To the Editor:
Fibrosing mediastinitis is an uncommon disorder characterized by inflammation and progressive fibrosis within the mediastinum (1–3). The resulting invasive obstruction of vital mediastinal structures is associated with significant morbidity (2, 4). Infectious and noninfectious inflammatory triggers have been reported (4). In North America, fibrosing mediastinitis is most commonly associated with histoplasmosis (4, 5). Although anecdotal success with antifungal, antiinflammatory, and antifibrotic therapy has been reported (5, 6), these results have not been confirmed in larger cases series (4). Furthermore, the success of surgical and nonsurgical interventions to relieve invasive vascular and airway obstruction has been variable, ranging from complete recovery to therapeutic failure with procedural mortality (4, 5, 7–10). New therapeutic approaches are urgently needed.
We previously demonstrated accumulation of CD20-positive B lymphocytes in fibrosing mediastinitis tissue samples (3). B lymphocytes characteristically form a peripheral rim, infiltrating sheets or follicular structures, and are closely associated with areas of fibrosis (3). They are increasingly linked to a number of fibroinflammatory diseases, including scleroderma and idiopathic pulmonary fibrosis, and have emerged as potential modulators of fibroblast activation (11, 12). Rituximab eliminates B lymphocytes by targeting the surface antigen CD20. On the basis of our hypothesis that tissue B lymphocytes play a pathogenic role in progressive fibrosing mediastinitis, we herein report the successful use of rituximab in three patients.
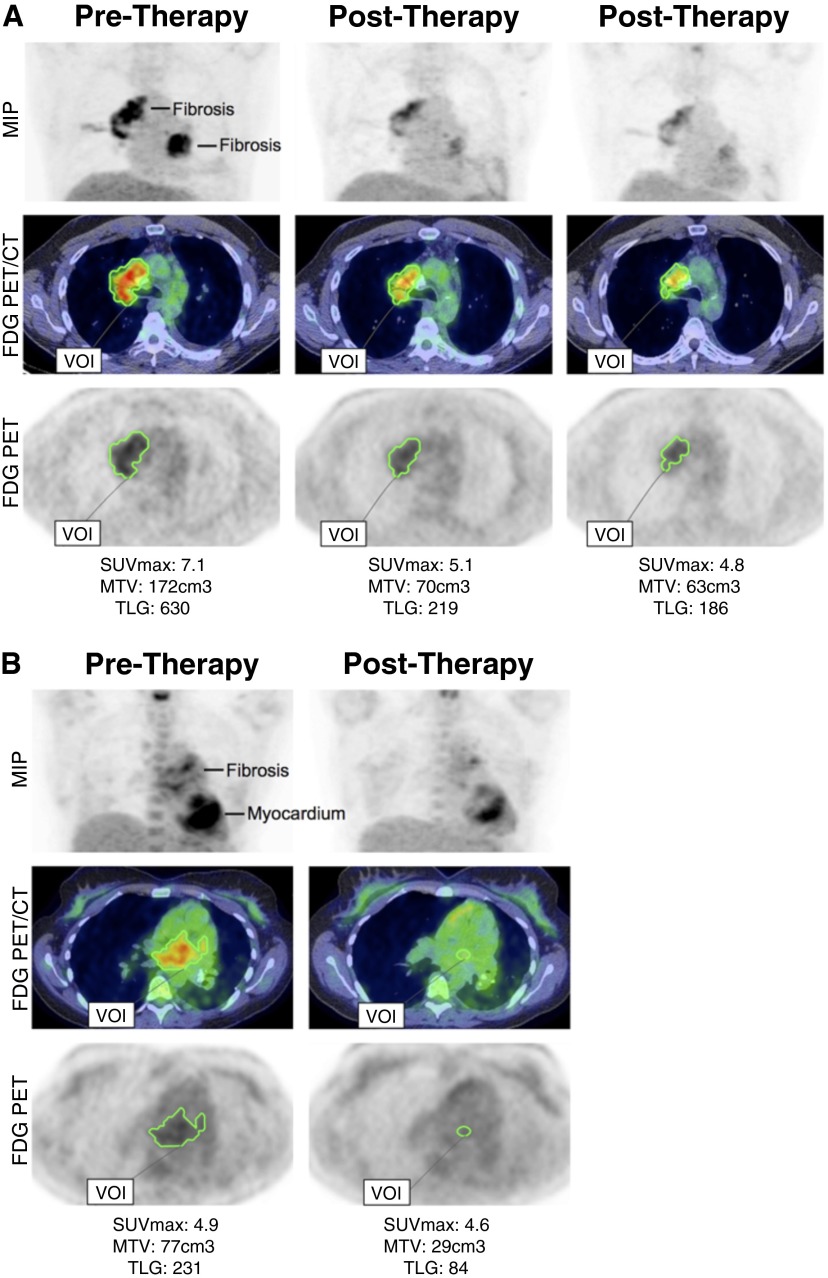
The demographics and clinical features of the three patients are listed in Table 1. Informed consent for off-label rituximab therapy was obtained from all patients, and all had biopsy evidence of chronic inflammation and fibrosis and increased metabolic activity by 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/computed tomography [CT]; Figure 1).
Table 1.
Patient Demographics and Clinical Characteristics
| Case | Age/Sex | Symptoms | Fibrosing Mediastinitis Involvement | Evidence of Histoplasmosis and Baseline Computed Tomography Findings | Progressive and/or Refractory Disease | Previous Treatment |
|---|---|---|---|---|---|---|
| 1 | 52/Male | Back pain | Right paravertebral mass, right hilum | Positive serology, noncalcified paravertebral mass | Recurrent symptoms during prednisone taper, persistent paravertebral mass | Chronic prednisone |
| 2 | 47/Male | Dyspnea on exertion, hemoptysis | Progressive bilateral mediastinal involvement. Occlusion left superior and inferior pulmonary veins and superior vena cava. Stenosis right superior pulmonary vein and right upper lobe bronchus. Recurrent large left pleural effusion. | Positive serology, calcified granulomas lung, calcified bilateral mediastinal mass | Over the course of 12 months: Severe dyspnea on exertion, large left pleural effusion, progressive bronchial and pulmonary vein obstruction, approximately 17% increase in size of mediastinal mass | Prednisone, itraconazole, indwelling pleural catheter |
| 3 | 38/Female | Dyspnea, cough, hemoptysis, palpitations | Progressive bilateral mediastinal involvement. Occlusion left superior and inferior pulmonary vein and left pulmonary artery. Stenosis left upper and lower lobe bronchus. | Positive serology, calcified granulomas lung, left and central noncalcified mass with calcified left hilar lymph nodes | Over the course of 12 months: Increased dyspnea, hemoptysis, left pulmonary artery and pulmonary vein obstruction, approximately 80% increase in size of mediastinal mass | Prednisone, itraconazole, bronchial artery embolization |
Figure 1.
Metabolic response by 18F-fluorodeoxyglucose positron emission tomography/computed tomography. (A) 18F-Fluorodeoxyglucose positron emission tomography/computed tomography for patient 2 at baseline (pretherapy; left) and at 6 (middle) and 12 (right) months of B-cell depletion (post-therapy). (B) 18F-Fluorodeoxyglucose positron emission tomography/computed tomography for patient 3 at baseline (pretherapy; left) and 7 months after B-cell depletion (post-therapy; right). MTV = metabolic tumor volume, TLG = total lesion glycolysis.
Rituximab Therapy
Patient 1 was treated with four weekly rituximab infusions of 375 mg/m2. Both patient 2 and patient 3 received an infusion of rituximab 1,000 mg on Days 0 and 14. Prednisone was prescribed at the time of the infusion and tapered off entirely in all three patients over the following 3–4 months. All patients received pneumocystis prophylaxis, and patients 2 and 3 also received itraconazole to prevent histoplasmosis reactivation.
Imaging Analysis
FDG-PET/CT (Discovery 690 scanner; GE Healthcare, Pewaukee, WI) used the standard clinical protocol. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were generated using a semiautomated process on an OsiriX v.5.5.2 64-bit workstation (Pixmeo, Bernex, Switzerland). FDG-PET/CT three-dimensional volumes of interest (VOIs) were obtained using a segmentation tool with a threshold standardized uptake value of 2.5 or a blood pool standardized uptake value maximum, whichever was higher. VOIs were grown around seed points within mediastinal fibrosis areas with intense FDG activity. Volumetric data were calculated with a Delaunay reconstruction filter. Each VOI was inspected and manually adjusted in PET and fused PET/CT axial images to ensure it did not extend beyond areas of fibrosis. Mediastinal vascular structures within VOI were not excluded. MTV for each scan was calculated by summing all VOIs. Lesion glycolysis was obtained by multiplying the mean standardized uptake value by the VOIs. TLG for each scan was calculated by summing the lesion glycolysis of all VOIs.
Results and Discussion
Circulating B lymphocytes, measured by flow cytometry, were completely depleted in all patients. Patient 2 experienced a pleural space infection during the period of B-lymphocyte depletion, which was attributed to a previously inserted intrapleural catheter. There were no other serious adverse events.
All patients had a favorable therapeutic response. In patient 1, the right paravertebral mass resolved entirely and repeat FDG-PET/CT showed no residual metabolic activity; prednisone was tapered successfully, and his back pain improved. He remains in remission after B-lymphocyte reconstitution 7 months after rituximab therapy. Patient 2 experienced an impressive decrease in his pleural fluid output over the course of 7 months (1,500 ml/d to 100 ml/wk), and the catheter was successfully removed. FDG-PET demonstrated a significant decrease in MTV and TLG (Figure 1A). Functional status and exertional dyspnea improved significantly. Mediastinal metabolic activity continues to improve. The patient remains B-lymphocyte-depleted after 2 additional doses of rituximab 17 months after the initial treatment. Patient 3 exhibited significant decrease in size on CT scan (3 months) and in MTV and TLG on FDG-PET imaging of her mediastinal mass (Figure 1B). In addition, subcarinal, prevascular, and superior mediastinal lymphadenopathy nearly resolved 2 months after rituximab therapy, and 6 months after rituximab therapy, her dyspnea on exertion and cough had resolved. She continues to be stable, and her B lymphocyte depleted 15 months later, after an additional treatment course of rituximab.
Current medical therapy for progressive fibrosing mediastinitis is ineffective (3). Rituximab is approved for the treatment of non-Hodgkin’s lymphoma, rheumatoid arthritis, chronic lymphocytic leukemia, and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Using rituximab, we successfully reduced lesion size and metabolic activity in three patients with progressive refractory fibrosing mediastinitis. The clinical improvement and reduction of fibroinflammatory tissue burden with rituximab therapy in these three patients with progressive fibrosing mediastinitis support the hypothesis that B lymphocytes detected in fibrosing mediastinitis tissue play a significant pathogenic role.
Although the precise pathophysiology of fibrosing mediastinitis remains poorly understood, evidence for a role of B lymphocytes in various fibrotic conditions, such as systemic sclerosis and diffuse fibrotic interstitial lung diseases including idiopathic pulmonary fibrosis, is growing (11–13). In addition, B lymphocyte–deficient animals are less susceptible to bleomycin-induced lung fibrosis, and activated B lymphocytes enhance the extracellular matrix production of dermal fibroblasts in normal control subjects and patients with systemic sclerosis in vitro (11, 14). Subsets of patients with fibrotic diseases have elevated levels of B lymphocyte–promoting cytokines, such as B lymphocyte–activating factor (12). As a consequence, the specific targeting of B lymphocytes has been explored as a potential treatment strategy for these fibrotic diseases (15–17).
Fibrosing mediastinitis is commonly diagnosed by its clinical and radiographic characteristics, and tissue confirmation is neither universally required nor performed (2, 3). FDG-PET/CT performed to evaluate for suspected malignancy frequently shows increased metabolic activity in fibrosing mediastinitis lesions (3). As a consequence, we hypothesized that tissue B lymphocytes are involved in the evolution of progressive fibrosing mediastinitis and contribute to the increased metabolic activity on FDG-PET/CT, and that therapy with rituximab can attenuate the increased metabolic activity and prevent disease progression. Depletion of B lymphocytes resulted not only in reduced metabolic activity of the fibrosing mediastinitis lesions but also in a significant reduction of the size of the fibrosing mediastinitis lesions and improvements in clinical symptoms. This novel mechanism-based treatment approach deserves further investigation.
Footnotes
Supported by K23CA159391 (T.P.).
Author Contributions: Conception and design: B.D.W., G.B.J., F.M., J.P.U., U.S., and T.P. Analysis and interpretation: B.D.W., G.B.J., F.M., J.P.U., U.S., and T.P. Drafting the manuscript for important intellectual content: B.D.W., G.B.J., F.M., J.P.U., U.S., and T.P.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Goodwin RA, Nickell JA, Des Prez RM. Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis. Medicine (Baltimore) 1972;51:227–246. doi: 10.1097/00005792-197205000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Davis AM, Pierson RN, Loyd JE. Mediastinal fibrosis. Semin Respir Infect. 2001;16:119–130. doi: 10.1053/srin.2001.24242. [DOI] [PubMed] [Google Scholar]
- 3.Peikert T, Colby TV, Midthun DE, Pairolero PC, Edell ES, Schroeder DR, Specks U. Fibrosing mediastinitis: clinical presentation, therapeutic outcomes, and adaptive immune response. Medicine (Baltimore) 2011;90:412–423. doi: 10.1097/MD.0b013e318237c8e6. [DOI] [PubMed] [Google Scholar]
- 4.Loyd JE, Tillman BF, Atkinson JB, Des Prez RM. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 1988;67:295–310. doi: 10.1097/00005792-198809000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Urschel HC, Jr, Razzuk MA, Netto GJ, Disiere J, Chung SY. Sclerosing mediastinitis: improved management with histoplasmosis titer and ketoconazole. Ann Thorac Surg. 1990;50:215–221. doi: 10.1016/0003-4975(90)90737-q. [DOI] [PubMed] [Google Scholar]
- 6.Savelli BA, Parshley M, Morganroth ML. Successful treatment of sclerosing cervicitis and fibrosing mediastinitis with tamoxifen. Chest. 1997;111:1137–1140. doi: 10.1378/chest.111.4.1137. [DOI] [PubMed] [Google Scholar]
- 7.Dines DE, Payne WS, Bernatz PE, Pairolero PC. Mediastinal granuloma and fibrosing mediastinitis. Chest. 1979;75:320–324. doi: 10.1378/chest.75.3.320. [DOI] [PubMed] [Google Scholar]
- 8.Doyle TP, Loyd JE, Robbins IM. Percutaneous pulmonary artery and vein stenting: a novel treatment for mediastinal fibrosis. Am J Respir Crit Care Med. 2001;164:657–660. doi: 10.1164/ajrccm.164.4.2012132. [DOI] [PubMed] [Google Scholar]
- 9.Garrett HE, Jr, Roper CL. Surgical intervention in histoplasmosis. Ann Thorac Surg. 1986;42:711–722. doi: 10.1016/s0003-4975(10)64619-x. [DOI] [PubMed] [Google Scholar]
- 10.Albers EL, Pugh ME, Hill KD, Wang L, Loyd JE, Doyle TP. Percutaneous vascular stent implantation as treatment for central vascular obstruction due to fibrosing mediastinitis. Circulation. 2011;123:1391–1399. doi: 10.1161/CIRCULATIONAHA.110.949180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.François A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, Gottenberg JE. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. 2013;15:R168. doi: 10.1186/ar4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue J, Kass DJ, Bon J, Vuga L, Tan J, Csizmadia E, Otterbein L, Soejima M, Levesque MC, Gibson KF, et al. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J Immunol. 2013;191:2089–2095. doi: 10.4049/jimmunol.1203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins SR, Turesson C, Myers JL, Tazelaar HD, Ryu JH, Matteson EL, Bongartz T. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum. 2006;54:635–641. doi: 10.1002/art.21758. [DOI] [PubMed] [Google Scholar]
- 14.Komura K, Yanaba K, Horikawa M, Ogawa F, Fujimoto M, Tedder TF, Sato S. CD19 regulates the development of bleomycin-induced pulmonary fibrosis in a mouse model. Arthritis Rheum. 2008;58:3574–3584. doi: 10.1002/art.23995. [DOI] [PubMed] [Google Scholar]
- 15.Matteson E, Bongartz T, Ryu JH, Crowson CS, Hartman TE, Dellaripa PF. Open-label, pilot study of the safety and clinical effects of rituximab in patients with rheumatoid arthritis-associated interstitial pneumonia. Open J Rheumatol Automimmune Dis. 2012;3:53–58. [Google Scholar]
- 16.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, Distler O on behalf of the EUSTAR Rituximab study group. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 17.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–359. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]