To the Editor:
Mechanical ventilation (MV) causes diaphragm inactivity and unloading, and this quiescence results in ventilator-induced diaphragm dysfunction (VIDD). VIDD is characterized by oxidative stress, muscle atrophy, and decreases in diaphragm fiber force, as documented in both animal models and humans (1). VIDD is a major clinical problem because the ensuing decreased ability to develop inspiratory pressure will contribute to the difficulties in weaning patients from MV. During open-chest cardiothoracic surgery in humans, a rapid form of VIDD has been observed, with 20–30% depression of diaphragm force occurring within 2 hours of commencing MV (2). Extensive experiments establishing a cause-and-effect relationship for the mechanisms leading to VIDD in animals are available (reviewed in Reference 3), but interventions in humans are scant.
Recently, intermittent phrenic nerve stimulation during MV has emerged as a potential VIDD countermeasure (4, 5). Specifically, hemidiaphragm stimulation during MV resulted in greater type 2 muscle fiber area in sheep (4), improved contractile properties in rats (6), and higher mitochondrial respiration rates in humans undergoing cardiothoracic surgery (5). In this preliminary study, we examined whether intermittent phrenic nerve stimulation during cardiothoracic surgery prevents diaphragm fiber contractile function impairment.
We obtained diaphragm biopsies from four patients undergoing sternotomy for elective cardiothoracic surgical procedures (Table 1). The University of Florida Institutional Review Board approved the protocol, and participants gave written consent. Patients received nondepolarizing neuromuscular blockers during intubation, but none during surgery. The experimental intervention consisted of unilateral of phrenic nerve stimulation using an external cardiac pacemaker (Medtronic 5388; Medtronic, Minneapolis, MN) with temporary wire electrodes (AE Medical Corp., Farmingdale, NJ). The stimulation parameters and protocol were reported previously (5) and are given in Table 1. Briefly, stimulations were conducted every 30 minutes as soon as the phrenic nerve and diaphragm were exposed. The contralateral hemidiaphragm served as intrasubject control. There were no complications from the stimulations or biopsies. All participants were successfully extubated on the first attempt.
Table 1.
Patient Characteristics and Stimulation Parameters
| Characteristic | Parameter |
|---|---|
| Sex | 1 female/3 male |
| Age, yr | 57 ± 10 |
| Height, cm | 176 ± 9 |
| Mass, kg | 95 ± 13 |
| Body mass index | 30 ± 3 |
| Smoker | 2+, 2− |
| Smoking history, pack-years* | 30 ± 52 |
| FVC, % predicted | 54 ± 19 |
| FEV1, % predicted | 54 ± 12 |
| Maximal inspiratory pressure, cm H2O | −62 ± 3 |
| Stimulation current, mA | 21 ± 4.0 |
| Total stimulations (train duration, 1 min; frequency, 0.5 Hz, 1.5-ms pulses) | 5.7 ± 2.1 |
| Esophageal temperature during stimulation, °C | 36 ± 1 |
| Intubation to first stimulation, min | 112 ± 34 |
| Intubation to biopsy, min | 281 ± 64 |
| Last stimulation bout to biopsy, min | 29 ± 1.5 |
| Intubation to extubation time, h | 25 ± 23 |
Data are mean ± standard deviation. Patients were undergoing elective cardiothoracic surgical procedures including aortic valve replacement, coronary artery bypass, aortic aneurysm repairs, or a combination of these procedures.
Smokers only.
Muscle biopsies from each hemidiaphragm were either frozen immediately and later prepared for protein immunoblotting (see online supplement) or placed in an ice-cold solution containing low calcium and high ATP concentration (“relaxing” solution) (7), processed for chemical permeabilization (7), and stored at −20°C in relaxing solution (50% glycerol) until measurements of contractile properties (≤3 wk of surgery). We carefully isolated single fibers from diaphragm bundles in ice-cold relaxing solution and clamped the fibers to a force transducer and length controller for mechanical testing at 15°C, using calcium activation (7). We discarded fibers with sarcomere length greater than 2.65 μm under slack conditions on final mounting on the mechanics apparatus. This was the only exclusion criterion we adopted to avoid selectively studying “healthy” fibers. In our hands, slack sarcomere length greater than 2.65 μm is a sign of damage imposed during isolation of the fiber. We studied fibers from each hemidiaphragm (stimulated or control) in all subjects and determined fiber types using myosin heavy chain gel electrophoresis (8). Incidentally, the vast majority of fibers were type 2 (38 fibers); only four fibers were type 1 (three control, one stimulated). Hence, we report here only findings for type 2 fibers. These fibers account for approximately half of the diaphragm fiber composition (2).
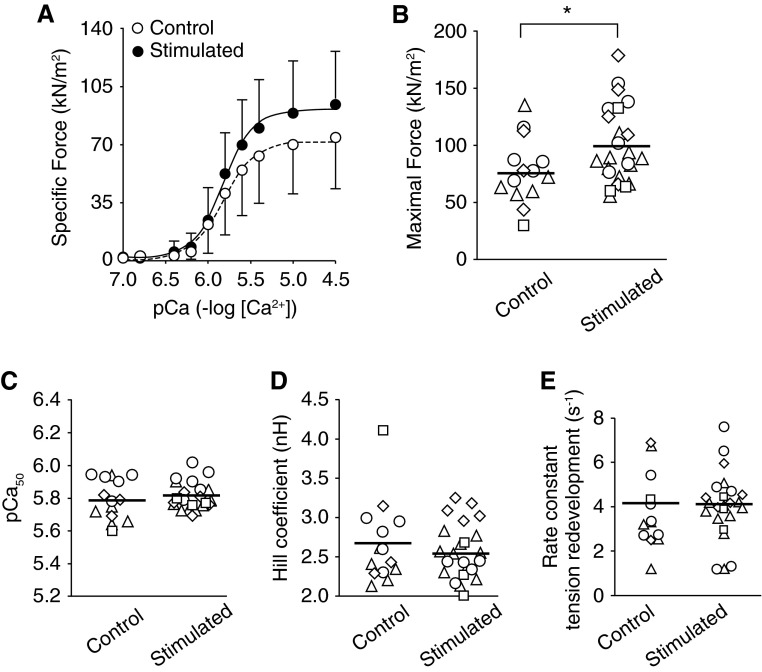
We analyzed the data from four patients with a varying number of fibers in each of the stimulated and control muscles, using a mixed effects model with random intercept for each individual and a fixed effect for group (stimulated or control), using the parametric bootstrap to assess statistical significance, as described by Faraway (9). This statistical approach takes into consideration the within-subject design in which different numbers of fibers are analyzed per subject in each condition. Specific force averaged 77 kN/m2 in control/inactive fibers compared with 100–150 kN/m2 reported in healthy active type 2 fibers [rodents (7), humans (2)]. Stimulated fibers elicited an approximately 30% greater specific force compared with the inactive fibers (P = 0.028; Figure 1). We found no differences between stimulated and control hemidiaphragms in other measures of isometric contractile properties (Figure 1). We also measured the abundance of protein carbonyls and ubiquitin conjugates (n = 3 patients), which, respectively, are general markers of oxidative damage and targeting for degradation by the ubiquitin-proteasome system. There was no difference between stimulated and control hemidiaphragms for protein carbonyls (control, 8.5 ± 2.8; stimulated, 9.2 ± 3.1) or ubiquitin conjugates (control, 2.3 ± 1.1; stimulated, 2.5 ± 1.0; Figures E1–E2 in the online supplement).
Figure 1.
Phrenic nerve stimulation increases diaphragm fiber force after cardiothoracic surgery. (A) Specific force versus pCa relationship for all fibers from control and stimulated hemidiaphragms. Specific force is absolute force (in kilonewtons) normalized to the fiber cross-sectional area (in meters squared). pCa is the negative log[Ca2+], and in the figure shown, [Ca2+] ranges from 0.1 to 32 μM. Symbols and error bars are mean ± standard deviation. Solid and dashed lines are best fit from Hill equation. (B) Maximal calcium-activated (pCa 4.5) specific force. (C) pCa50 is pCa that elicits 50% maximal force and represents calcium sensitivity of the contractile apparatus. (D) Hill coefficient is equivalent to the slope of force–pCa relationship and represents the cooperativity of thin-filament activation. (E) Rate constant of tension redevelopment (ktr) obtained from quick-release and restretch test. The ktr is an indicator of cross-bridge cycling kinetics. (B–E) Data are for each individual fiber studied (symbols) and mean for condition (solid line). The four unique symbols shown represent fibers from each patient. *P < 0.05 determined by linear mixed model as detailed in the text.
Several mechanisms are involved in VIDD, including activation of proteolytic pathways, oxidative stress, impaired excitation–contraction coupling, and posttranslational modification of sarcomeric proteins. Our preliminary findings suggest that intermittent phrenic nerve stimulation preserves human diaphragm fiber contractile function, possibly by inhibiting proteolysis and/or posttranslational modification of sarcomeric proteins seen after MV (3, 10). However, the protection does not appear to be mediated by changes in protein carbonyls or ubiquitination. The resolution of specific cellular and molecular mechanisms will require a larger number of subjects and different analysis techniques. It is possible that indirect stretch imposed onto the control hemidiaphragm by stimulation of the treated side conferred some protection against VIDD (11). Therefore, our data may underestimate the benefits of intermittent phrenic nerve stimulation to diaphragm fiber function. However, our intrasubject design was sufficiently sensitive to detect significant differences in function that may be obscured by the large variability in an intersubject design resulting from factors such as age, sex, genetics, duration of surgery, and so on.
We cannot determine whether a 30% increase in diaphragm fiber force is clinically relevant. Of note, an increase in inspiratory/diaphragm muscle strength elicited by inspiratory strength training is associated with greater weaning rate than standard care in chronic failure-to-wean patients (12). Phrenic nerve stimulation has been shown to increase the type 2 fiber cross-sectional area in sheep (4), mitochondrial respiration rates (5), and specific force in rats (6) and humans, as shown for the first time to our knowledge in the present study. These observations suggest that intermittent diaphragm activity during MV offers some protection against VIDD. Thus, intermittent diaphragm activity may ultimately prove to be a useful clinical strategy to prevent or attenuate VIDD in a subset of patients that has yet to be identified in larger trials.
Footnotes
L.F.F. was supported by National Institutes of Health grant HL098453. A.D.M. and C.L. are supported by National Institutes of Health grant GM111152.
Author Contributions: Tissue collection and processing: B.A., S.A., T.M., T.B., B.K.S., P.H., and A.D.M. Study design and intervention: A.D.M., T.M., T.B., P.H., S.A., C.L., and B.K.S. Patient inclusion and study coordination: A.D.M., T.M., T.B., B.K.S., and P.H. Experimental design and data analysis: A.D.M., T.B., B.A.B., and L.F.F. Data collection and interpretation: B.A., and L.F.F. Writing and critical revisions: B.A., A.D.M., B.K.S., C.L., L.F.F., and T.B.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 2.Welvaart WN, Paul MA, Stienen GJ, van Hees HW, Loer SA, Bouwman R, Niessen H, de Man FS, Witt CC, Granzier H, et al. Selective diaphragm muscle weakness after contractile inactivity during thoracic surgery. Ann Surg. 2011;254:1044–1049. doi: 10.1097/SLA.0b013e318232e75b. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol. 2013;305:R464–R477. doi: 10.1152/ajpregu.00231.2013. [DOI] [PubMed] [Google Scholar]
- 4.Masmoudi H, Coirault C, Demoule A, Mayaux J, Beuvin M, Romero N, Assouad J, Similowski T. Can phrenic stimulation protect the diaphragm from mechanical ventilation-induced damage? Eur Respir J. 2013;42:280–283. doi: 10.1183/09031936.00045613. [DOI] [PubMed] [Google Scholar]
- 5.Martin AD, Joseph AM, Beaver TM, Smith BK, Martin TD, Berg K, Hess PJ, Deoghare HV, Leeuwenburgh C. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med. 2014;42:e152–e156. doi: 10.1097/CCM.0b013e3182a63fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Wang H, Han G, Chen L, Huang L, Jiang J, Li S. Phrenic nerve stimulation protects against mechanical ventilation-induced diaphragm dysfunction in rats. Muscle Nerve. 2013;48:958–962. doi: 10.1002/mus.23850. [DOI] [PubMed] [Google Scholar]
- 7.Roberts BM, Ahn B, Smuder AJ, Al-Rajhi M, Gill LC, Beharry AW, Powers SK, Fuller DD, Ferreira LF, Judge AR. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J. 2013;27:2600–2610. doi: 10.1096/fj.12-222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tikunov BA, Sweeney HL, Rome LC. Quantitative electrophoretic analysis of myosin heavy chains in single muscle fibers. J Appl Physiol (1985) 2001;90:1927–1935. doi: 10.1152/jappl.2001.90.5.1927. [DOI] [PubMed] [Google Scholar]
- 9.Faraway JJ.Extending the linear model with R: generalized linear, mixed effects, and nonparametric regression models. Boca Raton, FL: Chapman & Hall/CRC; 2006 [Google Scholar]
- 10.van Hees HW, Schellekens WJ, Andrade Acuña GL, Linkels M, Hafmans T, Ottenheijm CA, Granzier HL, Scheffer GJ, van der Hoeven JG, Dekhuijzen PN, et al. Titin and diaphragm dysfunction in mechanically ventilated rats. Intensive Care Med. 2012;38:702–709. doi: 10.1007/s00134-012-2504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooijman PE, Ottenheijm CA. Passive stretch of the diaphragm following unilateral phrenic nerve stimulation. Eur Respir J. 2014;43:1533–1534. doi: 10.1183/09031936.00161713. [DOI] [PubMed] [Google Scholar]
- 12.Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, Layon AJ, Banner MJ, Caruso LJ, Deoghare H, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]