased adiposity, are associated with increased 1-year mortality after lung transplantation even after accounting for BMI. Taken together, these results suggest that it is reasonable to relax the upper threshold for BMI when considering lung transplant candidacy, while still considering an increased burden of adiposity as a relative contraindication to transplant surgery.
Acknowledgments
Acknowledgment
The authors express their appreciation for the thousands of patients and caregivers who have participated in the Lung Transplant Outcomes Group and Lung Transplant Body Composition studies. This work is dedicated in memory of Renee.
Footnotes
Supported by National Heart, Lung, and Blood Institute (K23 HL111115) and a Nina Ireland Program in Lung Health Award (J.P.S.); National Heart, Lung, and Blood Institute (R01 HL114626, D.J.L. and J.D.C.), R01 HL087115 and R01 HL081619 (J.D.C.), and HL081332 and HL088263 (L.B.W.). This project was supported in part by an Irving Pilot Award from the Irving Institute for Clinical and Translational Research. A portion of this study was supported by the National Center for Advancing Translational Sciences (previously the National Center for Research Resources) at the National Institutes of Health (UCSF-CTSI UL1 RR024131; CUMC-CTSA UL1 TR000040). A portion of this study was supported by Health Resources and Services Administration contract 231-00-01115.
Author Contributions: J.P.S., E.R.P., M.E.S., L.B.W., J.D.C., and D.J.L. made substantial contributions to the conception and design of the work. All authors made substantial contributions to the acquisition, analysis, or interpretation of data for the work. J.P.S. wrote the first draft of the manuscript. J.P.S., E.R.P., M.E.S., P.P.K., J.A.G., J.K., S.H., S.M.P., L.B.W., J.D.C., and D.J.L. revised the manuscript for important intellectual content. All authors approved the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201405-0973OC on September 18, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, et al. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, et al. Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.World Health OrganizationObesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000 [PubMed] [Google Scholar]
- 4.Lederer DJ, Wilt JS, D’Ovidio F, Bacchetta MD, Shah L, Ravichandran S, Lenoir J, Klein B, Sonett JR, Arcasoy SM. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180:887–895. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanasky WF, Jr, Anton SD, Rodrigue JR, Perri MG, Szwed T, Baz MA. Impact of body weight on long-term survival after lung transplantation. Chest. 2002;121:401–406. doi: 10.1378/chest.121.2.401. [DOI] [PubMed] [Google Scholar]
- 6.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, et al. Lung Transplant Outcomes Group. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184:1055–1061. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis. 2012;22:741–747. doi: 10.1016/j.numecd.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526, e9–e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 16.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 17.Singer JP, Peterson ER, Golden J, Snyder ME, Lim B, Desai A, Sonett JR, Kukreja J, Arcasoy SM, Katz PP, et al. The impact of body composition on survival after adult lung transplantation. J Heart Lung Transplant. 2014;33:S79. [Google Scholar]
- 18.Snyder ME, Peterson ER, Singer J, Shah R, Golden J, Shin J, Wickersham N, Kawut SM, Desai A, Katz P, et al. Evolution of the association between obesity and survival after adult lung transplantation in the lung allocation score era. J Heart Lung Transplant. 2014;33:S79. [Google Scholar]
- 19.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et al. Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Consultation WHO WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body composition by dual energy X-ray absorptiometry (DEXA) Clin Physiol. 1991;11:331–341. doi: 10.1111/j.1475-097x.1991.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 22.Heymsfield SB, Wang J, Heshka S, Kehayias JJ, Pierson RN. Dual-photon absorptiometry: comparison of bone mineral and soft tissue mass measurements in vivo with established methods. Am J Clin Nutr. 1989;49:1283–1289. doi: 10.1093/ajcn/49.6.1283. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 24.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin MR, Arcasoy SM, Shah A, Schulze PC, Sze J, Sonett JR, Lederer DJ. Hypoalbuminemia and early mortality after lung transplantation: a cohort study. Am J Transplant. 2012;12:1256–1267. doi: 10.1111/j.1600-6143.2011.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo MJ, Iribarne A, Hong KN, Davies RR, Xydas S, Takayama H, Ibrahimiye A, Gelijns AC, Bacchetta MD, D’Ovidio F, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137:651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuang WM, Vock DM, Finlen Copeland CA, Lederer DJ, Palmer SM. An acute change in lung allocation score and survival after lung transplantation: a cohort study. Ann Intern Med. 2013;158:650–657. doi: 10.7326/0003-4819-158-9-201305070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyle UG, Genton L, Hans D, Karsegard VL, Michel JP, Slosman DO, Pichard C. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. J Am Geriatr Soc. 2001;49:1633–1640. doi: 10.1046/j.1532-5415.2001.t01-1-49272.x. [DOI] [PubMed] [Google Scholar]
- 31.Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr. 2013;56:270–278. doi: 10.1016/j.archger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB.Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study Am J Clin Nutr 2005. 82:872–878; quiz 915–876 [DOI] [PubMed] [Google Scholar]
- 33.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects ≥60 years of age (from the Third National Health and Nutrition Examination Survey) Am J Cardiol. 2013;112:1592–1598. doi: 10.1016/j.amjcard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 35.Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–824. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takabatake N, Nakamura H, Abe S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. Circulating leptin in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1215–1219. doi: 10.1164/ajrccm.159.4.9806134. [DOI] [PubMed] [Google Scholar]
- 37.Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 38.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, Shaked A, Wille K, Lama VN, Milstone A, et al. Lung Transplant Outcomes Group. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9:389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathur A, Baz M, Staples ED, Bonnell M, Speckman JM, Hess PJ, Jr, Klodell CT, Knauf DG, Moldawer LL, Beaver TM.Cytokine profile after lung transplantation: correlation with allograft injury Ann Thorac Surg 2006811844–1849.discussion 1849–1850 [DOI] [PubMed] [Google Scholar]
- 42.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 43.Congleton J. The pulmonary cachexia syndrome: aspects of energy balance. Proc Nutr Soc. 1999;58:321–328. doi: 10.1017/s0029665199000439. [DOI] [PubMed] [Google Scholar]
- 44.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 45.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 47.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 48.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, et al. CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 51.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 52.Singer JP, Blanc PD, Hoopes C, Golden JA, Koff JL, Leard LE, Cheng S, Chen H. The impact of pretransplant mechanical ventilation on short- and long-term survival after lung transplantation. Am J Transplant. 2011;11:2197–2204. doi: 10.1111/j.1600-6143.2011.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]