Abstract
Appropriate interneuron migration and distribution is essential for the construction of functional neuronal circuitry and the maintenance of excitatory/inhibitory balance in the brain. GABAergic interneurons originating from ventral telencephalon choreograph a complex pattern of migration to reach their target destinations within the developing brain. This review examines the cellular and molecular underpinnings of the major decision-making steps involved in this process of oriental navigation of cortical interneurons.
Keywords: Interneurons, migration, laminar organization, neural circuitry, cerebral cortex
Introduction
The functions of the central nervous system (CNS) requires balanced and coordinated activities between the excitatory, glutamatergic projection neurons and inhibitory GABAergic (gamma-aminobutyric-acid) interneurons. In contrast to the projection neurons that are generated in the dorsal telencephalon (pallium) and migrate radially over a relatively shorter distance into the developing cortical plate, interneurons originate from distinct regions of the subpallium and migrate tangentially in multiple streams, across areal boundaries of the developing telencephalon, to reach their intended destinations in the neocortex, striatum, hippocampus, and olfactory bulb (OB)1. During this process, interneurons precisely integrate their cell-intrinsic characteristics with input from local environmental cues to facilitate decisions that are necessary for appropriate patterns of migration (Text Box 1). This review provides a summary of the major decision-making steps involved in interneuron migration and the cellular and molecular mechanisms underlying each of these steps. In particular, we focus on the determinant steps that enable cortical interneurons to navigate towards and incorporate into defined neural microcircuitry in the cortex and the challenges remaining in our understanding of this process.
Text Box 1. Origins and migratory routes of interneurons in the developing brain.
Interneurons are highly heterogeneous and diverse neuronal population that arises from progenitor pools within the lateral ganglionic eminence (LGE), medial ganglionic eminence (MGE), caudal ganglionic eminence (CGE), preoptic area (POa), and septal anlage of the subpallium in the developing telencephalon2–6. Post-mitotic interneurons from these distinct proliferative domains exit through distinct pathways7–12, dorsally to the cortex, ventrolaterally to the striatum, caudally to the hippocampus and rostrally to the OB, to reach their final target destinations.
The most extensively studied of these pathways is the GE-derived GABAergic interneurons migrating towards the dorsal cortex. Early tracing studies have demonstrated that different streams of interneurons arising from GE are able to transit across the cortico-subpallial boundary, and course tangentially into the cortex. An early stream of interneurons (~ E11.5 in mouse) from MGE migrate dorsolaterally onto the top of the preplate, where many of them eventually become layer I Cajal-Retzius neurons2. Later during corticogenesis (~ E13-E15 in mouse), a second and more prominent stream of interneurons, mainly from MGE, rapidly migrate into the neocortex, through the intermediate zone (IZ)2. This latter stream is joined by interneurons from LGE, although much less robustly and via a more restricted route through the cortical proliferative zone1, 2. At later stages of corticogenesis, interneurons enter the cortex via multiple streams, largely through lower IZ and subventricular zone (SVZ), as well as through migratory streams in subplate (SP) and marginal zone (MZ). Additionally, CGE has been shown to be another major source of cortical interneurons. 3-D profile of cortical interneuron migration indicates that simultaneous with the MGE derived streams, a wave of interneurons originating from CGE migrate in a lateral and medial direction to enter the caudal-most end of the cerebral cortex8, 9, 13 (Figure 1).
Subpallially originating interneurons also tangentially migrate toward other destinations within the developing brain: ventrolaterally to the striatum, caudally to the hippocampus and rostrally to the OB. MGE together with the adjacent POa gives rise to striatal interneurons that migrate tangentially into the developing striatum, where they differentiate and integrate into the local striatal neural circuitry7. CGE is the largest source of hippocampal interneurons. By E13.5 in mouse, a stream of CGE-derived interneurons rapidly migrate towards the caudal end of telencephalon, where they enter MZ and eventually settle down in the hippocampus9, 13. In contrast, LGE give rise to most if not all interneurons that migrate rostrally and populate both the glomerular and granule cell layers of the olfactory bulb7, 10, 14, 15. The migration of olfactory interneuron precursors continues throughout postnatal period and adulthood, providing a constant supply of interneurons to the local neural circuits of the olfactory bulb16, 17. The subventricular zone (SVZ), a mitotically active region in the dorsal-medial corner of striatum that is derived from embryonic LGE, gives rise to these postnatal olfactory interneurons18, 19. Compared to the embryonic stages of olfactory interneuron migration, during which loosely associated neurons disperse through the extracellular space, new born interneurons in neonates and adults organize into a network of interlinked chains, surrounded by astroglial tubes, to migrate in a restricted and highly oriented route named rostral migration stream (RMS) 16, 17.
Decision-making steps of interneuron migration
The cellular dynamics (Text Boxes 1, 2) underlying the navigation of interneurons from their sites of birth to their final areal and laminar destinations (Text Box 3) can be broadly divided into six decision-making steps and the mechanisms serving each of them are examined below.
Text Box 2. Cellular dynamics of migrating interneurons.
Unlike the stereotypical migratory behavior of many neurons that extend a single leading process in the direction of migration, interneurons search for guidance signals by vigorously and continuously extending multiple, diverging branches from the leading process to better sense and align with the source of the orienting gradients20, 21. The branch that best aligns with the net gradient of the guidance cues then is stabilized, while other branches retract, and the nucleus moves in the direction of the stabilized leading process. Further, interneurons can also alter migratory direction by reversing their polarity, i. e., by converting the trailing process directly into a leading process while the previous leading process retracts like a trailing process22.
Once the migratory direction is decided, interneurons advance forward by performing a repeated cycle of two-phase nucleokinesis23. First, as the leading process is stabilized, organelles including the centrioles and Golgi apparatus within the perinuclear cytoplasm form a presomal swelling and extend into the leading process. In the second phase, the nucleus translocates toward the presomal swelling as the trailing process retracts toward the new position of the cell soma. This two-phase nucleokinesis results in the characteristic saltatory mode of interneuron migration, alternating between a resting phase, when the leading process is actively extending and exploring, and a moving phase, when the cell soma translocates in a new direction23, 24. Two sets of cellular forces facilitate the nuclear movement in migrating interneurons: the microtubule-dependent pulling force and the actomyosin-dependent pushing force. The pulling force is generated by the microtubule “perinuclear cage”, which envelops the nucleus and is tethered to the centrosome to couple the nuclear movement with the direction set by the leading process23. In contrast, non-muscle myosin II that accumulates at the rear end of the cell body provides the contractile pushing force for the forward movement of nucleus23. This pattern of coordinated leading process/nucleokinesis dynamics is repeated to facilitate directional movement of interneurons.
Text Box 3. Laminar and areal allocation of cortical interneurons.
Once in the dorsal cortex, interneurons employ multiple modes of migration as they move to specific areal and laminar locations within the emerging CP22, 25–28. The local migration within the dorsal cerebral wall is crucial in determining the final positioning of cortical interneurons. For example, interneurons migrating in MZ stream undergo multidirectional local migration, actively contacting radial glial endfeet, before turning inwards and moving radially towards the CP8, 25–28. Interneurons in SP or IZ/SVZ streams also switch their mode of migration from tangential to radial, and extensively contact the radial glial processes as they migrate up towards the CP8, 26–28. Moreover, a subpopulation of interneurons within IZ exhibit “ventricle-oriented migration”, during which they migrate radially into VZ, and pause at the bottom of VZ, extending multiple processes to scan the ventricular surface, possibly to obtain positional information or modulate progenitor proliferation, prior to migrating up radially towards the CP22, 26.
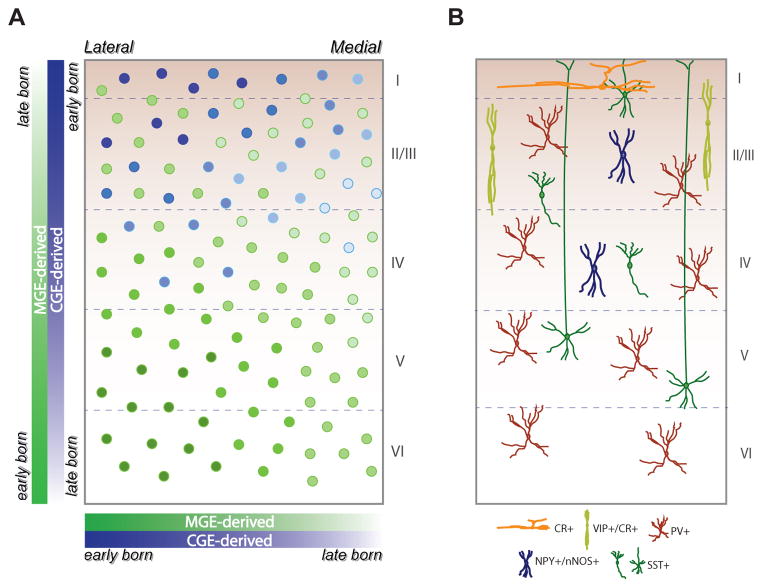
Interneurons follow a lateral to medial gradient to colonize the neocortex, with younger neurons arriving at the lateral cortical domains earlier than the medial regions29 (Figure I. A). After arriving at the appropriate cortical area, interneurons settle into specific laminar positions prior to forming functional synaptic contacts with appropriate projection neuronal partners. Birthdate analysis of specific interneuron subtypes suggests that interneurons follow heterogeneous developmental rules for laminar positioning8, 30–34. MGE and POa derived somatostatin+(SST+), parvalbumin+(PV+), and calbindin+(CB+) subtypes show a time-dependent, inside-out pattern of positioning that is similar to projection neurons. In contrast, CGE-derived calretinin+ interneurons show an outside-in placement pattern8, 29. Further, vasoactive intestinal polypeptide+ (VIP+) and neuropeptide Y+ (NPY+) interneurons do not show a strict inside-out layering pattern, but preferentially localize to superficial layers or scatter widely within the cortex, respectively29, 34(Figure I. A, B). The final cortical distribution of interneurons therefore depends on the temporal and spatial origin of interneurons, subtype specification, as well as on interactions with radial glial scaffold and projection neurons.
Figure I. The developmental distribution of interneuron subtypes.
(A) Schematic of a coronal section through the mouse neonatal cerebral cortex showing the areal and laminar positioning of MGE- and CGE-derived GABAergic interneurons. Both MGE- and CGE-derived interneurons reach their final areal positions in a lateral to medial gradient (i.e., arriving first in laternal regions of cortex). MGE-derived interneurons show an inside-out pattern of distribution, whereas CGE-derived interneurons exhibit an outside-in pattern of distribution. MGE-derived interneurons distribute relatively evenly in the neocortex, whereas CGE-derived interneurons preferentially distribute in superficial layers. (B) Laminar distribution of main subtypes of interneurons. PV+ interneurons are abundant throughout cortical layers II–VI. SST+ interneurons mainly localize to layers II–V. CR+ interneurons preferentially distribute in layer I. VIP+/CR+ interneurons preferentially distribute through layer II/III. NPY+/nNOS+ interneurons mainly localize to layers II–IV. PV, parvalbumin; SST, somatostatin; CR, calretinin; VIP, vasoactive intestinal polypeptide; NPY, neuropeptide Y; nNOS, neuronal nitric oxide synthase. I–VI: cortical layers.
Exit from the proliferative zone and initiation of migration
Newborn interneurons cluster around radial glial fibers or coalesce as migratory stream as they exit from the subpallial proliferative zone (Figure 1)4, 35. Newborn interneurons initiate their exit away from the proliferative zone in subpallium by utilizing a combination of chemorepulsive guidance cues and motogenic factors36, 37. Chemorepulsive cues play a key role in guiding the path of exit of migrating interneurons away from the VZ of GE. Diffusible guidance proteins Slit1 and Netrin1, known chemorepulsive cues for axonal growth and guidance, have been shown in vitro to repel interneurons from GE region, although, in vivo genetic models failed to provide direct evidence supporting their repulsive influence on interneuron migration38–40. Further, a recent study has demonstrated that guidance molecule Ephrin-A5 acts as the repellent force to facilitate the exit of newborn interneurons from GE41. Ephrin-A5 is expressed in the VZ of GE, while its signaling receptor EphA4 is strongly expressed in newborn, GE-derived interneurons41. In vitro assays showed that down-regulated Ephrin-A5 in the VZ of GE led to ectopic invasion of interneurons into VZ41. In contrast, exogenously applied Ephrin-A5 recombinant protein restores the avoidance of VZ by migrating interneurons41.
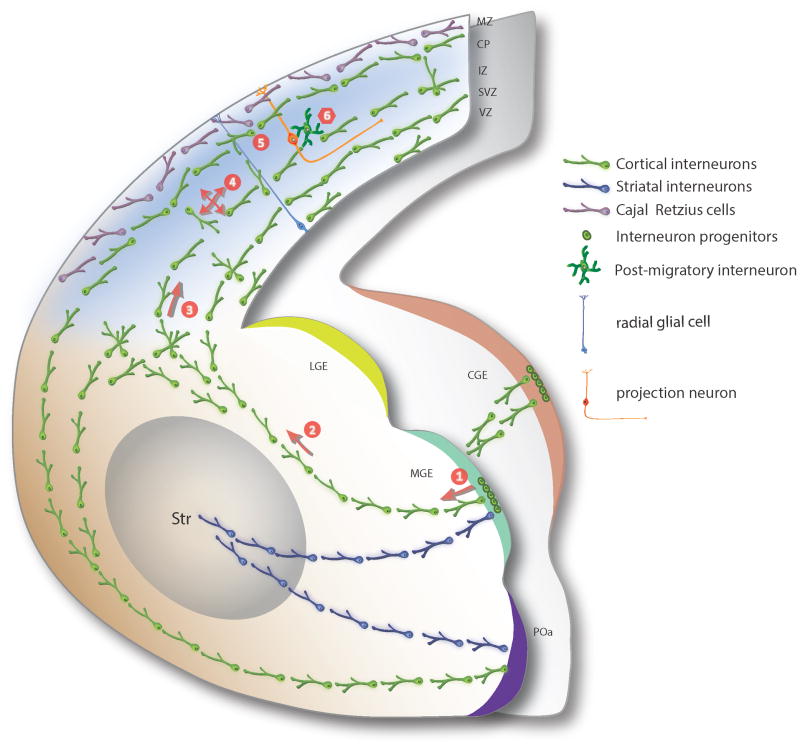
Figure 1. Patterns of interneuron migration in the developing telencephalon.
This schema shows rostral and caudal hemi-section through the mouse telencephalon at mid-embryonic (E15) stage. The major decision-making steps (1-6) involved in the migration of cortical interneurons derived from the subpallium are illustrated. Interneurons derived from MGE (green), POa (purple), or CGE (orange) exit the proliferative zones and initiate their migration towards the developing neocortex and striatum. Cortical interneurons traverse around the developing striatum, transit across the cortico-subpallial boundary, and course tangentially into the cortex, whereas striatal interneurons ventrolaterally migrate into the developing striatum. Cortical interneurons transit the neocortex mainly through the MZ, SP, IZ/SVZ migratory streams. Once in the neocortex, tangentially migrating interneurons undergo multi-modal local migration as they reach and settle in specific areal and laminar locations within the emerging CP, prior to forming functional synaptic contacts with appropriate projection neuron partners. Multiple decision-making steps are involved in this process. These include: (1) exit from the proliferative zone and initiation of migration in the subpallium, (2) selection of migratory route towards the dorsal cortex, (3) choice of migratory streams within the neocortex, (4) local orientation of migration within the cerebral wall, (5) identification of the final areal and laminar location, and (6) termination of migration at the appropriate cortical layer. Arrows indicate net directionality of movement. LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; POa, preoptic area; Str, striatum; MZ, marginal zone; CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone.
Once repelled away from the proliferative zone, several motogenic factors have been identified to stimulate the migration of newborn interneurons from GE24, 42. Of these, dysfunction of hepatocyte growth factor/scatter factor (HGF/SF) signaling resulted in impaired cell mobility and reduced interneuron migration into the cortex42. Other growth factors including brain-derived neurotrophic factor (BDNF), neurotrophin 4 (NT4) and glial cell line-derived neurotrophic factor (GDNF) have also been suggested to be potent motogenic factors for newborn interneurons in GE24, 43. Although genetic evidence is still lacking to conclude a direct role for these molecules in the initiation of interneuron migration in vivo, several in vitro experiments using isolated interneurons and cortical slices have clearly suggested their influence on interneuron motility42–45. Together, these observations suggest a combination of chemorepellent and motogenic cues present in the proliferative zones of the GE may impel newborn interneurons to exit GE and initiate their migration.
Selection of migratory route towards dorsal cortex
Once migration is underway, interneurons face the challenge of selecting a specific migratory route into the dorsal or ventral cortex (Figure 1). Interneurons with different temporal and spatial origin in the subpallium follow specific migratory routes, suggesting that distinct origins of interneurons help prespecify their migratory routes. Indeed, the results of isochronic and heterochronic transplantation experiments have shown that interneurons are cell-autonomously committed to their specific migratory fate as early as E11.5 for LGE-derived interneurons and E13.5 for MGE and CGE-derived interneurons9, 13, 15, 46. The intrinsic migratory fate of interneurons are specified by the combinatorial expression of several key transcription factors that are expressed within the progenitor domains of the subpallium22, 47–51. These transcription factors not only define subpallial patterning and interneuron differentiation, but also provide migratory route instructions for the newborn interneurons22, 47–53. One of these transcription factors is Nkx2.1. Its expression is maintained in newborn interneurons migrating into striatum, but is downregulated in interneurons destined for the cortex. This differential Nkx2.1 expression is necessary for interneurons to migrate into cortex and serves as a sorting mechanism for directional migration of cortical and striatal interneurons52. In contrast, COUP transcription factor II (COUP-TFII), preferentially expressed in the CGE, is required for the CGE-derived interneuron migration in the caudal direction54. Notably, overexpression of COUP-TFII in MGE interneurons is sufficient to change their migratory orientation to caudal direction when transplanted into the CGE environment, thus providing an example of how a single, locally expressed transcription factor activity is capable of determining the migratory fate of interneurons in its local environment54.
It is likely that transcription factors specify the intrinsic migratory fate of interneurons by modulating the expression of signaling receptors and cytoskeletal components that impart them with competence to respond selectively to route specific environmental cues. For example, the MGE-derived cortical interneurons avoid ventral POa and lateral striatum as they migrate toward dorsal cortex39, 55. Chemorepulsive cues play an essential role in establishing this pattern. EphrinB3 expressed in POa and its derivatives acts as a repulsive cue by binding to EphA4 receptor expressed by MGE-derived cortical interneurons56. This repellent activity prevents MGE interneurons from migrating in a ventral direction and is possibly responsible for their dorsal orientation toward the cortex56. Also, the repellent activity mediated by class 3 semaphorins (Sema3A and Sema3F) present in the developing striatum is largely responsible for the sorting between MGE-derived cortical interneurons and striatal interneurons. The expression of Neuropilin 1 (Nrp) and Nrp2 receptors by MGE-derived interneurons destined to cortex, but not by striatal interneurons, ensures cortical interneurons are competent to respond to the repulsive actions of Sema3A and Sema3F, and thus enabling them to migrate around the developing striatum and enter the neocortex57. Importantly, Nkx2.1 has been shown to directly repress Nrp levels57. Thus, the downregulation of Nkx2.1 expression in MGE-derived interneurons renders them sensitive to Sema3A/Sema3F repellent cue, and facilitates their choice of specific migratory route. The downregulation of Nkx2.1 in cortical interneurons requires transcription factor Sip1. Sip1 also contributes to the sorting of cortical vs. striatal interneurons by repressing Netrin1 receptor Unc5b expression in cortical interneurons to facilitate their entry into the neocortex58, 59.
In addition to repulsive cues, GE-derived interneurons also utilize gradients of permissive and attractant cues to migrate towards cortex39. Two isoforms of Neuregulin-1 (Nrg1), a membrane-bound isoform, CRD-Nrg1, and a diffusible isoform, Ig-Nrg1, have been shown to act as short-range permissive and long-range chemoattractant cue, respectively, for cortical interneurons60, 61. CRD-Nrg1 is expressed throughout the LGE from the VZ to the developing striatal mantal zone, providing a permissive corridor from the MGE to the pallial-subpallial boundary60. In contrast, Ig-Nrg1 is released in the neocortex, providing a diffusible cue that attracts cortical interneurons towards the neocortex as they exit the CRD-Nrg1+ permissive corridor60. The function of Nrg1 requires activity of ErbB4 receptors60, 61. Consistently, perturbation of ErbB4 signaling decreases the number of interneurons entering the neocortex60, 61.
Further, recent evidence suggests that neurotransmitters including ambient GABA, glycine, glutamate and dopamine promote interneuron migration and their entry into the neocortex62–74. Glycine functions through GlyRs to regulate interneuron migration velocity and nucleokinesis by controlling actomyosin contractility74. Acute loss of GlyR function impairs interneuron corticostriatal boundary crossing and entry into the neocortex74. In contrast, migrating interneurons appear to activate their response to ambient GABA signal or glutamate once they reach the neocortex. This switch-on response is accomplished by altering the expression profile of distinct GABAA receptor subunits (increased expression of the α1-, α2-, γ5-, γ2-, and γ3-subunits) and activation of AMPA receptors, respectively, as interneurons navigate from subpallium to the neocortex62, 68, 69, 71, 72. Moreover, a balance in distinct dopamine receptor activities differentially modulates interneuron migration from GE to cortex: D1 receptor activation promotes, whereas D2 receptor activation decreases interneuron migration67. Taken together, interneurons integrate their transcription factor and signaling receptor expression profile with extrinsic environmental cues (e.g., chemorepulsive cues, chemoattractive cues, and neurotransmitters) to facilitate the selection of a migratory route from the GE to the cortex.
Choices of migratory streams within the neocortex
Interneurons form specific migratory streams through MZ, SP and IZ/SVZ as they traverse the neocortex22, 26 (Figure 1). This migratory pattern raises the question of whether interneurons randomly distribute in these streams or actively choose one of these three streams. If the latter is true, what are the factors that determine the choice of the migratory stream and does the selective path of migration plays a role in the eventual emergence of the interneuron subtype identity?
Cell intrinsic determinants are thought to play an essential role in migratory stream choices of interneurons. For example, transplantation experiments with retinoblastoma (Rb) mutant interneurons showed a dramatic failure of mutant neurons to migrate along the MZ stream in the wild type brain, suggesting a cell-autonomous requirement for Rb protein in interneuronal migration in the MZ stream75. Further, pharmacological blockade of the GABAB receptor resulted in accumulation of interneurons migrating in the SVZ/VZ stream and fewer interneurons in the MZ stream66. In contrast, dysfunction of GlyR α2 subunit specifically decreased interneurons migrating in the SVZ stream74. In addition, loss of Dopamine D1 receptor signaling significantly decreased the migration of interneurons in IZ and VZ/SVZ streams, whereas loss of D2 Dopamine receptors led to an increase of interneurons migrating in these streams66, 67. These results suggest that cell-intrinsic characteristics dictate interneuronal route preferences within the neocortex.
Aside from cell-intrinsic determinants, regionally localized environmental cues also influence the interneuron migration routes within the neocortex. For example, Netrin1 is produced in the cortical MZ and Netrin1’s binding to α3β1 integrin is required for the migration of interneurons through the MZ stream in the neocortex76. Consistently, in Netrin1/α3β1 integrin double mutants, significantly fewer interneurons migrate through the MZ stream and increased number of interneurons ectopically migrate through the VZ76. Further, Cajal-Retzius (CR) cells may provide positional cues for the interneurons migrating in close apposition below them in the MZ stream. It has been shown that either loss of CR cells or abnormal distribution of CR cells disrupt interneuron migration along the cortical MZ77–79. Gene expression profile analysis has revealed that a large number of genes including signaling receptors (e.g. Cdh8, Epha3, Robo2) and intracellular signaling modulators (e.g. Cdc42ep3, Plcb1, Rasgdf1b) are differentially expressed between the interneurons that migrate through either the MZ or the IZ stream in the neocortex80. Thus, it is likely that distinct intrinsic characteristics of migrating interneurons, either acquired prior to or after their entry into the cortex, in combination with extracellular cues released within the cerebral wall, dictate the choice of distinct interneuron migratory routes within the cerebral wall.
Determination of local orientation of migration in neocortex
The directional steering of migrating interneurons within or in between streams is achieved by biased choices of leading process branches. This choice correlates tightly with rapid changes in growth cones dynamics. In particular, the stabilized leading branch of a migrating interneuron displays an elaborate growth cone, whereas the growth cones of non-selected branches rapidly collapse prior to branch retraction20. Growth cones serve to elongate or retract the branches by receiving various environmental guidance cues and relaying this guidance information to the two main cytoskeletal networks: actin filaments and microtubules20. The dynamic interplay between the pushing force exerted via microtubule assembly and the actin-driven pulling force at the leading edge of the growth cone is required for process extension and retraction. Semaphorin signaling in the growth cone provides an illustrative model of how guidance cues coordinate the cytoskeletal rearrangement necessary for local directional migration. Semaphorins function as chemorepellent cues by inducing growth cone collapse via Rho GTPases and associated proteins81–88. Semaphorin regulated activation of Rho GTPases Rac1 or RhoA lead to either decreased actin turnover or increased actin contractility, respectively, resulting ultimately in growth cone collapse81, 82, 84. Alternatively, semaphorin-mediated signaling could also regulate microtubule dynamics via GSK3β activity, leading to microtubule destabilization and growth cone collapse86, 88. As a result, only the branch that is oriented farthest away from source of the repulsive cue gets stabilized and subsequently facilitates the nucleokinesis of the neurons away from the repellent cue. Conversely, the presence of chemoattractants (e.g. Nrg1) influences the initial orientation of the newly extended branches towards the chemoattractant gradients and helps to selectively stabilize the leading process branch that is in closest proximity to the source of the attractant, and thus enables efficient directional change20.
Although little is known about the molecular mechanisms that directly transfer extracellular guidance cue information to the underlying cytoskeleton in motile interneurons, doublecortin (DCX), a microtubule associated protein known to stabilize mircrotubles, has been shown to play an important role in regulating growth cone dynamics and process stability in migrating interneurons89–91. In DCX-deficient interneurons, the leading processes exhibit increased growth cone formation and branching89–91. As a result, their ability to make directional changes in response to environmental cues is compromised and DCX-deficient interneurons migrate in a less organized manner from GE into the neocortex91. In addition, other cytoskeletal regulators such as microtubule associated protein Lissencephaly 1 (Lis1), Doublecortin-like kinases (DCLKs), their upstream regulators CDK5/p35, and transcription factors such as Dlx1/2, are also known to modulate the oriented extension of the leading process during interneuronal migration59, 88, 91–97.
Interneuron nucleokinesis, which follows leading process stabilization, also relies on rapid cytoskeletal rearrangements involving both microtubules and actin networks. Nuclear translocation requires centrosome-nucleus coupling by the microtubule perinuclear cage. Consistently, in DCX mutant interneurons, the branching defects are coupled with nucleokinesis defects, the latter being characterized by shorter nuclear displacements and abnormal perisomal swelling dynamics91. The mechanism of nuclear translocation in interneurons is also heavily dependent upon myosin II-mediated actin contractability at the rear of the cell23. Notably, Nonmuscle myosin II inhibition efficiently blocked nuclear translocation in migrating interneurons23. Recent studies have also suggested that primary cilium, a microtubule-based sensory organelle, is essential for sensing and integrating networks of signaling pathways necessary for oriented interneuron migration98, 99. The membrane of primary cilium is enriched with signaling receptors that enable it to act as a sensor of shallow gradients during oriented interneuronal migration. The proximity and linkage of the primary cilium to the nucleus and centrosomes may facilitate its ability to efficiently convey determinant signals necessary for nucleokinesis. Coordination of branching dynamics and nucleokinesis by signals emanating from different domains of interneurons may thus help set the local migratory direction of interneurons.
Intracortical dispersion of interneurons
Upon traversing the neocortex in different streams, interneurons radially invade the CP once in their appropriate cortical areas100 (Figure 1). Chemoattractant activity mediated by signals such as chemokine CXCL12 normally confines interneurons within the migratory streams and may regulate their appropriate exit from the streams. CXCL12 is strongly expressed within MZ and SVZ, and at a lower level in SP78, 100–104. CXCL12 signaling restricts the migrating cortical interneurons into confined streams by suppressing the leading process branching and thereby maintaining their tangential migratory direction105, 106. The expression of both receptors CXCR4 and CXCR7 are required for interneurons to respond to CXCL12102–104. In CXCR4 or CXCR7 mutants, interneurons display frequent branching, defects in forming organized migratory streams through MZ and SVZ, and prematurely invade the developing CP100–103, 105, 107, 108. Thus, CXCL12 signaling not only confines interneurons into tangential migratory streams, but may also prevent them from invading into the developing CP prematurely.
Within the MZ stream, interneurons exhibit a particular migratory behavior called “random walk”, leading to constant, multidirectional changes25. This behavior of interneurons is believed to contribute to the tangential dispersion of interneurons to appropriate cortical areal positions. Layer I Cajal-Retzius cells and interneurons in the MZ stream both show similar multidirectional migration with their leading processes arranged in similar orientations25, 27, 28. CR cells occupy the entire surface of the cerebral cortex and arrive through tangential migration at earlier stages of corticogenesis109. Repetitive, random cell-cell repulsive interaction mediated by Eph/ephrin signaling appears to be essential for the even dispersion and final distribution of CR cells in the cerebral cortex109. This contact repulsion process is also required to establish and stabilize the boundaries between different territories of subgroups of CRs that are born in discrete regions (cortical hem, pallial septum and ventral pallium)109–111. Further, contact between interneurons and radial glial endfeet is known to alter the migratory patterns of subtypes of interneurons26–28. It is tempting to speculate that similar contact repulsive interactions may exist between individual interneurons within MZ stream, between CR cells and interneurons, or between interneurons and radial glial endfeet, and may thus contribute to the appropriate dispersion of interneurons within the cerebral cortex.
The final stages of intracortical dispersion of interneurons depend on a tangential to radial switch of the interneuronal migratory mode. To date, the mechanisms coordinating this switch remain largely unclear. A series of isochronic or heterochronic transplant experiments have demonstrated that interneurons of different birthdates remain within the tangential migration streams for similar amount of time (~ 48 hours)100. The temporally regulated loss of responsiveness to CXCL12 signaling seems to be critical for this process since the interneurons that radially invade the CP no longer respond to CXCL12 signaling24, 102, 105. These observations led to the suggestion that interneurons and the cortical environment might undergo stage-dependent and synchronized maturation to coordinate tangential to radial switch and interneuronal entry into the developing CP.
Further, it is likely that radial glial scaffold is instructive in interneurons’ tangential to radial migration transition24, 26. The adhesion protein Connexin 43 (CX43) has been shown to be required for the interaction between interneurons and radial glia and deletion of CX43 significantly retards the tangential to radial transition of interneurons112. In order to make the tangential to radial directional switch, interneurons rapidly extend new branches that are oriented orthogonally to their tangential migratory direction 20, 24, 26. Changes in the dynamics of interneuron branching appear to be critical for this transition. For instance, over-activation of PAK3, a member of the p21-activated serine/threonine kinases (PAKs) family, in Dlx1/2 mutant interneurons contributes to decreased branching, excessive leading process extension and the resultant defect in tangential to radial migration transition97. Consequently, Dlx1/2 mutant interneurons accumulate in the MZ and IZ in the neocortex97. Similarly, inhibition of RhoA/ROCK signaling also leads to leading process elongation, reduced branching, and impaired tangential to radial transition20. Recently, Sonic hedgehog signaling mediated by primary cilia was shown to coordinate nucleokinesis and leading process extension dynamics necessary for tangential to radial transition, further highlighting the importance of coordination of these two cellular events for intracortical migration of interneurons99.
Termination of migration
Once within the CP, interneurons are directed to their final laminar positions (Figure 1). Several lines of evidence suggest that projection neurons with distinct layer identities selectively affect the distribution of subtypes of interneurons that are destined to populate the same cortical layers. First, majority of MGE-derived interneurons settle down with their coetaneous projection neurons in the same laminar layer, in an inside-out manner (i.e., later born interneurons migrating past earlier born populations to occupy more superficial laminar layer)30, 33, 113. A notable exception is the CGE-derived cortical interneurons which tend to populate the superficial layers regardless of their birthdates114 (Text Box 3). Second, heterochronic transplantations of MGE cells have suggested that both early- and late-born interneuron progenitors are able to switch their laminar fates in their new cortical environment, suggesting that the exposure to cortical environmental cues, can influence interneuronal laminar fate33. Interneurons delay their invasion into the CP until their pyramidal neuronal counterparts have acquired their laminar identities31, 100, 115. Consistently, mutants that exhibit premature invasion of interneruons into the CP also show disrupted final laminar and regional distribution of interneurons100. Third, interneurons distribute abnormally in the cortex of mutants with projection neuron positioning defects31, 92, 116–118. Finally, projection neurons with different layer identities differentially affect the laminar distribution of distinct interneuron subtypes31, 115, 116, 118. Clonally related interneurons, similar to projection neuron clones, do not randomly disperse but are frequently arranged into vertical or horizontal clusters in the neocortex35, 119. It is possible that coordinated interactions between identity matched, spatially organized clones of inhibitory interneurons and excitatory projection neurons may contribute to the appropriate placement of neurons necessary for a lineage-dependent organization of microcircuits in the neocortex.
In addition to signals from projection neurons, postnatal neuronal activity can also affect interneuron positioning120. Once at the final laminar localization, interneurons cease migration by altering their intracellular calcium transients in response to ambient GABA and glutamate signal64. KCC2, a potassium/chloride exchanger, is the deciding factor during this process. The upregulation of KCC2 in interneurons as they arrive at their laminar locations triggers a depolarization to hyperpolarizion switch, thereby altering their response to ambient GABA and glutamate from motogenic to stop signal64. In CGE-derived interneurons, induced overexpression of the potassium channel Kir2.1 between postnatal days 0–3 alters their excitability and results in an aberrant increase in the localization of CGE-derived Calretinin+ interneurons in deeper layers120. Further, participation of cortical interneurons in the emergence of synchronized glutamate-dependent cortical network oscillations during early postnatal stages may also influence the laminar positioning of interneurons65, 121–123. Together, these observations suggest that interneurons integrate information about their temporal and spatial origin, subtype identity, and extrinsic signals from projection neurons and CP environment to establish their final laminar fate.
Concluding remarks
Although significant advances have been made in delineating the various molecular mechanisms underlying interneuron migration, many questions about the decision-making aspects of this process remain open. The current approaches to the study interneuron migration use fixed tissue analysis of limited cortical regions, dissociated neurons in vitro, or focus on movement of tens of neurons in small areas of often undefined embryonic cortical regions. While these approaches have provided insights into the modalities and molecular control of neuronal migration, they do not help us understand how specific subtypes of interneurons navigate and achieve their laminar and areal positions at the right time in right numbers within the entirety of cerebral cortex. This parceling out of appropriate numbers and types of cortical interneurons to distinct cortical areas is fundamental to the emergence of functional specification and connectivity of cerebral cortex. New methods that can track the behavior of large cohorts of interneurons from the time of birth to the final placement in distinct cortical areas35, 124 will be necessary to gain comprehensive insights into the impact of interneuron migration in the emergence of neuronal connectome. Combining such approaches with examination of signaling dynamics in developing interneurons will also facilitate answers to several other related outstanding issues. For example, do interneurons at different decision points along their migration route utilize different signaling networks to mediate their choices? What are the hierarchical relationships between the different signaling networks used to make different choices during the process of migration? STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) analysis of proteins regulating interneuron migration indicates that the strength of interactions between genes in specific signaling pathways predominate over others94. But how these distinct signaling networks are recruited seamlessly to facilitate different stages of interneuron migration and epigenetic regulation of these mechanisms remain to be deciphered. Furthermore, how do interneurons sense and coordinate environmental guidance cues with intracellular signal transduction and cytoskeletal rearrangements necessary for oriental cellular movement? Signaling emanating from different cellular compartments (e.g., growth cone, cilium, cell soma etc.) may differentially affect the migratory behavior or decisions of interneurons. On a system wide basis, subtypes of interneurons appear to coordinate their communication with radial glial scaffold and projection neurons to achieve their final area and laminar fate. Elucidation of signaling network dynamics in developing interneurons will help us understand how these patterns of coordination are achieved. Lastly, developmental disruptions of interneurons and the resultant changes in excitatory/inhibitory balance of cortical circuits are thought to be an underlying cause of neurobehavioral disorders125. Thus it will be informative to examine (a) if susceptibility genes associated with interneuronal dysfunction in diseases such as schizophrenia, autism, and related neuropsychiatric disorders affect selective steps of interneuron migration? (b) the epigenetic deregulation of these interneuron related developmental pathways in neuropsychiatric disorders, and (c) how such perturbations affect the emergence of excitatory/inhibitory balance of cortical circuits? Answers to these questions will not only lead to a richer understanding of the process of interneuron migration, but will also help illuminate its relevance for normal cortical development and aberrant brain functions in neurodevelopmental disorders.
Highlights.
Process of migration regulates placement and differentiation of interneurons.
Interneuronal placement affects excitatory/inhibitory balance in cortex.
This review evaluates the decision making steps of cortical interneuron migration.
Acknowledgments
This research was supported by NIH grants MH060929 and MH094735 to E.S.A. We thank G.Wilkins, T-Y. Eom, and C. Plestant for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development (Cambridge, England) 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 2.Marín O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 3.Defelipe J, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature Reviews Neuroscience. 2013 doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen DV, et al. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nature neuroscience. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma T, et al. Subcortical origins of human and monkey neocortical interneurons. Nature neuroscience. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 6.Molnár Z, Butt SJB. Best-laid schemes for interneuron origin of mice and men. Nature neuroscience. 2013;16:1512–1514. doi: 10.1038/nn.3557. [DOI] [PubMed] [Google Scholar]
- 7.Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 8.Ang ESBC, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nature neuroscience. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 10.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development (Cambridge, England) 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 11.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nature Reviews Neuroscience. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 12.Gelman DM, Marín O. Generation of interneuron diversity in the mouse cerebral cortex. The European Journal of Neuroscience. 2010;31:2136–2141. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- 13.Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development (Cambridge, England) 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- 15.Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nature neuroscience. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 16.Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- 17.Lois C, García-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 18.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 19.Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martini FJ, et al. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development (Cambridge, England) 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- 21.Métin C, Vallee RB, Rakic P, Bhide PG. Modes and mishaps of neuronal migration in the mammalian brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11746–11752. doi: 10.1523/JNEUROSCI.3860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadarajah B, Alifragis P, Wong ROL, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nature neuroscience. 2002;5:218–224. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- 23.Bellion A, Baudoin JP, Alvarez C, Bornens M, Métin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka DH, et al. Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex. J Neurosci. 2009;29:1300–1311. doi: 10.1523/JNEUROSCI.5446-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokota Y, et al. Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS ONE. 2007;2:e794. doi: 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development (Cambridge, England) 2003;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development (Cambridge, England) 2006;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- 29.Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- 30.McConnell S, Kaznowski C. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 31.Pla R, Borrell V, Flames N, Marín O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. The Journal of neuroscience. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yozu M, Tabata H, Nakajima K. Birth-date dependent alignment of GABAergic neurons occurs in a different pattern from that of non-GABAergic neurons in the developing mouse visual cortex. Neurosci Res. 2004;49:395–403. doi: 10.1016/j.neures.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Métin C, Baudoin JP, Raki S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. The European Journal of Neuroscience. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- 35.Brown KN, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faux C, Rakic S, Andrews W, Britto JM. Neurons on the move: migration and lamination of cortical interneurons. Neurosignals. 2012;20:168–189. doi: 10.1159/000334489. [DOI] [PubMed] [Google Scholar]
- 37.Marín O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. The European Journal of Neuroscience. 2013;38:2019–2029. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- 38.Hamasaki T, Goto S, Nishikawa S, Ushio Y. Neuronal cell migration for the developmental formation of the mammalian striatum. Brain Res Brain Res Rev. 2003;41:1–12. doi: 10.1016/s0165-0173(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 39.Marín O, et al. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130:1889–1901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Li H-s, Zhou L, Wu J, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 41.Zimmer G, et al. Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. Eur J Neurosci. 2008;28:62–73. doi: 10.1111/j.1460-9568.2008.06320.x. [DOI] [PubMed] [Google Scholar]
- 42.Powell E, Mars W, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 43.Pozas E, Ibáñez C. GDNF and GFRα1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Huertas C, Rico B. CREB-dependent regulation of GAD65 transcription by BDNF/TrkB in cortical interneurons. Cerebral Cortex. 2011;21:777–788. doi: 10.1093/cercor/bhq150. [DOI] [PubMed] [Google Scholar]
- 45.Canty A, et al. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRα1 signaling. The Journal of neuroscience. 2009;29:10695–10705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker E, Polleux F, Lamantia AS. Position and time specify the migration of a pioneering population of olfactory bulb interneurons. Dev Biol. 2006;297:387–401. doi: 10.1016/j.ydbio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Briscoe J, et al. Homeobox gene Nkx2. 2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 48.Cobos I, Broccoli V, Rubenstein J. The vertebrate ortholog of Aristaless is regulated by Dlx genes in the developing forebrain. Journal of Comparative Neurology. 2005;483:292–303. doi: 10.1002/cne.20405. [DOI] [PubMed] [Google Scholar]
- 49.Flandin P, et al. Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors. Neuron. 2011;70:939–950. doi: 10.1016/j.neuron.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long JE, Cobos I, Potter GB, Rubenstein JLR. Dlx1&2 and Mash1 Transcription Factors Control MGE and CGE Patterning and Differentiation through Parallel and Overlapping Pathways. Cerebral Cortex (New York, NY) 19:i96. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flames N, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. The Journal of neuroscience. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nóbrega-Pereira S, et al. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chédotal A, Rijli FM. Transcriptional regulation of tangential neuronal migration in the developing forebrain. Curr Opin Neurobiol. 2009;19:139–145. doi: 10.1016/j.conb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. The Journal of Neuroscience. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wichterle H, Alvarez-Dolado M, Erskine L, Alvarez-Buylla A. Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:727–732. doi: 10.1073/pnas.242721899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmer G, et al. Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence-and preoptic area-derived interneurons in the deep and superficial migratory stream. The Journal of Neuroscience. 2011;31:18364–18380. doi: 10.1523/JNEUROSCI.4690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 58.van den Berghe V, et al. Directed migration of cortical interneurons depends on the cell-autonomous action of sip1. Neuron. 2013;77:70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 59.McKinsey GL, et al. Dlx1&2-Dependent Expression of Zfhx1b (Sip1, Zeb2) Regulates the Fate Switch between Cortical and Striatal Interneurons. Neuron. 2013;77:83–98. doi: 10.1016/j.neuron.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flames N, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 62.Cuzon V, Yeh P, Cheng Q, Yeh H. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cerebral Cortex. 2006;16:1377–1388. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- 63.Inada H, et al. GABA regulates the multidirectional tangential migration of GABAergic interneurons in living neonatal mice. PLoS ONE. 2011;6:e27048. doi: 10.1371/journal.pone.0027048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manent JB, Jorquera I, Ben-Ari Y, Aniksztejn L, Represa A. Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. The Journal of neuroscience. 2006;26:5901–5909. doi: 10.1523/JNEUROSCI.1033-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.López-Bendito G, et al. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- 67.Crandall JE, et al. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. Journal of Neurophysiology. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- 69.Cuzon Carlson VC, Yeh HH. GABAA receptor subunit profiles of tangentially migrating neurons derived from the medial ganglionic eminence. Cereb Cortex. 2011;21:1792–1802. doi: 10.1093/cercor/bhq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poluch S, et al. AMPA receptor activation leads to neurite retraction in tangentially migrating neurons in the intermediate zone of the embryonic rat neocortex. Journal of neuroscience research. 2001;63:35–44. doi: 10.1002/1097-4547(20010101)63:1<35::AID-JNR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Yozu M, Tabata H, Konig N, Nakajima K. Migratory behavior of presumptive interneurons is affected by AMPA receptor activation in slice cultures of embryonic mouse neocortex. Dev Neurosci. 2008;30:105–116. doi: 10.1159/000109856. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen L, et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 74.Avila A, et al. Glycine Receptor α2 Subunit Activation Promotes Cortical Interneuron Migration. Cell Rep. 2013;4:738–750. doi: 10.1016/j.celrep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferguson K, et al. A cell-autonomous requirement for the cell cycle regulatory protein, Rb, in neuronal migration. The EMBO Journal. 2005;24:4381–4391. doi: 10.1038/sj.emboj.7600887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stanco A, et al. Netrin-1–α3β1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proceedings of the National Academy of Sciences. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinozaki K, et al. Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential cell migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development (Cambridge, England) 2002;129:3479–3492. doi: 10.1242/dev.129.14.3479. [DOI] [PubMed] [Google Scholar]
- 78.Zarbalis K, Choe Y, Siegenthaler JA, Orosco LA, Pleasure SJ. Meningeal defects alter the tangential migration of cortical interneurons in Foxc1hith/hith mice. Neural Development. 2012;7:2. doi: 10.1186/1749-8104-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alcántara S, Pozas E, Ibañez C, Soriano E. BDNF-modulated spatial organization of Cajal–Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cerebral Cortex. 2006;16:487–499. doi: 10.1093/cercor/bhi128. [DOI] [PubMed] [Google Scholar]
- 80.Antypa M, Faux C, Eichele G, Parnavelas J, Andrews W. Differential gene expression in migratory streams of cortical interneurons. European Journal of Neuroscience. 2011;34:1584–1594. doi: 10.1111/j.1460-9568.2011.07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Västrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A-induced growth-cone collapse is mediated by Rac1 amino acids 17–32. Curr Biol. 1999;9:991–998. doi: 10.1016/s0960-9822(99)80447-3. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura F, Kalb RG, Strittmatter SM. Molecular basis of semaphorin-mediated axon guidance. J Neurobiol. 2000;44:219–229. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 83.Aizawa H, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nature neuroscience. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- 84.Liu B, Strittmatter S. Semaphorin-mediated axonal guidance via Rho-related G proteins. Current opinion in cell biology. 2001;13:619–626. doi: 10.1016/s0955-0674(00)00260-x. [DOI] [PubMed] [Google Scholar]
- 85.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Y, Gunput RAF, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Science Signaling. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uchida Y, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 89.Bai J, et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nature neuroscience. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- 90.Kappeler C, et al. Branching and nucleokinesis defects in migrating interneurons derived from doublecortin knockout mice. Human Molecular Genetics. 2006;15:1387–1400. doi: 10.1093/hmg/ddl062. [DOI] [PubMed] [Google Scholar]
- 91.Friocourt G, et al. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3875–3883. doi: 10.1523/JNEUROSCI.4530-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raki S, et al. Cortical interneurons require p35/Cdk5 for their migration and laminar organization. Cereb Cortex. 2009;19:1857–1869. doi: 10.1093/cercor/bhn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Colasante G, et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. The Journal of neuroscience. 2008;28:10674–10686. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Evsyukova I, Plestant C, Anton ES. Integrative Mechanisms of Oriented Neuronal Migration in the Developing Brain. Annu Rev Cell Dev Biol. 2013;29:299–353. doi: 10.1146/annurev-cellbio-101512-122400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohshima T, et al. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development (Cambridge, England) 2007;134:2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- 96.Nasrallah IM, McManus MF, Pancoast MM, Wynshaw-Boris A, Golden JA. Analysis of non-radial interneuron migration dynamics and its disruption in Lis1+/− mice. J Comp Neurol. 2006;496:847–858. doi: 10.1002/cne.20966. [DOI] [PubMed] [Google Scholar]
- 97.Cobos I, Borello U, Rubenstein J. Dlx Transcription Factors Promote Migration through Repression of Axon and Dendrite Growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Higginbotham H, et al. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Developmental Cell. 2012;23:925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baudoin JP, et al. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 2012;76:1108–1122. doi: 10.1016/j.neuron.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 100.López-Bendito G, et al. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tiveron MC, et al. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li G, et al. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sánchez-Alcañiz JA, et al. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Stumm R, et al. CXCR4 regulates interneuron migration in the developing neocortex. The Journal of neuroscience. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lysko DE, Putt M, Golden JA. SDF1 regulates leading process branching and speed of migrating interneurons. J Neurosci. 2011;31:1739–1745. doi: 10.1523/JNEUROSCI.3118-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caronia-Brown G, Grove EA. Timing of cortical interneuron migration is influenced by the cortical hem. Cereb Cortex. 2011;21:748–755. doi: 10.1093/cercor/bhq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanaka DH, et al. CXCR4 is required for proper regional and laminar distribution of cortical somatostatin-, calretinin-, and neuropeptide Y-expressing GABAergic interneurons. Cereb Cortex. 2010;20:2810–2817. doi: 10.1093/cercor/bhq027. [DOI] [PubMed] [Google Scholar]
- 108.Wang Y, et al. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villar-Cerviño V, et al. Contact repulsion controls the dispersion and final distribution of Cajal-Retzius cells. Neuron. 2013;77:457–471. doi: 10.1016/j.neuron.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Griveau A, et al. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bielle F, et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 112.Elias LAB, Turmaine M, Parnavelas JG, Kriegstein AR. Connexin 43 mediates the tangential to radial migratory switch in ventrally derived cortical interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7072–7077. doi: 10.1523/JNEUROSCI.5728-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miyoshi G, Butt SJB, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lodato S, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. The European Journal of Neuroscience. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- 118.Hammond V, et al. Layer positioning of late-born cortical interneurons is dependent on Reelin but not p35 signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1646–1655. doi: 10.1523/JNEUROSCI.3651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ciceri G, et al. Lineage-specific laminar organization of cortical GABAergic interneurons. Nature neuroscience. 2013;16:1199–1210. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- 120.De Marco García NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 122.McCabe AK, Chisholm SL, Picken-Bahrey HL, Moody WJ. The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. The Journal of Physiology. 2006;577:155–167. doi: 10.1113/jphysiol.2006.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bartolini G, Ciceri G, Marín O. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. NEURON. 2013;79:849–864. doi: 10.1016/j.neuron.2013.08.014. [DOI] [PubMed] [Google Scholar]