Abstract
Rationale: Transfusion of erythrocytes stored for prolonged periods is associated with increased mortality. Erythrocytes undergo hemolysis during storage and after transfusion. Plasma hemoglobin scavenges endogenous nitric oxide leading to systemic and pulmonary vasoconstriction.
Objectives: We hypothesized that transfusion of autologous blood stored for 40 days would increase the pulmonary artery pressure in volunteers with endothelial dysfunction (impaired endothelial production of nitric oxide). We also tested whether breathing nitric oxide before and during transfusion could prevent the increase of pulmonary artery pressure.
Methods: Fourteen obese adults with endothelial dysfunction were enrolled in a randomized crossover study of transfusing autologous, leukoreduced blood stored for either 3 or 40 days. Volunteers were transfused with 3-day blood, 40-day blood, and 40-day blood while breathing 80 ppm nitric oxide.
Measurements and Main Results: The age of volunteers was 41 ± 4 years (mean ± SEM), and their body mass index was 33.4 ± 1.3 kg/m2. Plasma hemoglobin concentrations increased after transfusion with 40-day and 40-day plus nitric oxide blood but not after transfusing 3-day blood. Mean pulmonary artery pressure, estimated by transthoracic echocardiography, increased after transfusing 40-day blood (18 ± 2 to 23 ± 2 mm Hg; P < 0.05) but did not change after transfusing 3-day blood (17 ± 2 to 18 ± 2 mm Hg; P = 0.5). Breathing nitric oxide decreased pulmonary artery pressure in volunteers transfused with 40-day blood (17 ± 2 to 12 ± 1 mm Hg; P < 0.05).
Conclusions: Transfusion of autologous leukoreduced blood stored for 40 days was associated with increased plasma hemoglobin levels and increased pulmonary artery pressure. Breathing nitric oxide prevents the increase of pulmonary artery pressure produced by transfusing stored blood.
Clinical trial registered with www.clinicaltrials.gov (NCT 01529502).
Keywords: hemoglobins, pulmonary hypertension, nitric oxide, endothelium, echocardiography
At a Glance Commentary
Scientific Knowledge on the Subject
Transfusion of stored red blood has been reported to increase pulmonary complications and the mortality rate of critically ill patients. During storage, red blood cells undergo progressive structural and functional changes reducing their oxygen transport capacity and viability, leading to hemolysis and the release of hemoglobin, heme, and iron into circulating plasma. In experimental studies, plasma hemoglobin causes vasoconstriction, inflammation, and thrombosis. By elucidating the mechanisms of injury caused by stored blood transfusion, novel therapies might be identified to prevent harm.
What This Study Adds to the Field
This study demonstrates an increased pulmonary artery pressure following transfusion of one unit of 40-day stored autologous blood in overweight subjects with endothelial dysfunction, which is prevented by breathing nitric oxide. This study did not find any evidence of systemic hemodynamic changes or systemic inflammation after stored blood transfusion.
Blood transfusion is a common medical intervention, with more than 14 million units of blood transfused annually in the United States (1). In developed countries, noninfectious hazards of transfusion, such as transfusion-related acute lung injury, have emerged as the leading complications of blood transfusion (2). In particular, the combination of transfusing red blood cells (RBC) stored for longer than 14 days and preexisting diseases, such as diabetes and atherosclerosis, in the recipient are associated with increased mortality (3–5). Conversely, other clinical studies have not reported adverse effects associated with transfusion of longer stored erythrocytes (6, 7).
The US Food and Drug Administration allows RBCs to be stored for up to 42 days (1). During storage, RBCs undergo progressive structural and functional changes that reduce oxygen-transport capacity and viability, leading to hemolysis and the release of microparticles, hemoglobin, heme, and iron into the circulation (8–10).
Nitric oxide (NO) is a potent, endogenous dilator of vascular smooth muscle. Circulating hemoglobin, both free in plasma and contained in shed microparticles, avidly scavenges NO via the dioxygenation reaction, depleting vascular NO levels and resulting in vasoconstriction (11). Because pulmonary vasoconstriction was produced in animal models by stored RBC transfusion and prevented by breathing NO (12), we previously tested whether breathing low levels of NO could selectively dilate the pulmonary circulation without producing systemic vasodilation or hypotension (13).
In awake mice with endothelial dysfunction, characterized by reduced vascular NO availability, transfusing cell-free hemoglobin produces marked systemic vasoconstriction and hypertension (14). In a subsequent study, Yu and coworkers (15) demonstrated that transfusing murine RBCs stored for 14 days induces systemic vasoconstriction and inflammation in diabetic mice with endothelial dysfunction and that breathing NO prevents the hypertension induced by transfusing stored RBCs. Breathing NO oxidizes plasma hemoglobin to methemoglobin, a form of hemoglobin that does not scavenge NO, blocking the vasoconstriction response (16). Hypertension and vasoconstriction were shown to be caused by transfusing the supernatant of stored murine RBCs, but transfusing washed 14-day stored RBCs did not cause vasoconstriction (15). In awake lambs, Baron and coworkers (12, 17) reported that autologous leukoreduced RBCs stored for 40 days induced pulmonary hypertension and inflammation, which was prevented by breathing NO.
In our previous study of healthy young volunteers (with intact endothelial function), transfusion of one unit of autologous, leukoreduced blood stored for 40 days neither changed systemic blood pressure nor impaired endothelial function, despite increasing plasma levels of cell-free hemoglobin. However, we did not assess pulmonary artery pressure (PAP) (10). In the present study, we selected overweight and obese volunteers with preexisting systemic endothelial dysfunction to assess the systemic and pulmonary vascular response to transfusion with stored autologous blood.
We hypothesized that transfusion of stored blood would increase pulmonary and/or systemic vasoconstriction by presenting a load of NO-scavenging hemoglobin in the presence of preexisting impaired endothelial function (18). We also tested the hypothesis that breathing NO, before and during transfusion, would prevent the vasoconstrictor effects of transfusing stored autologous blood.
Methods
Population and Study Protocol
The inclusion criteria to participate in this trial were age older than 18 and younger than 60 years; body mass index between 27 and 45 kg/m2; and evidence of impaired systemic endothelial function, as assessed by reduced postischemic vasodilation (natural log [Ln] of reactive hyperemia index [RHI] ≤0.75) (19).
The study protocol was a three-arm crossover study of autologous blood transfusion in volunteers with systemic endothelial dysfunction. Volunteers received in random order autologous leukoreduced blood stored between 2 and 3 days (3-d challenge), autologous leukoreduced blood stored for 38–42 days 40-d challenge), or autologous leukoreduced blood stored for 38–42 days while breathing NO (80 ppm; 40-d+NO challenge).
Venous blood was sampled before and 10 minutes, 1 hour, 4 hours, and 24 hours after completion of each blood transfusion to study plasma and serum biomarkers of hemolysis, inflammation, and organ injury.
Echocardiography and Hemodynamic Measurements
Transthoracic echocardiography was performed before blood transfusion and again 15 minutes before the end of the blood transfusion (see Figure E1 in the online supplement). In most of the volunteers, because a tricuspid regurgitation jet was not present for analysis, the pulmonary artery acceleration time (PAAT) was measured to estimate the mean PAP (20, 21). PAAT was defined as the interval between the onset of systolic pulmonary arterial flow and the peak flow velocity. PAAT was also normalized by the right ventricular ejection time (RVET; interval between the onset of right ventricular ejection and the time of systolic pulmonary arterial flow cessation). To estimate mean PAP, we used a validated formula (21). All measurements were performed by the study cardiologist and reviewed by a second cardiologist, both of whom were masked to the treatment.
Statistical Analysis
We anticipated enrolling a total of 14 subjects in this crossover-randomized study (42 blood challenges). Sample size calculation and statistical analyses were performed using STATA-12 software (StataCorp LP, College Station, TX) value as detailed in the online supplement. Continuous variables are expressed as mean ± SEM or median and interquartile range, as appropriate. Statistical significance was reached with P less than 0.05.
Additional details on the study protocol, NO delivery, estimation of systolic and mean PAP, and statistical methods can be found in the online supplement.
Results
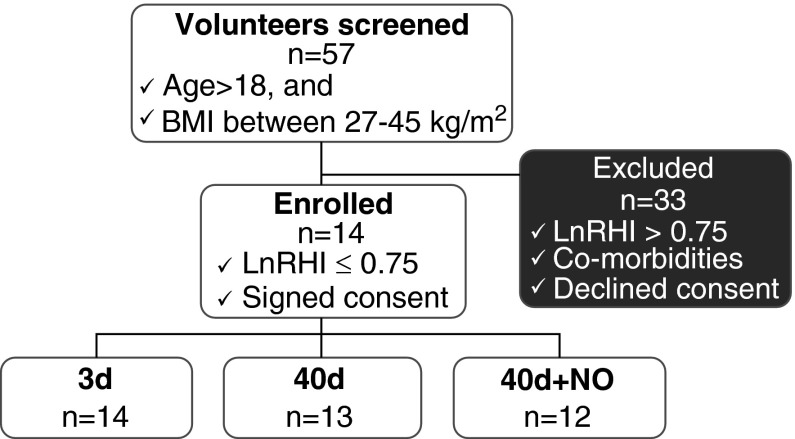
Fifty-seven volunteers were screened for a reduced RHI, and 14 were enrolled in the study (nine males and five females). Twelve volunteers completed the study, and two left the study before finishing all three phases (Figure 1). Demographic characteristics are shown in Table 1. Endothelial dysfunction was present in all participants, as reflected by a low RHI at the time of screening (LnRHI = 0.46 ± 0.03) (18), and baseline (before transfusion). Estimated mean PAP at baseline was 18 ± 1 mm Hg, and systolic PAP was 39 ± 2 mm Hg (Figure 2, Table 2).
Figure 1.
Study screening, enrollment, and blood challenges. BMI = body mass index; LnRHI = natural logarithm of reactive hyperemia index; NO = nitric oxide.
Table 1.
Baseline Characteristics
| Characteristics | n = 14 |
|---|---|
| Male, n (%) | 9 (64%) |
| Age, yr | 41 ± 4 |
| Race, n (%) | |
| White | 12 (86%) |
| African-American | 1 (7%) |
| Asian | 1 (7%) |
| Hispanic | 0 |
| ABO blood group, n (%) | |
| A | 3 (22%) |
| B | 2 (14%) |
| AB | 2 (14%) |
| O | 7 (50%) |
| BMI, kg/m2 | 33.4 ± 1.3 |
| LnRHI | 0.46 ± 0.03 |
Definition of abbreviations: BMI = body mass index; LnRHI = natural log of reactive hyperemia index.
Data are shown as mean ± SEM or count (%).
Figure 2.
Breathing 80 ppm nitric oxide (NO) prevents transfusion-related increase of pulmonary artery pressure (PAP). (A) Transfusion of 40-day stored blood decreased pulmonary artery acceleration time (PAAT) (P = 0.006, 40-d PAAT pretransfusion vs. PAAT during transfusion), whereas transfusion 40-day+NO stored blood increased PAAT (P = 0.004). (B) Mean PAP increased during 40-day challenge (P = 0.009) and decreased during 40-day+NO challenge (P = 0.005). Data presented as mean ± SEM. *P < 0.01, paired t test pretransfusion versus during-transfusion measurements.
Table 2.
Hemodynamic and Transthoracic Echocardiography Variables before and during Transfusion
| 3 Days (n = 9) |
40 Days (n = 11) |
40 Days+NO (n = 12) |
||||
|---|---|---|---|---|---|---|
| Pre | During | Pre | During | Pre | During | |
| PAAT, ms | 132 ± 9 | 129 ± 8 | 127 ± 8 | 110 ± 6* | 132 ± 7 | 154 ± 7* |
| PAAT/RVET | 0.40 ± 0.03 | 0.39 ± 0.02 | 0.40 ± 0.03 | 0.32 ± 0.02* | 0.39 ± 0.02 | 0.46 ± 0.02* |
| Mean PAP, mm Hg | 17 ± 2 | 18 ± 2 | 18 ± 2 | 23 ± 2* | 17 ± 2 | 12 ± 1* |
| Systolic PAP, mm Hg | 38 ± 3 | 39 ± 3 | 40 ± 3 | 46 ± 3* | 38 ± 3 | 31 ± 2* |
| CO, L/min | 5.3 ± 0.4 | 5.0 ± 0.3 | 4.5 ± 0.4 | 4.6 ± 0.3 | 4.5 ± 0.3 | 4.4 ± 0.2 |
| LVEF, % | 65 ± 1 | 68 ± 2 | 63 ± 1 | 63 ± 1 | 64 ± 2 | 65 ± 1 |
| SV, ml | 87 ± 8 | 82 ± 7 | 77 ± 8 | 78 ± 8 | 75 ± 6 | 76 ± 5 |
| LnRHI | 0.74 ± 0.09 | 0.87 ± 0.06 | 0.74 ± 0.07 | 0.90 ± 0.06 | 0.67 ± 0.07 | 0.78 ± 0.06 |
Definition of abbreviations: CO = cardiac output; LnRHI = natural log of reactive hyperemia index; LVEF = left ventricular ejection fraction; NO = nitric oxide; PAAT = pulmonary artery acceleration time; PAP = pulmonary artery pressure; RVET = right ventricular ejection time; SV = stroke volume.
Data are shown as mean ± SEM.
P < 0.01 paired t test values pretransfusion versus during transfusion.
Pulmonary Artery Pressure Is Increased during Transfusion of 40-Day Stored Blood, Unchanged after 3 Days, and Decreased during 40-Day+NO
Blood transfusion with 3-day challenge showed no systemic or pulmonary hemodynamic changes except for a transient decrease in heart rate following transfusion (Table 2; see Table E1).
Similarly, blood transfusion with 40-day challenge did not change systemic blood pressure, cardiac output, or RHI and only transiently decreased heart rate following transfusion. However, during transfusion, we measured a decreased PAAT and PAAT/RVET (P = 0.006 and P = 0.004, respectively) corresponding to an increase of mean and systolic PAP of 5 and 6 mm Hg, respectively (P = 0.009 and P = 0.006, respectively) (Figure 2, Table 2; see Table E1).
When volunteers breathed NO before and during transfusion of 40-day stored blood (40-d+NO challenge), the PAAT and PAAT/RVET increased (P = 0.004 and P = 0.009, respectively) and the mean PAP decreased by 5 mm Hg, and the systolic PAP decreased by 7 mm Hg (P = 0.005 and P = 0.004, respectively), whereas systemic vascular hemodynamic measurements were unchanged (Figure 2, Table 2; see Table E1).
When mean PAP values were compared, we found that during transfusion PAP tended to be greater in the 40-day challenge group as compared with PAP in the 3-day challenge group (40-d vs. 3-d; P = 0.054); also, PAP was lower in the 40-day+NO group compared with PAP in the 3-day group (40-d+NO vs. 3-d; P = 0.007); and, the PAP was markedly greater in the 40-day challenge group as compared with PAP in 40-day+NO (40-d vs. 40-d+NO; P = 0.001) (Figure 2, Table 2).
Transfusion of Blood Stored for 40 Days Is Associated with an Increased Plasma Hemoglobin Level, and Plasma NO consumption Is Increased Only after Transfusing 40-Day Stored Blood
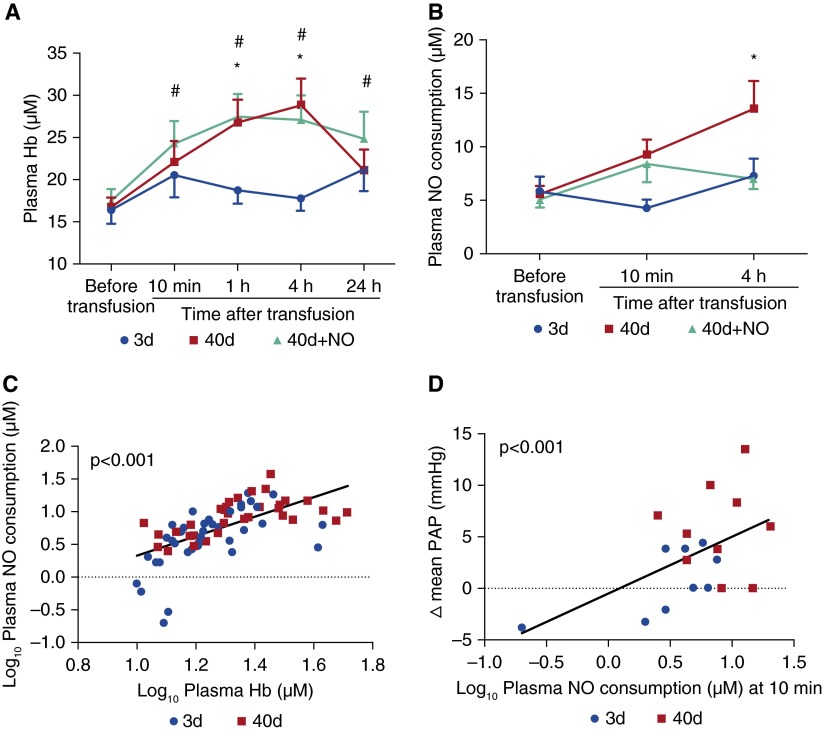
Transfusion with 3-day challenge did not change the levels of plasma hemoglobin, plasma NO consumption (Figure 3), serum levels of iron, and indirect bilirubin (see Figure E3), indicating the absence of hemolysis.
Figure 3.
Plasma levels of hemoglobin (Hb), plasma nitric oxide (NO) consumption, and changes of mean pulmonary artery pressure (PAP). (A) Plasma Hb increased after 40-day transfusion challenge (*P < 0.01 plasma Hb at 1-h and 4-h measurements compared with baseline [= before transfusion]), and after 40-day+NO challenge (#P < 0.01 plasma Hb at 10-min, 1-h, 4-h, 24-h measurements compared with baseline in 40-d challenge). No significant changes were found after challenge with 3-day transfusion. (B) Plasma NO consumption increased only at 4 hours after transfusion of 40-day stored blood (*P = 0.001). (C) Plasma Hb concentration at baseline, 10 minutes, and 4 hours after transfusion in volunteers receiving 3-day and 40-day challenges correlates with NO consumption (P < 0.001). (D) Plasma NO consumption at 10 minutes after 3-day and 40-day challenges correlates with ∆ mean PAP (mm Hg) (P < 0.001). Data are presented as mean ± SEM. In A and B, a two-way analysis of variance for repeated measures with post hoc Bonferroni correction. In C and D, measurements of plasma Hb and NO consumption were transformed into a log10 scale.
Plasma hemoglobin levels almost doubled between 1 and 4 hours after transfusion with 40-day challenge (plasma hemoglobin at 4 h differs from plasma hemoglobin before transfusion; P = 0.001) (Figures 3A and 3B). Plasma NO consumption increased at 4 hours after transfusion (P < 0.001) (Figure 3B). Hemolysis associated with transfusion of 40-day challenge was confirmed by increased serum levels of iron and indirect bilirubin, which peaked at 4 hours (see Figures E3A and E3B).
After transfusion with 40-day+NO challenge, plasma hemoglobin levels increased between 1 and 4 hours (at 4 h after initiation of transfusion vs. before transfusion; P < 0.001). Serum iron levels and indirect bilirubin increased similarly between 1 and 4 hours (Figure 3A; see Figures E3A and E3B). However, in volunteers breathing NO, there was no increase in plasma NO consumption (Figure 3B).
Because breathing NO oxidizes plasma hemoglobin to methemoglobin, thereby reducing the scavenging of NO, we measured the values of plasma NO consumption in the 3-day and 40-day transfusion challenges and correlated them with circulating levels of plasma hemoglobin (P < 0.001, correlation between log10 plasma hemoglobin levels vs. log10 plasma NO consumption) (Figure 3C). Moreover, plasma NO consumption at 10 minutes after transfusion in the 3-day and 40-day challenge groups was associated with the change (∆) in mean PAP during blood transfusion from baseline (P < 0.001, correlation between log10 plasma NO consumption levels at 10 minutes vs. ∆ mean PAP) (Figure 3D).
As expected, in the 40-day+NO challenge, there was no correlation between plasma NO consumption and plasma hemoglobin levels or between plasma NO consumption at 10 minutes and PAP (data not shown).
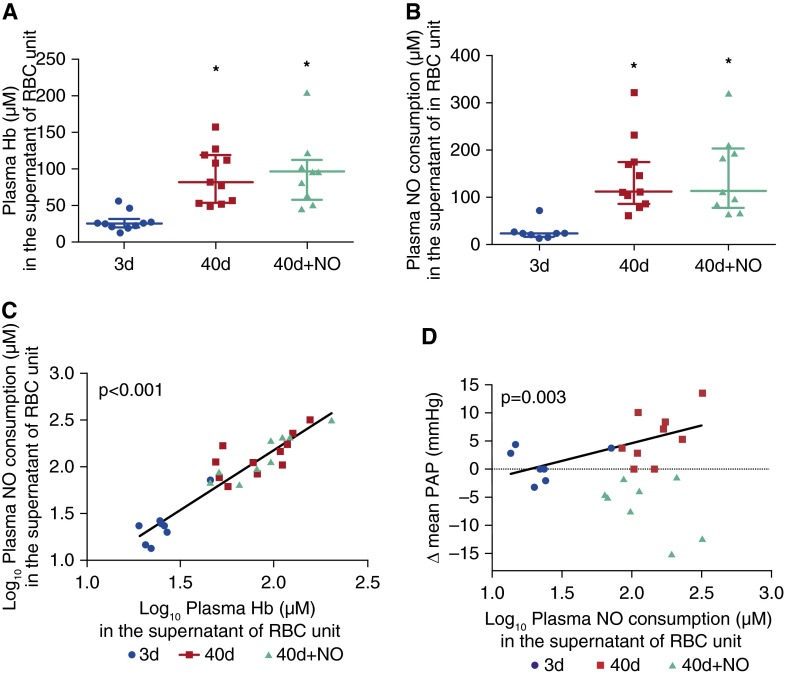
Supernatant Levels of Hemoglobin in the PRBC Units Are Increased with Storage Time and Correlate Closely with Supernatant NO Consumption
Supernatant hemoglobin and NO consumption levels in the RBC units of the 40-day and 40-day+NO challenges were approximately four times higher than those in the 3-day RBC units (Figures 4A and 4B). NO consumption in the supernatant of the RBCs units of the 3-day, 40-day, and 40-day+NO challenges closely correlated with supernatant levels of hemoglobin (P < 0.001, correlation between log10 supernatant hemoglobin vs. log10 supernatant NO consumption) (Figure 4C). The ∆ mean PAP in the 3-day and 40-day challenge groups correlated with the levels of NO consumption in the supernatant of the transfused RBC unit (P = 0.003, correlation of the log supernatant NO consumption vs. ∆ mean PAP) (Figure 4D). In the 40-day+NO challenge group the ∆ mean PAP did not correlate with the level of NO consumption in the supernatant of the transfused RBC units.
Figure 4.
Plasma hemoglobin (Hb) and plasma nitric oxide (NO) consumption in the supernatant of red blood cell (RBC) unit, and changes of mean pulmonary artery pressure (PAP). (A) Supernatant Hb levels contained in the 40-day and 40-day+NO stored blood units are higher than those in the supernatant of the 3-day stored units (*P < 0.01). (B) Supernatant NO consumption is higher in the 40-day and 40-day+NO stored units as compared with the NO consumption of the 3-day stored units (*P < 0.01). (C) Supernatant NO consumption correlates with supernatant Hb levels from all RBC units (P < 0.001). (D) Supernatant NO consumption correlates with change of mean PAP after 3-day and 40-day challenges (P = 0.003). Despite high NO consumption levels of the supernatant of the 40-day+NO stored units, breathing NO decreases mean PAP. Data presented as median and interquartile range. In A and B, the P value was calculated with a Kruskal-Wallis rank test. In C and D, measurements of Hb in the supernatant and NO consumption were transformed to a log10 scale. Note that there are three fewer points in D compared with Figure 3D: two samples from the 3-day stored blood units and one sample from the 40-day stored unit were not obtained.
Endothelial Dysfunction Is Associated with Low Plasma Levels of NO Metabolites; Breathing NO Immediately Increases Plasma Nitrate Levels but Not Plasma Nitrite Levels
At baseline (before transfusion), endothelial dysfunction in our overweight-obese volunteers was characterized by a reduced RHI (decreased availability of endothelial NO) (22) and confirmed by extremely low plasma levels of nitrite and nitroso compounds compared with prior reports in the literature (see Table E3) (10, 23, 24). Other NO metabolites were comparable with the levels that we previously reported in young healthy human volunteers (see Table E3) (10).
Levels of NO metabolites in plasma and erythrocytes showed similar trends after transfusion with 3-day and 40-day challenges (see Figure E4). There was no significant decrease in plasma and erythrocyte nitrate levels after transfusion with 3-day and 40-day challenges. Plasma nitrite levels increased slightly, whereas erythrocyte nitrite levels halved immediately following transfusion, and there was no change in the plasma or erythrocyte levels of nitroso compounds after transfusion with either 3-day or 40-day stored RBCs.
After transfusion with 40-day+NO stored blood, nitrate levels in plasma and erythrocytes increased immediately after inhalation of NO and returned to baseline levels at 24 hours (see Figures E4A–E4D). Plasma levels of nitrite increased only after 24 hours in the 40-day+NO challenge group (see Figure E4). Concentrations of other NO metabolites (RXNO, NOx) in plasma and erythrocytes after NO breathing did not significantly differ from the values after 3-day or 40-day transfusions (without NO breathing).
Discussion
We investigated the effects of transfusing stored autologous RBCs on systemic and pulmonary hemodynamics and systemic endothelial function in obese adult volunteers with endothelial dysfunction. The principal finding of our study was that autotransfusion of one unit of leukoreduced blood stored for 40 days increased the PAP, as estimated by transthoracic echocardiography. Inhalation of 80 ppm NO prevented the increase in PAP induced by transfusing 40-day stored blood. A second important finding was the absence of systemic hypertension, inflammation, or organ injury after transfusion of 40-day stored blood in our volunteers with preexisting systemic endothelial dysfunction. We previously reported that diabetic mice with endothelial dysfunction were sensitized to systemic vasoconstriction after the transfusion of stored syngeneic blood (15). We also noted that both diabetic and high-fat diet-fed mice developed an enhanced systemic inflammatory response after stored blood transfusion (15, 25). Thus, we were surprised that our obese volunteers with a markedly reduced RHI did not show any systemic vasoconstriction or inflammation after stored 40-day blood transfusion. Similar findings of an absence of inflammation have been reported after transfusion of stored blood into healthy (nonobese) volunteers (10, 26) and intensive care unit patients (27).
We measured the PAAT by transthoracic echocardiography and calculated an estimated mean and systolic PAP (20, 21). Our volunteers with endothelial dysfunction exhibited an increased PAP at baseline (before transfusion). These observations are in agreement with those of McQuillan and coworkers (27), who reported that patients with a higher body mass index had an increased systolic PAP. It is possible that the combination of obesity and systemic endothelial dysfunction placed our volunteers at higher risk for pulmonary vasoconstriction when challenged with a stored blood transfusion. Because transfusion with blood stored for 40 days did not change the cardiac output, the increase of PAP that we measured is most likely explained by an increase of pulmonary vascular resistance caused by pulmonary vasoconstriction. Without inserting a pulmonary artery catheter, we are unable to determine whether transfusion altered the pulmonary capillary wedge pressure. However, because no change in PAP was measured following transfusion with blood stored for 3 days, it is unlikely that the wedge pressure was changed by transfusing one unit of 40-day RBCs.
Transfusion of blood stored for prolonged periods into either lambs or dogs produces acute pulmonary hypertension (12, 28–30). Baron and coworkers (12) developed a model of autologous blood transfusion in the awake lamb and reported that top-load transfusion of one unit of blood stored for 40 days induced transient pulmonary vasoconstriction and an increased PAP. Treating lambs with a low dose of L-NAME to mimic endothelial dysfunction markedly augmented the pulmonary vasoconstriction following transfusion with stored blood (12). In a subsequent study of lambs resuscitated with autologous stored blood after hemorrhagic shock, Baron and colleagues (17) reported enhanced pulmonary vasoconstriction and hypertension. Moreover, Solomon and coworkers (28) described persistent pulmonary hypertension in a septic canine model subjected to an exchange transfusion with stored canine donor blood. In elegant studies, Stevens and coworkers showed that the relevant hydrostatic pressure for alveolar edema formation is the transcapillary pressure, which depending on the model may or may not correlate with PAP (31, 32). In Solomon’s canine study, the increase of pulmonary hypertension after stored blood transfusion was associated with more severe lung damage than the fresh blood transfused control, evidenced by increased necrosis, hemorrhage, and thrombosis (28). Similarly, Baron and coworkers (17) reported that two of six animals transfused with stored blood developed severe pulmonary edema. Thus, any connection between the increased PAP and lung edema, necrosis, hemorrhage, and thrombosis especially in patients with acute lung injury receiving stored blood should be closely examined.
To determine the degree of depletion of NO in plasma after blood transfusion and the benefits of breathing NO, we measured NO consumption in both stored RBC supernatant and the recipient’s plasma after transfusion. During storage, we found that the NO consumption of the supernatant was markedly increased, as others have reported (9, 17). Supernatant NO consumption closely correlated with the level of plasma hemoglobin in the supernatant. The increase of PAP during transfusion correlated with both the level of NO consumption of the supernatant in the transfused RBC unit and with the level of NO consumption in the transfusion recipient’s plasma measured 10 minutes after completing the transfusion. It is likely that the increase of PAP during transfusion of blood stored for 40 days is caused by the transfusion of hemoglobin contained in the blood storage unit and by hemolysis of circulating transfused erythrocytes. The peak level of plasma hemoglobin in our volunteers occurred between 1 and 4 hours after the transfusion, consistent with ongoing hemolysis after transfusion.
Breathing NO is an efficacious and safe method to selectively reduce pulmonary hypertension. Minneci and coworkers (16) demonstrated that inhaled NO reversed the pulmonary hypertension produced by hemoglobin infusion in dogs. Many thousands of patients with acute pulmonary hypertension have been successfully treated with inhaled NO (13, 33). In children with pulmonary endothelial dysfunction proved by a lack of response to acetylcholine infusion, and pulmonary hypertension following extracorporeal bypass, Wessel and coworkers (34) showed that PAP was normalized by breathing NO. In our study, breathing NO before and during transfusion abolished the acute pulmonary pressure increase induced by transfusing blood stored for 40 days in volunteers with endothelial dysfunction. Thus, we believe that inhaled NO prevented or reversed any vasoconstriction caused by NO scavenging by hemoglobin. We reviewed other possible sources for the increase of PAP with stored blood transfusion, such as reduced red cell deformability (35), and presumably increased viscosity. There is limited evidence that NO exposure can reduce red cell deformity (36). If reactive oxygen species are elevated by iron released by stored blood and help produce vasoconstriction, it is possible that inhaled NO could scavenge oxygen free radicals (37). These results suggest that patients with endothelial dysfunction that receive stored blood transfusions could be treated with inhaled NO to prevent an increased pulmonary pressure.
Finally, to further investigate the response to inhaled NO after transfusion of stored blood in volunteers with endothelial dysfunction, we measured NO metabolites in plasma and erythrocytes. We report that baseline levels of nitrite were three times lower than the levels we measured in healthy young volunteers without endothelial dysfunction (10). Unlike our previous study in healthy volunteers, volunteers with endothelial dysfunction showed no change of NO metabolite levels after transfusion of either 3-day or 40-day stored blood challenges. In particular, plasma nitrite levels, despite a slight increase immediately after transfusion of 3-day or 40-day blood, remained at extremely low levels when compared with healthy individuals. It is likely that the diseased endothelium of our obese volunteers was incapable of increasing NO synthesis after transfusing free hemoglobin. This is in contrast to our previous study of healthy volunteers where circulating nitrite levels were increased after stored blood transfusion. Breathing NO produced an immediate increase of erythrocytic and plasma levels of nitrate but not nitrite. Thus, it is unlikely that breathing NO produced its effects via nitrite release.
Our study has several limitations. First, this is an exploratory study conducted with only 14 human volunteers. Larger clinical trials of stored blood transfusion may eventually clarify whether an increase in PAP manifests after multiple transfusions with stored blood and if this translates to increased adverse pulmonary and cardiac events. Second, we did not explore the effects of breathing NO in the absence of a transfusion in overweight volunteers with systemic endothelial dysfunction, we also did not study the effects of a volume challenge (saline or albumin) on systemic and pulmonary hemodynamics, and we did not investigate the effects of breathing NO during transfusion of 3-day stored blood. These additional control groups need to be examined in future studies. Third, without continuous monitoring of PAP by a pulmonary catheter, we do not know if the peak of in vivo hemolysis at 1–4 hours correlates with an increased PAP. Future studies should investigate whether in vivo hemolysis causes persistent pulmonary vasoconstriction. Lastly, we did not evaluate other possible noxious effects of stored blood transfusion, such as iron and heme toxicity, microparticles, protein oxidation, or lipid peroxidation of the RBC membrane.
In conclusion, in our randomized crossover trial of autologous leukoreduced stored blood transfusion in overweight and obese adult volunteers with systemic endothelial dysfunction, we found that transfusion of leukoreduced erythrocytes stored for 40 days was associated with an increased PAP, most likely caused by NO depletion by plasma hemoglobin. Selective pulmonary vasodilation induced by breathing 80 ppm NO prevented the increase of PAP after the transfusion of 40-day stored red cells. This study demonstrates an increase in PAP following transfusion of 40-day stored blood and provides a safe and practical pharmacologic intervention to avoid the increase of PAP. Clinical trials of stored blood transfusion should prospectively search for pulmonary hypertension and any adverse effect of augmenting pulmonary edema, especially in critically ill patients with endothelial dysfunction and acute lung injury.
Acknowledgments
Acknowledgment
The authors thank Hui Zheng, Ph.D., Biostatistics Center, Massachusetts General Hospital, and Rajeev Malhotra, M.D., Cardiology Division, Department of Medicine, Massachusetts General Hospital, for statistical support. Drs. Zheng and Malhotra had access to the primary data and served as coanalysts for the investigators.
Footnotes
Supported by the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital. Inhaled nitric oxide was supplied by iNO Therapeutics, Ikaria (Hampton, NJ). L.B. was supported by National Institutes of Health (NIH) grant Research Training for Anesthetists T32GM007592. K.D.B. was supported by NIH R01 grant HL074352 and a grant from the Foundation LeDucq. M.F. acknowledges support from the Faculty of Medicine, University of Southampton. E.A.H. has received grant support from NIH K08-HL103756. M.S.-C. was supported by NIH R21 DK092909.
Author Contributions: L.B., R.P., C.M., and W.M.Z. made substantial contributions to the conception and design of the work and the acquisition, analysis, interpretation of data for the work. L.B. and W.M.Z. drafted the work, revising it critically for important intellectual content. R.P., B.Y., and C.M. revised it critically for important intellectual content. C.P.S., L.W., B.Y., B.O.F., M.F., E.A.H., D.C., M.S.-C., and K.D.B. made substantial contributions to the conception or design of the work; interpretation of data for the work; and revised it critically for important intellectual content. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201405-0850OC on August 27, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.The US Department of Health and Human Services 2011National Blood Collection and Utilization Survey Report[accessed 2014 Sept 17]Available from: http://www.hhs.gov/ash/bloodsafety/nbcus/
- 2.Bolton-Maggs P, Thomas DW, Cohen H.editors. SHOT Annual Report 2012[accessed 2014 Sept 17]Available from: http://www.shotuk.org/home/
- 3.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 4.Pettilä V, Westbrook AJ, Nichol AD, Bailey MJ, Wood EM, Syres G, Phillips LE, Street A, French C, Murray L, et al. Blood Observational Study Investigators for ANZICS Clinical Trials Group. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15:R116. doi: 10.1186/cc10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middelburg RA, Borkent-Raven BA, Janssen MP, van de Watering LMG, Wiersum-Osselton JC, Schipperus MR, Beckers EAM, Briët E, van der Bom JG. Storage time of blood products and transfusion-related acute lung injury. Transfusion. 2012;52:658–667. doi: 10.1111/j.1537-2995.2011.03352.x. [DOI] [PubMed] [Google Scholar]
- 7.Lelubre C, Vincent J-L. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care. 2013;17:R66. doi: 10.1186/cc12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berra L, Coppadoro A, Yu B, Lei C, Spagnolli E, Steinbicker AU, Bloch KD, Lin T, Sammy FY, Warren HS, et al. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117:56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 12.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloch KD, Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007;75:339–348. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012;52:1410–1422. doi: 10.1111/j.1537-2995.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron DM, Beloiartsev A, Nakagawa A, Martyn T, Stowell CP, Malhotra R, Mayeur C, Bloch KD, Zapol WM. Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs. Crit Care Med. 2013;41:2492–2501. doi: 10.1097/CCM.0b013e31828cf456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yared K, Noseworthy P, Weyman AE, McCabe E, Picard MH, Baggish AL. Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J Am Soc Echocardiogr. 2011;24:687–692. doi: 10.1016/j.echo.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 22.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 23.Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, Kelm M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006;47:573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- 24.Cannon RO, III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology. 2012;117:1190–1202. doi: 10.1097/ALN.0b013e318272d866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds JD, Bennett KM, Cina AJ, Diesen DL, Henderson MB, Matto F, Plante A, Williamson RA, Zandinejad K, Demchenko IT, et al. S-nitrosylation therapy to improve oxygen delivery of banked blood. Proc Natl Acad Sci USA. 2013;110:11529–11534. doi: 10.1073/pnas.1306489110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortés-Puch I, Wang D, Sun J, Solomon SB, Remy KE, Fernandez M, Feng J, Kanias T, Bellavia L, Sinchar D, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–1411. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochoa CD, Stevens T. Studies on the cell biology of interendothelial cell gaps. Am J Physiol Lung Cell Mol Physiol. 2012;302:L275–L286. doi: 10.1152/ajplung.00215.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chetham PM, Babál P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol. 1999;276:L41–L50. doi: 10.1152/ajplung.1999.276.1.L41. [DOI] [PubMed] [Google Scholar]
- 33.Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 34.Wessel DL, Adatia I, Giglia TM, Thompson JE, Kulik TJ. Use of inhaled nitric oxide and acetylcholine in the evaluation of pulmonary hypertension and endothelial function after cardiopulmonary bypass. Circulation. 1993;88:2128–2138. doi: 10.1161/01.cir.88.5.2128. [DOI] [PubMed] [Google Scholar]
- 35.Frank SM, Abazyan B, Ono M, Hogue CW, Cohen DB, Berkowitz DE, Ness PM, Barodka VM. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;116:975–981. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol. 2003;284:H1577–H1584. doi: 10.1152/ajpheart.00665.2002. [DOI] [PubMed] [Google Scholar]
- 37.Kiefmann R, Rifkind JM, Nagababu E, Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood. 2008;111:5205–5214. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]