Abstract
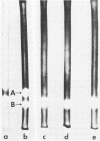
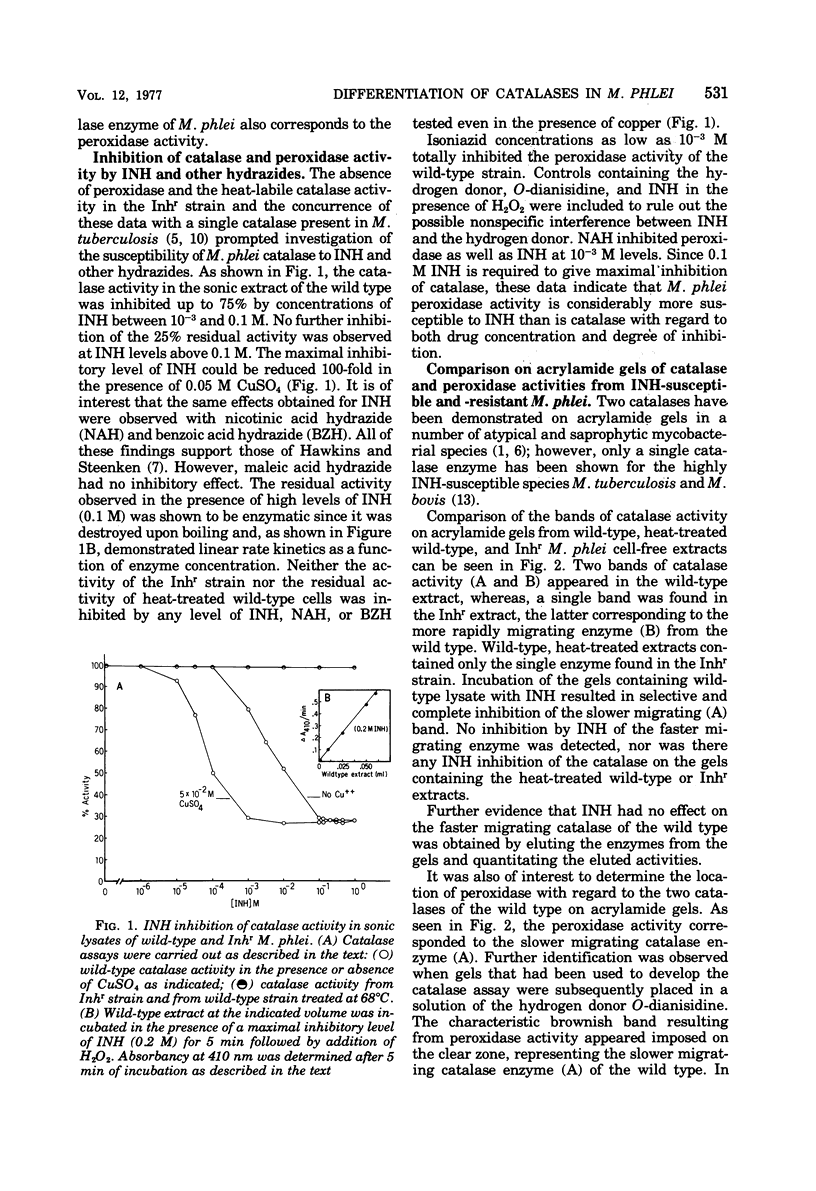
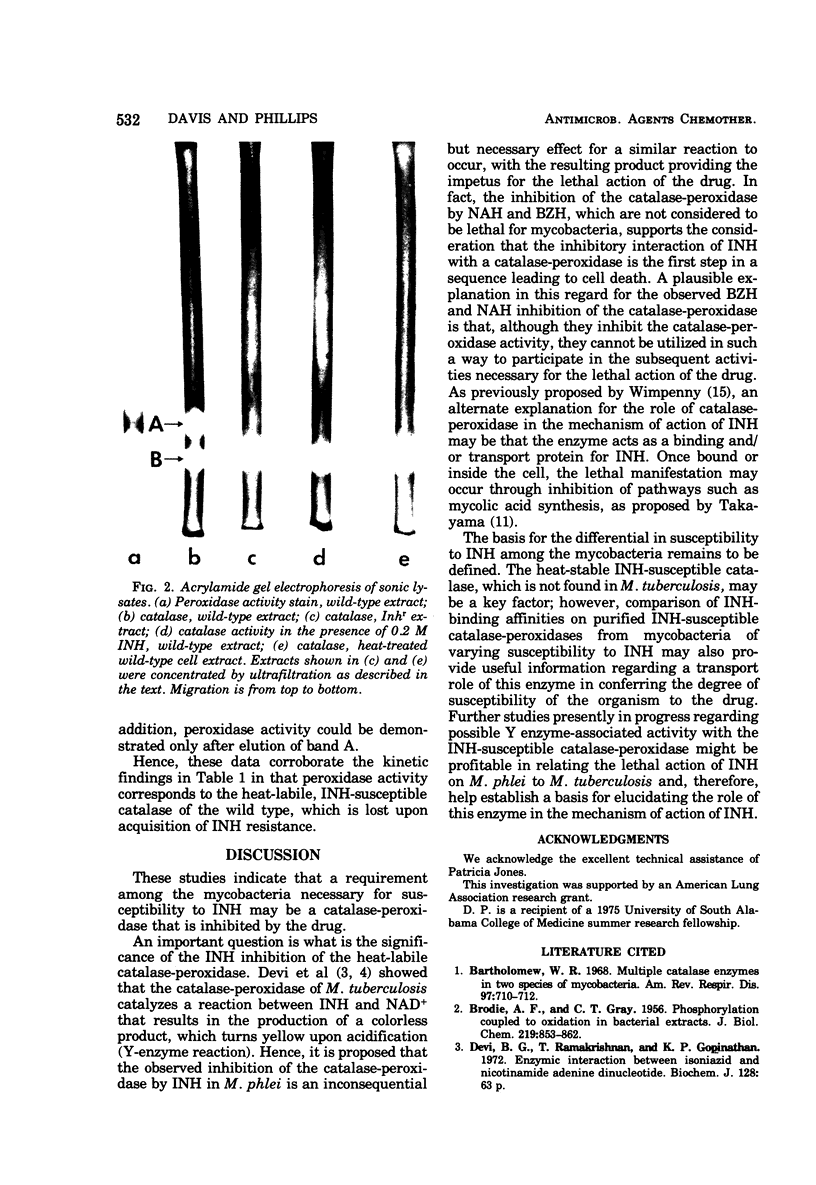
Mycobacterium phlei contains two catalase activities and a single peroxidase activity. The latter is associated with one of the catalases. The single catalase-peroxidase enzyme accounted for 75% of the total catalase activity and was lost upon acquisition of resistance to the antitubercular drug isoniazid (INH). Heat-treated (68°C) wild-type cells showed similar decreases in catalase activity as well as complete loss of peroxidase activity. Catalase activity in the INH-resistant strain of M. phlei (Inhr) was unaffected by heating. The heat-sensitive catalase of the wild-type M. phlei was completely inhibited by 0.1 M INH, and Cu2+ enhanced this inhibitory effect by 100-fold. No inhibition of activity was found with the heat-stable enzyme. Equivalent inhibition of catalase was also observed with nicotinic acid hydrazide and benzoic acid hydrazide. Peroxidase activity was also completely inhibited by any one of the three hydrazides, either INH, benzoic acid hydrazide, or nicotinic acid hydrazide at 10−3 M. The presence of two catalase activities and the loss of one (catalase-peroxidase) on acquiring INH resistance or heating wild-type cells was confirmed by acrylamide gel electrophoresis of the cell-free extracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE A. F., GRAY C. T. Phosphorylation coupled to oxidation in bacterial extracts. J Biol Chem. 1956 Apr;219(2):853–862. [PubMed] [Google Scholar]
- Bartholomew W. R. Multiple catalase enzymes in two species of mycobacteria. Am Rev Respir Dis. 1968 Apr;97(4):710–712. doi: 10.1164/arrd.1968.97.4.710. [DOI] [PubMed] [Google Scholar]
- Devi B. G., Shaila M. S., Ramakrishnan T., Gopinathan K. P. The purification and properties of peroxidase in Mycobacterium tuberculosis H37Rv and its possible role in the mechanism of action of isonicotinic acid hydrazide. Biochem J. 1975 Jul;149(1):187–197. doi: 10.1042/bj1490187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz G. A., Wayne L. G. Isolation and characterization of catalase produced by Mycobacterium tuberculosis. Am Rev Respir Dis. 1974 Sep;110(3):312–319. doi: 10.1164/arrd.1974.110.3.312. [DOI] [PubMed] [Google Scholar]
- Gruft H., Gaafar H. A. Multiple catalases of mycobacteria: differences in molecular weight. Am Rev Respir Dis. 1974 Sep;110(3):320–323. doi: 10.1164/arrd.1974.110.3.320. [DOI] [PubMed] [Google Scholar]
- HAWKINS J. E., STEENKEN W., Jr Inhibition of catalase activity by isoniazid and the effect of vitamin B6. Proc Soc Exp Biol Med. 1963 Jan;112:30–33. doi: 10.3181/00379727-112-27941. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIDDLEBROOK G. Isoniazid-resistance and catalase activity of tubercle bacilli; a preliminary report. Am Rev Tuberc. 1954 Mar;69(3):471–472. doi: 10.1164/art.1954.69.3.471. [DOI] [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]
- Takayama K., Wang L., David H. L. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1972 Jul;2(1):29–35. doi: 10.1128/aac.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDER F. Catalase and peroxidase in mycobacteria. Possible relationship to the mode of action of isoniazid. Am Rev Respir Dis. 1960 Jan;81:68–78. doi: 10.1164/arrd.1960.81.1P1.68. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Diaz G. A., Doubek J. R. Acquired isoniazid resistance and catalase activity of myobacteria. Am Rev Respir Dis. 1968 May;97(5):909–913. doi: 10.1164/arrd.1968.97.5.909. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wimpenny J. W. The uptake and fate of isoniazid in Mycobacterium tuberculosis var. bovis BCG. J Gen Microbiol. 1967 Jun;47(3):389–403. doi: 10.1099/00221287-47-3-389. [DOI] [PubMed] [Google Scholar]
- Youatt J. A review of the action of isoniazid. Am Rev Respir Dis. 1969 May;99(5):729–749. doi: 10.1164/arrd.1969.99.5.729. [DOI] [PubMed] [Google Scholar]