Abstract
Rationale: Apnea of prematurity is a common condition that is usually treated with caffeine, an adenosine receptor blocker that has powerful influences on the central nervous system. However, little is known about the long-term effects of caffeine on sleep in the developing brain.
Objectives: We hypothesized that neonatal caffeine use resulted in long-term abnormalities in sleep architecture and breathing during sleep.
Methods: A total of 201 ex-preterm children aged 5–12 years who participated as neonates in a double-blind, randomized, controlled clinical trial of caffeine versus placebo underwent actigraphy, polysomnography, and parental sleep questionnaires. Coprimary outcomes were total sleep time on actigraphy and apnea–hypopnea index on polysomnography.
Measurements and Main Results: There were no significant differences in primary outcomes between the caffeine group and the placebo (adjusted mean difference of −6.7 [95% confidence interval (CI) = −15.3 to 2.0 min]; P = 0.13 for actigraphic total sleep time; and adjusted rate ratio [caffeine/placebo] for apnea–hypopnea index of 0.89 [95% CI = 0.55–1.43]; P = 0.63). Polysomnographic total recording time and total sleep time were longer in the caffeine group, but there was no difference in sleep efficiency between groups. The percentage of children with obstructive sleep apnea (8.2% of caffeine group versus 11.0% of placebo; P = 0.22) or elevated periodic limb movements of sleep (17.5% in caffeine group versus 11% in placebo group) was high, but did not differ significantly between groups.
Conclusions: Therapeutic neonatal caffeine administration has no long-term effects on sleep duration or sleep apnea during childhood. Ex-preterm infants, regardless of caffeine status, are at risk for obstructive sleep apnea and periodic limb movements in later childhood.
Keywords: methylxanthines, apnea, periodic limb movements during sleep
At a Glance Commentary
Scientific Knowledge on the Subject
Therapeutic caffeine administration for apnea of prematurity has been shown to have beneficial short- and long-term effects on survival and neurodevelopmental disabilities. Animal studies have suggested that neonatal caffeine has long-term detrimental effects on sleep and control of breathing, but corresponding human studies have not been performed.
What This Study Adds to the Field
This study shows that therapeutic neonatal caffeine administration has no long-term effects on sleep quality or quantity, or on pathologic conditions during sleep. This finding should allay concerns about the long-term effects of the drug on sleep. In addition, this study demonstrates a higher-than-expected prevalence of obstructive sleep apnea syndrome and periodic limb movements during sleep in ex-preterm infants.
Apnea of prematurity is common, occurring in more than three-quarters of infants born before 30 weeks of gestation (1). It is typically treated with methylxanthines, primarily caffeine. Indeed, a study of 220 hospitals showed that caffeine was the most commonly used drug in infants under 32 weeks of gestation (2). The Caffeine for Apnea of Prematurity (CAP) trial randomized 2,006 preterm infants to neonatal caffeine therapy or placebo and showed beneficial results, with caffeine resulting in lower rates of bronchopulmonary dysplasia and severe retinopathy of prematurity before discharge, and reduced risks of cerebral palsy and cognitive delay at 18 months compared with placebo (3, 4); by 5 years of age, benefits on cognitive development were attenuated, but there were still benefits on motor development (5, 6). However, little is known about the long-term effects of caffeine, which is an adenosine receptor blocker, on sleep regulation in the developing brain. In particular, it is not known whether caffeine has long-term adverse effects on sleep architecture and ventilatory control, which could result in an increased prevalence of sleep disorders later in childhood, such as insomnia or obstructive sleep apnea syndrome (OSAS).
Although the acute sleep-suppressing effects of caffeine administration are well known in daily life and from research (7), studies have not evaluated the effects of chronic caffeine use on sleep. Studies across the age spectrum have shown that methylxanthines result in decreased sleep time and fragmented sleep (8, 9), and can also lead to decreased rapid eye movement/active sleep in neonates (10). This may have important clinical consequences, as sleep, and in particular rapid eye movement sleep, is thought to be important for memory and learning (11). Methylxanthines are also known to modulate ventilatory control, which is a determinant of upper airway collapsibility (12). Animal studies have shown that neonatal caffeine administration can chronically affect ventilation during adulthood, resulting in increased baseline ventilation and a decreased ventilatory response to hypercapnia (13). Children who were born prematurely have an elevated risk of developing OSAS (14), but it is not known whether this is due, at least in part, to the effect of neonatal caffeine exposure on ventilatory control.
We hypothesized that caffeine therapy for apnea of prematurity, although beneficial in the short term, would result in long-term abnormalities in sleep patterns and in breathing during sleep. We therefore obtained both subjective and objective measures of sleep in a cohort of children who had been randomized to masked caffeine or placebo as neonates in the CAP trial.
Some of these data were presented in abstract form at the American Thoracic Society, May 2014, and the SLEEP 2014 conference, June 2014 (15).
Methods
See the online supplement for additional details.
This was a prospective follow-up study of the CAP trial. A subsample of four CAP sites was selected based on recruitment/retention rates and geographic clustering. The research ethics board at Children's Hospital of Philadelphia and each clinical site approved the study, and written informed consent was obtained from parents/guardians. Children underwent 2 weeks of actigraphy and 1 night of comprehensive ambulatory home polysomnography; data were scored and interpreted centrally. Caregivers completed sleep diaries and questionnaires. Investigators and subjects/families remained blinded as to whether the subject had received caffeine or placebo as a neonate. The coprimary outcomes were average total sleep time based on multiple nights of actigraphy, and the obstructive apnea–hypopnea index (AHI) from polysomnography.
Children were studied at age 5–12 years. Subjects were born prematurely, weighed 500–1,250 g at birth, did not have major congenital anomalies or syndromes, were considered by their clinicians to be candidates for methylxanthine therapy during the first 10 days of life, and were randomized to caffeine or placebo for a median of 6 weeks during the neonatal period (3). The study sample included six (3%) children with moderate to severe cerebral palsy or developmental delays.
Actigraphy was performed for 2 weeks (Actiwatch 2; Philips Respironics, Bend, OR). Actigraphy is a validated technique for measuring sleep versus wakefulness based on movement, using a wristwatch-like device, and was used primarily to assess sleep quality and quantity during normal daily life (16). The actigraph was worn on the nondominant wrist. The Actiwatch 2 has been validated compared with in-laboratory polysomnography in this age group (17). However, further validation was performed by an epoch-by-epoch comparison of actigraphy and simultaneous ambulatory polysomnography in a random sample of 20 subjects.
Subjects underwent 1 night of unattended, ambulatory polysomnography. The primary purpose of polysomnography was to detect sleep-related medical conditions, such as OSAS, rather than sleep quality, as sleep quality may be affected by instrumentation. Details of the polysomnography technique and quality have been published previously (18). In brief, technologists went to the home to apply and remove monitoring leads at the child’s usual bed/wake times. Electroencephalograms, electro-oculograms, submental and tibial electromyograms, chest and abdominal wall movement, electrocardiogram, airflow (nasal pressure and oronasal thermistor), and arterial oxygen saturation with pulse waveform were monitored. Studies were scored using standard pediatric rules (19).
The caregiver completed the National Sleep Foundation 2004 Sleep In America questionnaire (20), the Pediatric Sleep Questionnaire Sleep Related Breathing Disorder Scale, and a restless legs syndrome questionnaire (21).
Statistical Analysis
AHI was compared between groups using a Poisson regression model incorporating adjustment for prespecified covariates of center, age, sex, race, and maternal education (22). Treatment effect and confidence interval (CI) were expressed as rate ratios. Average total sleep time was compared between groups with a weighted (inverse variance) regression model (23) with adjustment for the above covariates, as well as weekend nights and season. Data were compared between groups using Student’s t tests (or equivalent nonparametric tests) for quantitative data and Chi-squared tests for categorical data. P values less than 0.05 were considered significant. The target sample size of 200 was anticipated to yield 90% power for a 2.5% decrease in actigraphic mean sleep time (0.21 h, which was considered clinically meaningful) and 80% power to detect a mean difference in AHI of 1.5 events/h.
Results
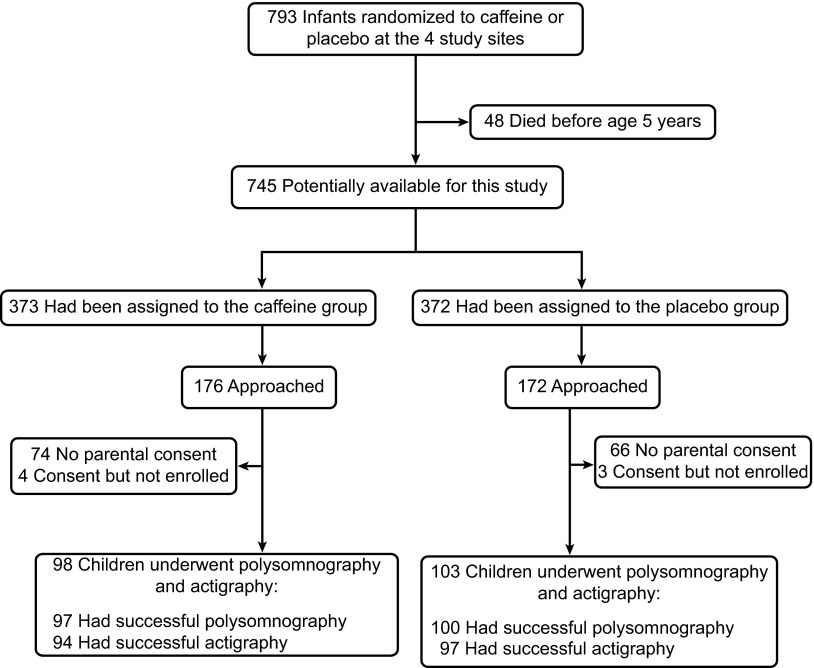
Study enrollment is shown in Figure 1; 201 subjects were recruited. The only demographic difference between those who enrolled versus those who declined was a higher maternal education level in those who enrolled (P = 0.028).
Figure 1.
Study enrollment. Details of study enrollment are shown. Planned enrollment was 200 subjects. Surviving children from the four participating Caffeine for Apnea of Prematurity (CAP) trial centers were eligible if they lived within an approximate 2-hour radius of the participating sleep center. Eligible families were consecutively approached, but recruitment was terminated when 201 subjects were recruited.
Subject characteristics are shown in Table 1. Reflective of the original CAP population (3), this was primarily a white population with a high level of maternal education.
Table 1.
Study Group
| Characteristic | Caffeine (n = 98) | Placebo (n = 103) | P Value |
|---|---|---|---|
| Age, yr | 9.1 ± 1.9 | 9.3 ± 2.1 | 0.58 |
| Males | 58 (59.1) | 57 (55.3) | 0.68 |
| Maternal race | |||
| White | 80 (81.6) | 88 (85.4) | 0.47 |
| Asian | 11 (11.2) | 9 (8.7) | |
| Black | 5 (5.1) | 6 (5.8) | |
| Other | 2 (2.0) | 0 (0.0) | |
| Site | |||
| Canada | 51 (52.0) | 62 (60.2) | 0.24 |
| Australia | 47 (48.0) | 41 (39.8) | |
| Gestational age, wk | 27.2 ± 1.6 | 27.3 ± 1.7 | 0.94 |
| Birth weight, g | 987 ± 164 | 953 ± 174 | 0.16 |
| BMI z-score* | −0.12 ± 1.33 | −0.17 ± 1.11 | 0.75 |
| Weight class* | |||
| Obese (BMI > 95th percentile) | 11 (11.2) | 4 (3.4) | 0.12 |
| BMI 5th–95th percentile | 76 (77.6) | 86 (84.3) | |
| Failure to thrive (BMI < 5th percentile) | 11 (11.2) | 12 (11.8) | |
| Time between study and anthropometric measurements, yr | 0.79 ± 1.87 | 1.18 ± 1.76 | 0.14 |
| Maternal education | |||
| Did not finish high school or equivalent | 18 (18.4) | 12 (11.7) | 0.29 |
| Completed high school or equivalent | 31 (31.6) | 41 (39.8) | |
| Attended college/university | 49 (50.0) | 50 (48.5) |
Definition of abbreviation: BMI = body mass index.
Data shown as mean ± SD or n (%).
n = 200.
Based on caregiver report, current caffeine intake of the children was low, with 26.0% of the caffeine group and 23.5% of the placebo group reporting any use (P = 0.74). In both groups, average intake was one caffeine-containing drink per day for those with any exposure to caffeine.
Actigraphy
Actigraphy was initially unsatisfactory in 26 (13%) of 201 subjects, and was repeated successfully in 16. For logistic reasons, actigraphy was not repeated in 10 subjects. Thus, there were 191 (95%) successful actigraphy studies (Figure 1). The actigraph was worn for 13.2 (±2.1) days in the caffeine group and 13.4 (±2.0) days in the placebo group (P = 0.60). The pilot study of actigraphy compared with polysomnography showed good sensitivity to detect sleep (0.87), but weak specificity to detect wake after sleep onset (0.48), similar to that reported in the literature (16). Actigraphy was less specific in children with periodic limb movements during sleep (PLMS); in participants with a periodic limb movement index greater than 5/h, wake after sleep onset was similar to those with an index of 5/h or less during polysomnography (50.1 ± 22.4 [SD] versus 45.4 ± 26.0 min, respectively; P = 0.46), but was significantly greater on actigraphy (113.4 ± 85.9 versus 83.2 ± 38.2 min; P = 0.010), indicating that movement from the PLMS (probably due to trunk or arm movements) resulted in false-positive wake time on actigraphy.
Summary actigraphy data (excluding the polysomnography night) are shown in Table 2. There was no significant difference between groups in the coprimary outcome of actigraphic total sleep time. There were no statistically significant differences in any of the secondary actigraphic outcomes, including sleep onset latency and sleep efficiency.
Table 2.
Actigraphy Results
| Outcome | Weighted Mean (SD) |
Unadjusted Difference* |
Adjusted for Covariates† |
|||
|---|---|---|---|---|---|---|
| Caffeine (n = 94) | Placebo (n = 97) | Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| Primary outcome | ||||||
| Total sleep time (duration of sleep in sleep period), min | 488.3 (3.7) | 493.3 (3.4) | −5.1 (−15.0 to 4.9) | 0.32 | −6.7 (−15.3 to 2.0) | 0.13 |
| Secondary outcomes | ||||||
| Bedtime (clock time)§ | 21:30 (5.4) | 21:32 (4.8) | −0.02 (−0.26 to 0.22) | 0.88 | −0.05 (−0.27 to 0.17) | 0.65 |
| Time in bed (from reported bedtime to wake time), min | 614.3 (4.0) | 614.3 (3.8) | −0.1 (−10.9 to 10.8) | 0.99 | −2.1 (−11.3 to 7.2) | 0.66 |
| Sleep period (from sleep onset to sleep offset), min | 564.3 (4.1) | 566.0 (3.9) | −1.7 (−12.8 to 9.4) | 0.76 | −4.4 (−12.8 to 4.0) | 0.30 |
| Sleep efficiency (total sleep time/time in bed), % | 79.7 (0.5) | 80.5 (0.5) | −0.8 (−2.2 to 0.6) | 0.26 | −0.8 (−2.2 to 0.6) | 0.26 |
| Sleep onset latency, min | 27.4 (1.6) | 26.6 (1.6) | 0.7 (−4.0 to 5.5) | 0.76 | 1.1 (−3.6 to 5.7) | 0.65 |
| Wake after sleep onset, min | 76.0 (2.4) | 72.7 (2.3) | 3.3 (−3.1 to 9.8) | 0.31 | 2.3 (−3.9 to 8.4) | 0.48 |
| Average activity count during sleep period | 16.8 (0.7) | 16.6 (1.1) | 0.2 (−2.3 to 2.7) | 0.88 | −0.1 (−2.7 to 2.5) | 0.93 |
Definition of abbreviation: CI = confidence interval.
Caffeine–placebo.
Covariates: center, age at assessment, sex, race, maternal education, proportion of weekend nights, season.
SD provided in minutes. Data obtained from sleep diary.
Polysomnography
Successful home polysomnography was obtained in 197 (98%) subjects (Figure 1) (18). Results are shown in Table 3. Polysomnographic total recording time and total sleep time were both longer in the caffeine group compared with placebo, but there was no difference in sleep efficiency or sleep architecture between the groups.
Table 3.
Polysomnography Results
| Variable | Unadjusted Results |
Adjusted for Covariates* |
||||
|---|---|---|---|---|---|---|
| Caffeine Mean (SD), Median (IQR), or n (%) (n = 97) | Placebo Mean (SD), Median (IQR), or n (%) (n = 100) | Mean Difference†, Rate Ratio‡, or Odds Ratio§ (95% CI) | P Value | Mean Difference†, Rate Ratio‡, or Odds Ratio§ (95% CI) | P Value | |
| Primary outcome | ||||||
| Obstructive apnea–hypopnea index, n/h | 0.3 (0.1 to 0.6) | 0.3 (0.1 to 0.8) | 0.95‡ (0.58 to 1.53) | 0.82 | 0.89‡ (0.55 to 1.43) | 0.63 |
| Secondary outcomes | ||||||
| Sleep architecture | ||||||
| Lights off (evening; clock time)|| | 21:23 (51) | 21:27 (62) | −4† (−21 to 12) | 0.60 | −4† (−19 to 10) | 0.59 |
| Lights on (morning; clock time)|| | 7:24 (44) | 7:09 (51) | 15† (1 to 28) | 0.034 | 12† (−1 to 26) | 0.077 |
| Total recording time, min¶ | 600.7 (52.7) | 579.6 (66.9) | 21.1† (3.9 to 38.3) | 0.017 | 19.2† (3.3 to 35.2) | 0.019 |
| TST, min¶ | 551.0 (52.7) | 531.9 (63.5) | 19.2† (2.5 to 35.8) | 0.024 | 18.3† (3.0 to 33.5) | 0.020 |
| Sleep efficiency, %¶ | 91.8 (4.9) | 91.8 (4.4) | −0.1† (−1.4 to 1.3) | 0.94 | 0.1† (−1.3 to 1.4) | 0.91 |
| Sleep latency, min¶ | 16.2 (18.6) | 17.7 (18.4) | −1.5† (−6.8 to 3.8) | 0.57 | −2.1† (−7.4 to 3.1) | 0.43 |
| Wake after sleep onset, min¶ | 33.4 (23.0) | 29.9 (20.4) | 3.5† (−2.7 to 9.7) | 0.27 | 3.2† (−3.2 to 9.7) | 0.33 |
| Stage N1, % TST¶ | 5.2 (2.1) | 4.7 (1.8) | 0.5† (−0.1 to 1.0) | 0.10 | 0.4† (−0.2 to 1.0) | 0.18 |
| Stage N2, % TST¶ | 38.2 (7.1) | 38.9 (6.8) | −0.7† (−2.7 to 1.3) | 0.51 | −0.4† (−2.5 to 1.6) | 0.69 |
| Stage N3, % TST¶ | 33.0 (6.4) | 33.9 (7.1) | −0.9† (−2.8 to 1.0) | 0.36 | −1.0† (−3.0 to 1.0) | 0.33 |
| Rapid eye movement sleep, % TST¶ | 23.6 (4.9) | 22.5 (4.5) | 1.1† (−0.2 to 2.5) | 0.11 | 1.0† (−0.1 to 2.4) | 0.14 |
| Arousal index, n/h | 9.1 (7.7 to 10.4) | 9.4 (7.6 to 10.6) | 1.02‡ (0.95 to 1.10) | 0.57 | 1.03‡ (0.95 to 1.11) | 0.47 |
| Ventilation | ||||||
| Subjects with obstructive apnea–hypopnea index ≥ 2/h | 8 (8.2%) | 11 (11.0%) | 0.73§ (0.28 to 1.89) | 0.51 | 0.51§ (0.18 to 1.48) | 0.22 |
| Subjects with obstructive apnea–hypopnea index ≥ 2/h and/or history of adenoidectomy/tonsillectomy | 23 (23.7%) | 29 (29.0%) | 0.76§ (0.40 to 1.44) | 0.40 | 0.73§ (0.38 to 1.42) | 0.35 |
| Central apnea index, n/h | 0.3 (0.1 to 0.6) | 0.3 (0.1 to 0.7) | 1.20‡ (0.91 to 1.59) | 0.19 | 1.16‡ (0.88 to 1.55) | 0.29 |
| Periodic breathing, % TST | 0.2 (0.5) | 0.1 (0.3) | 0.06† (−0.05 to 0.17) | 0.30 | 0.06† (−0.05 to 0.18) | 0.29 |
| SpO2 nadir, % | 91.8 (2.4) | 91.3 (3.5) | 0.5† (−0.3 to 1.4) | 0.21 | 0.6† (−0.3 to 1.4) | 0.19 |
| Time with SpO2 < 90%, % TST | 0.0 (0.1) | 0.3 (1.7) | −0.23† (−0.57 to 0.11) | 0.18 | −0.16† (−0.51 to 0.19) | 0.37 |
| Limb movements | ||||||
| Periodic limb movement index, n/h | 0.7 (0.0 to 7.4) | 0.6 (0.0 to 1.8) | 1.56‡ (1.01 to 2.39) | 0.043 | 1.47‡ (0.98 to 2.21) | 0.066 |
| Subjects with periodic limb movement index > 5/h | 17 (17.5%) | 11 (11.0%) | 1.72§ (0.76 to 3.89) | 0.19 | 1.62§ (0.69 to 3.85) | 0.27 |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; SpO2 = oxygen saturation as measured by pulse oximetry; TST = total sleep time.
Covariates: center, age at assessment, sex, race, maternal education.
Quantitative data shown as caffeine − placebo mean difference, estimated by a multiple linear regression model.
Indices are shown as rate ratios, caffeine/placebo, estimated by a Poisson regression model.
Dichotomized data shown as odds ratios, caffeine/placebo, estimated by a logistic regression model.
SD provided in minutes.
N = 95 for caffeine and 96 for placebo as data from several subjects with short total recording times due to power or child-related issues were excluded from sleep architecture analyses.
The coprimary outcome of the AHI did not differ significantly between groups (Table 3). A large proportion of children in both groups had OSAS (defined as an AHI ≥ 2/h [24]): 8.2% of the caffeine group and 11.0% of the placebo group (total of 9.6%). The highest AHI was 47.6/h. Furthermore, 24% of the caffeine group and 29% of the placebo group had either an elevated AHI and/or a history of adenoidectomy/tonsillectomy, the usual treatment for childhood OSAS (25). However, the proportion of children with OSAS did not differ between groups. There was no significant relationship between AHI and obesity (P = 0.28 with the categorical variable, and P = 0.59 for body mass index–Z score). Central apneas were rare, with the highest central apnea index being 3.3/h.
PLMS were common. The PLMS index was higher in the caffeine group than the placebo group, but this difference was no longer significant when corrected for covariates (Table 3). The proportion of subjects with a PLMS index in the pathologic range (>5/h [26]) was high (17.5% in the caffeine group versus 11% in the placebo group; 14% overall; Table 3), but did not differ significantly between groups. Seven (3.5%) children had a PLMS index in the severe range (>15/h), with the highest index being 35/h.
Questionnaires
The National Sleep Foundation Questionnaire data corroborated the actigraphy results. Most children in both groups usually went to bed between 20:00 and 20:59 and awoke between 7:00 and 7:29.
Although questionnaire data did not show significant differences in usual nocturnal (579 ± 99 min versus 563 ± 91 min; P = 0.22) or nap (29 ± 114 min versus 27 ± 186 min; P = 0.93) sleep time between caffeine and control subjects, respectively, caregivers in the caffeine group thought that their child needed more sleep than control caregivers (610 ± 73 min versus 584 ± 64 min; P = 0.007).
A total of 21.1% of caregivers in the caffeine group versus 19.0% in the placebo group (P = 0.73) answered “yes” to the broad question “Do you think your child has a sleep problem?” The mean Pediatric Sleep Questionnaire scores were in the normal range, and did not differ between groups (Table 4). There was no difference in the restless legs syndrome score between groups (Table 4).
Table 4.
Questionnaire Results
| Variable | Unadjusted Results |
Adjusted for Covariates* |
||||
|---|---|---|---|---|---|---|
| Caffeine Mean (SD) | Placebo Mean (SD) | Mean Difference† (95% CI) | P Value | Mean Difference† (95% CI) | P Value | |
| Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder Scale | ||||||
| n | 94 | 99 | ||||
| Result | 0.23 (0.19) | 0.27 (0.20) | −0.03† (−0.09 to 0.02) | 0.23 | −0.04† (−0.10 to 0.02) | 0.15 |
| Restless Legs Syndrome Questionnaire | ||||||
| n‡ | 87 | 82 | ||||
| Result | 3.7 (4.0) | 4.5 (5.0) | −0.8† (−2.2 to 0.5) | 0.23 | −1.0† (−2.3 to 0.4) | 0.17 |
Definition of abbreviation: CI = confidence interval.
Covariates: center, age at assessment, sex, race, and maternal education.
Quantitative data shown as caffeine − placebo mean difference, estimated by a multiple linear regression model.
The Restless Legs Syndrome Questionnaire was added later in the study, resulting in a lower n.
Discussion
This randomized, controlled, double-blind study of ex-preterm infants found no long-term effect of neonatal caffeine therapy on objective or subjective sleep measures at school age, but did show that ex-preterm infants had a high prevalence of OSAS and PLMS compared with the general population (27, 28).
Caffeine is the standard treatment for apnea of prematurity, and is used widely in neonatal care. Nevertheless, despite the marked effect of caffeine upon sleep regulation, the long-term effects of therapeutic caffeine administration on sleep and ventilatory control in ex-preterm infants have not been studied. Caffeine blocks adenosine, a sleep-promoting agent (29). In rodents, caffeine administration during early life results in permanent changes in adenosine receptor function during adulthood (30). Rats who received neonatal caffeine have sleep disruption as adults, including increased sleep latency, decreased sleep time, and sleep fragmentation (13). Furthermore, long-term animal studies have shown that rats who received methylxanthines during early life had increased resting ventilation and blunted hypercapnic ventilatory responses during adulthood (13). Thus, animal studies suggest that neonatal caffeine administration can lead to permanent abnormalities in sleep regulation and ventilatory control. However, corresponding human studies had not previously been performed.
In the current study, there was no difference in sleep quality or quantity on actigraphy between children exposed to caffeine and those exposed to placebo. Given the tightness of the 95% CIs, it is highly unlikely that this study failed to detect a true clinically important adverse effect of caffeine on sleep. Actigraphy and questionnaires showed bedtimes and total time in bed similar to those reported in the literature for school-aged children (20). Consistent with the literature, there was a discrepancy between the actigraphic and polysomnographic measures of sleep architecture (16, 17). Although time in bed (actigraphy) and total recording time (polysomnography) were similar, actigraphy demonstrated a lower sleep efficiency, longer sleep latency, and increased wake time after sleep onset. This demonstrates a known limitation of actigraphy use in school-aged children. Young children move frequently during sleep, and, therefore, actigraphy is sensitive at detecting sleep, but has decreased specificity in discriminating between sleep and wakefulness (16, 17); the specificity was most likely lowered further by the high prevalence of PLMS. Nevertheless, the actigraphy data supported the questionnaire data showing no difference in actual sleep time between caffeine and placebo groups.
Polysomnography showed a longer total recording time and longer total sleep time in the caffeine group compared with the placebo group, although sleep efficiency was the same. The reason for this longer sleep time, even when controlled for center, is unclear. Polysomnographic recordings were started and ended at the child’s usual bedtime and wake time, and questionnaires and actigraphy (which are better indicators of habitual sleep times) showed no significant difference in bedtimes, wake times, or total sleep time between groups, although caregivers of the caffeine group did note that they thought their children needed more sleep. Furthermore, polysomnography recorded sleep duration during a single night, whereas actigraphy recorded sleep duration for 2 weeks. Thus, the estimate of sleep duration obtained with actigraphy is bound to be more accurate and representative of the children’s actual sleep duration. Thus, it is likely that these finding were due to chance. There were no other differences in polysomnographic parameters between groups, and no difference in the coprimary outcome of AHI. The observed standard deviation of AHI was somewhat larger than the value used to determine sample size. Post hoc power calculations indicate that the study had 80% power to detect a true mean AHI difference of 2.8 events/h.
Of interest, however, was the high prevalence of both OSAS and periodic limb movements in both groups of children. The prevalence of OSAS in the school-aged population is estimated at 1–4% (27). In contrast, polysomnography demonstrated OSAS in 9.6% (95% CI = 5.9–14.7%) of children in the current study. This may underestimate the incidence of OSAS, as many children received prior treatment with adenotonsillectomy. Adenotonsillectomy is the primary treatment for childhood OSAS (25); 26% of children in this study had either an elevated AHI or a prior history of adenotonsillectomy. Although the indications for adenotonsillectomy in these subjects were not available, in general, the commonest indication for adenotonsillectomy is obstructed breathing (31), with 59–69% of tonsillectomies in the United States being performed for obstruction (32). Thus, these data suggest that as many as one-quarter of very low birth weight infants may develop OSAS. Several studies have indicated that ex-preterm infants have a higher prevalence of OSAS during the school-aged years (14, 33, 34). However, these studies showed an interaction between the known OSAS risk factors of African American race (35, 36), obesity (35), low socioeconomic status (37), and prematurity. The current study differs in that the majority of subjects was white, nonobese, and of higher socioeconomic status (based on maternal education), and yet still showed a markedly increased prevalence of OSAS, indicating that prematurity was the most important risk factor. Possible factors that may be hypothesized to contribute to OSAS in ex-preterm infants include palatal deformation secondary to intubation (38), hypotonia, and abnormalities in ventilatory control. Hibbs and colleagues (39) reported that neonatal methylxanthine use was associated with a twofold increase in childhood OSAS, which was not borne out in the current randomized, controlled trial.
The current study is the first, to our knowledge, demonstrating a high prevalence of PLMS in ex-preterm infants. PLMS are repetitive, stereotypical movements of the legs during sleep, which may be associated with wake symptoms of restless legs. The significance of PLMS is controversial, but they have been reported to be associated with sleep fragmentation, excessive daytime sleepiness, and autonomic disturbances (40). Studies of healthy children have shown a PLMS prevalence of 5–8% (28, 41). In comparison, 14.2% (95% CI = 9.7–19.9%) of children in the current study had PLMS in the pathologic range. The reasons for this increased prevalence are unclear. High caffeine intake in adults has been reported to be a risk factor for restless legs syndrome (42) and PLMS (43). Nevertheless, although children in the caffeine group had a tendency toward a higher PLMS index, this was not statistically significant. Both groups of children had minimal caffeine intake at the time of the study. PLMS in children are frequently associated with iron deficiency (44), for which ex-preterm children may potentially be at increased risk.
Strengths of this study include the randomized, blinded design, and the large cohort. A limitation is that, as with any study, only a portion of patients approached agreed to the study, and hence it is possible that those with concerns about their child’s sleep may have been more likely to consent. Nevertheless, no differences were noted between the caffeine and placebo groups.
Neonatal caffeine use for apnea of prematurity has been shown to decrease morbidity in premature infants, and is thus used widely in neonatal intensive care units. This study has shown that therapeutic neonatal caffeine administration has no long-term effects on sleep quality or quantity, or on pathologic conditions during sleep, such as OSAS. This finding should allay concerns about the long-term effects of the drug on sleep. In addition, this study demonstrated a higher-than-expected prevalence of OSAS and PLMS in ex-preterm infants.
Acknowledgments
Acknowledgment
The authors are grateful to the children and their families for their enthusiastic participation in this study. They thank Petrina Wong, M.B.B.S., for her assistance with the actigraphy validation analyses, and Drs. Aida Bairam, Avi Sadeh, Jodi Mindell, and Susan Redline for their input in the planning of this study.
Footnotes
Supported by National Institutes of Health grant R01 HL098045 and Canadian Institutes of Health Research grant MCT 13288. Philips Respironics, Inc. provided actigraphy devices.
Author Contributions: C.L.M. participated in the design of the study, collection of the data, interpretation of the data, drafted and edited the manuscript, and approved the final manuscript as submitted; L.J.M. participated in the design of the study, analysis of the actigraphic data, interpretation of the data, drafted and edited the manuscript, and approved the final manuscript as submitted; R.S.R. was the statistician who was primarily responsible for analyzing and interpreting the data, edited the manuscript, and approved the final manuscript as submitted; J.T. participated in collection of the data, interpretation of the data, drafted and edited the manuscript, and approved the final manuscript as submitted; J. Dix, J. D’ilario, M.C., J.G., and L.C. participated in collection of the data, edited the manuscript, and approved the final manuscript as submitted; participated in collection of the data, edited the manuscript, and approved the final manuscript as submitted; E.A., G.O., L.W.D., G.M.N., I.N., and R. Bhattacharjee participated in the design of the study, collection of the data, interpretation of the data, edited the manuscript, and approved the final manuscript as submitted; S.N.B., M.D., and R.S.C.H. participated in the collection of the data, interpretation of the data, edited the manuscript, and approved the final manuscript as submitted; R. Bradford participated in collection of the data, edited the manuscript, and approved the final manuscript as submitted; and B.S. participated in the design of the study, collection of the data, interpretation of the data, edited the manuscript, and approved the final manuscript as submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201406-1092OC on August 29, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Henderson-Smart DJ. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J. 1981;17:273–276. doi: 10.1111/j.1440-1754.1981.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 2.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, Davis PG, Tin W, Moddemann D, Solimano A, et al. Caffeine for Apnea of Prematurity (CAP) Trial Investigators. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LW, Schmidt B, Anderson PJ, Davis PG, Moddemann D, Grunau RE, O’Brien K, Sankaran K, Herlenius E, Roberts R Caffeine for Apnea of Prematurity Trial investigators. Reduction in developmental coordination disorder with neonatal caffeine therapy. J Pediatr. 2014;165:356–, e2. doi: 10.1016/j.jpeds.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen SJ.Drugs of abuse and sleep: stimulants. In: Opp MR, editor. SRS basics of sleep guide. Westchester: Sleep Research Society; 2005. 159–164 [Google Scholar]
- 8.Kaplan J, Fredrickson PA, Renaux SA, O’Brien PC. Theophylline effect on sleep in normal subjects. Chest. 1993;103:193–195. doi: 10.1378/chest.103.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Avital A, Sanchez I, Holbrow J, Kryger M, Chernick V. Effect of theophylline on lung function tests, sleep quality, and nighttime SaO2 in children with cystic fibrosis. Am Rev Respir Dis. 1991;144:1245–1249. doi: 10.1164/ajrccm/144.6.1245. [DOI] [PubMed] [Google Scholar]
- 10.Hayes MJ, Akilesh MR, Fukumizu M, Gilles AA, Sallinen BA, Troese M, Paul JA. Apneic preterms and methylxanthines: arousal deficits, sleep fragmentation and suppressed spontaneous movements. J Perinatol. 2007;27:782–789. doi: 10.1038/sj.jp.7211820. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Han L, Feng S, Tian S, Hu B. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–1192. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 12.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 13.Montandon G, Horner RL, Kinkead R, Bairam A. Caffeine in the neonatal period induces long-lasting changes in sleep and breathing in adult rats. J Physiol. 2009;587:5493–5507. doi: 10.1113/jphysiol.2009.171918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Meltzer LJ, Roberts RS, et al. Longterm effects of caffeine therapy for apnea of prematurity on sleep [abstract] Sleep. 2014;37:A302. doi: 10.1164/rccm.201406-1092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–166. doi: 10.5665/sleep.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus CL, Traylor J, Biggs SN, Roberts RS, Nixon GM, Narang I, Bhattacharjee R, Davey MJ, Horne RS, Cheshire M, et al. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10:913–918. doi: 10.5664/jcsm.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. Iber C, editor. Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 20.Mindell JA, Meltzer LJ, Carskadon MA, Chervin RD. Developmental aspects of sleep hygiene: findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009;10:771–779. doi: 10.1016/j.sleep.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Mindell JA, Owens JA.A clinical guide to pediatric sleep. 2nd ed. Philadelphia: Lipincott Williams & Wilkins; 2010 [Google Scholar]
- 22.Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118:392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- 23.Kleinbaum DG, Kupper LL, Muller KE.Applied regression analysis and other multivariable methods. 2nd ed. Boston: PWS-Kent; 1988 [Google Scholar]
- 24.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, et al. Childhood Adenotonsillectomy Trial (CHAT) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Sheldon SH, Spruyt K, Ward SD, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine. The international classification of sleep disorders. 2nd ed. Westchester: American Academy of Sleep Medicine; 2005 [Google Scholar]
- 27.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus CL, Traylor J, Gallagher PR, Brooks LJ, Huang J, Koren D, Katz L, Mason TB, Tapia IE. Prevalence of periodic limb movements during sleep in normal children. Sleep. 2014;37:1349–1352. doi: 10.5665/sleep.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillet R, Kellogg C. Neonatal exposure to therapeutic caffeine alters the ontogeny of adenosine A1 receptors in brain of rats. Neuropharmacology. 1991;30:489–496. doi: 10.1016/0028-3908(91)90011-y. [DOI] [PubMed] [Google Scholar]
- 31.Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, Darrow DH, Giordano T, Litman RS, Li KK, et al. American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1) Suppl:S1–S30. doi: 10.1177/0194599810389949. [DOI] [PubMed] [Google Scholar]
- 32.Boss EF, Marsteller JA, Simon AE. Outpatient tonsillectomy in children: demographic and geographic variation in the United States, 2006. J Pediatr. 2012;160:814–819. doi: 10.1016/j.jpeds.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 33.Calhoun SL, Vgontzas AN, Mayes SD, Tsaoussoglou M, Sauder K, Mahr F, Karippot A, Wisner K, Bixler EO. Prenatal and perinatal complications: is it the link between race and SES and childhood sleep disordered breathing? J Clin Sleep Med. 2010;6:264–269. [PMC free article] [PubMed] [Google Scholar]
- 34.Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep. 2012;35:1475–1480. doi: 10.5665/sleep.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 36.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 37.Spilsbury JC, Storfer-Isser A, Kirchner HL, Nelson L, Rosen CL, Drotar D, Redline S. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 38.Ash SP, Moss JP. An investigation of the features of the pre-term infant palate and the effect of prolonged orotracheal intubation with and without protective appliances. Br J Orthod. 1987;14:253–261. doi: 10.1179/bjo.14.4.253. [DOI] [PubMed] [Google Scholar]
- 39.Hibbs AM, Johnson NL, Rosen CL, Kirchner HL, Martin R, Storfer-Isser A, Redline S. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. J Pediatr. 2008;153:176–182. doi: 10.1016/j.jpeds.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wing YK, Zhang J, Ho CK, Au CT, Li AM. Periodic limb movement during sleep is associated with nocturnal hypertension in children. Sleep. 2010;33:759–765. doi: 10.1093/sleep/33.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 42.Lutz EG. Restless legs, anxiety and caffeinism. J Clin Psychiatry. 1978;39:693–698. [PubMed] [Google Scholar]
- 43.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 44.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26:735–738. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]