Abstract
Lower respiratory tract infections caused by bacteria are a major cause of death in humans irrespective of sex, race, or geography. Indeed, accumulated data indicate greater mortality and morbidity due to these infections than cancer, malaria, or HIV infection. Successful recognition of, followed by an appropriate response to, bacterial pathogens in the lungs is crucial for effective pulmonary host defense. Although the early recruitment and activation of neutrophils in the lungs is key in the response against invading microbial pathogens, other sentinels, such as alveolar macrophages, epithelial cells, dendritic cells, and CD4+ T cells, also contribute to the elimination of the bacterial burden. Pattern recognition receptors, such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain–like receptors, are important for recognizing and responding to microbes during pulmonary infections. However, bacterial pathogens have acquired crafty evasive strategies to circumvent the pattern recognition receptor response and thus establish infection. Increased understanding of the function of TLRs and evasive mechanisms used by pathogens during pulmonary infection will deepen our knowledge of immunopathogenesis and is crucial for developing effective therapeutic and/or prophylactic measures. This review summarizes current knowledge of the multiple roles of TLRs in bacterial lung infections and highlights the mechanisms used by pathogens to modulate or interfere with TLR signaling in the lungs.
Keywords: lung, bacterial infection, Toll-like receptor, evasion of Toll-like receptor signaling
Innate and Adaptive Immune Responses during Bacterial Lung Infections
Lower respiratory tract infections, particularly pneumonia, are a major threat to human health (1). The global burden of pneumonia and pneumonia-associated complications in humans is staggering, particularly in children, as more than 2 million children across the world annually die from pneumonia (2). Bacteria are frequent causes of severe pneumonia (1). Bacterial pneumonia can be superimposed on existing host inflammatory conditions such as chronic obstructive pulmonary disease (3) or influenza (4). Although the pulmonary immune system has sophisticated mechanisms for generating innate and acquired immune responses against infection (4), bacteria often breach host defense, leading to rapid bacterial multiplication in the lungs and dissemination to distant organs. Therefore, an effective early immune response in the lungs is critical for a successful elimination of bacteria.
The major cellular players of innate immunity against respiratory infections are the phagocytes, such as neutrophils and macrophages, as well as epithelial cells, dendritic cells (DCs), γ delta (γδ) T cells, and natural killer (NK) T cells (4, 5). When bacteria enter the lung, circulatory neutrophils recruit to the lung (5). Although neutrophils are critical for host defense, excessive neutrophil accumulation leads to parenchymal damage that can contribute to poor disease outcomes (3, 5). Recruited neutrophils clear the engulfed bacteria by producing bactericidal agents, including, reactive oxygen species, antimicrobial proteins, and proteolytic enzymes (e.g., elastase, cathepsins, etc.) (5). In addition, neutrophils also form neutrophil extracellular traps, which are chromatin networks containing antibacterial proteins that can trap and kill extracellular bacteria (5). Although epithelial cells and DCs regulate the inflammatory response in lung during infection, γδ and NK T cells provide defense against phospho-antigens and glycolipid antigens produced by multiple respiratory bacterial pathogens, including Streptococcus pneumoniae (4). Moreover, adaptive immune cells, particularly CD4+ T cells, are required for effective bacterial clearance in the lung and for the host to acquire long-term immunity to infection or vaccines. CD4+ Th1 cells are primarily involved in pulmonary immunity to intracellular bacteria, whereas IL-17–producing CD4+ T cells are critical for host defense against intracellular and extracellular bacteria. The key role of CD4+ T cells in pulmonary defense is unequivocally demonstrated in humans in whom either primary or acquired (during HIV infection) deficiency of these cells enhances susceptibility to severe bacterial lung infections (4).
Toll-like Receptors
Innate immunity relies on the signals generated from membrane-bound and cytosolic pattern recognition receptors (PRRs). Toll-like receptors (TLRs) were the first PRRs to be identified and have been subsequently well characterized. TLRs are germ-line–encoded receptors that sense distinct pathogen-associated molecular patterns derived from pathogens (6). PRRs also detect molecules released by stressed or damaged host cells, known as damage-associated molecular patterns (5). TLR-mediated innate immune responses are one of the primary mechanisms for containment of bacterial growth at the site of infection and consequently for minimizing bacterial dissemination. A wide range of bacteria are eliminated by antimicrobial pathways triggered by the various membrane-bound and/or cytosolic TLRs (3, 5). Each TLR is composed of a cytoplasmic signaling domain, called a Toll/IL-1 receptor domain, a central transmembrane domain, and an extracellular domain containing a leucine-rich repeat (6). To date, 12 functional TLRs in mice and 10 TLRs in humans have been identified along with several TLR ligands and adaptor molecules (6). Engagement of specific adaptor proteins results in activation of two distinct signaling pathways downstream of TLRs, a MyD88-dependent pathway involving TLR1, 2, 4, 5, 6, 7, 8, 9, and 10 and a TIR-domain–containing adapter-inducing IFN-β (TRIF)-dependent pathway involving TLR 3 and 4 (6) (Figure 1).
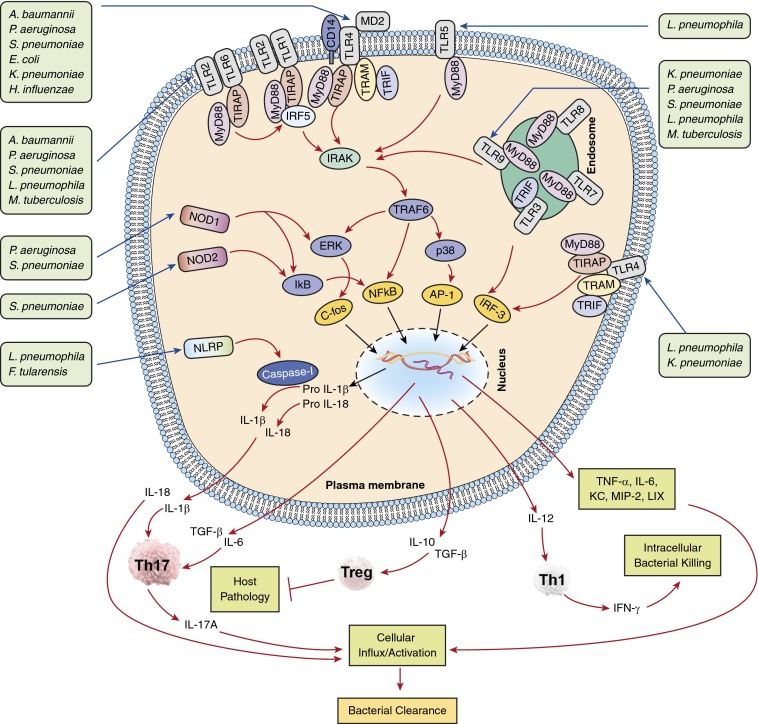
Figure 1.
Signaling cascades on activation of pattern recognition receptors by pulmonary pathogens. Plasma membrane-bound Toll-like receptors (TLRs) (TLR1, 2, 4, 5) and endosome membrane-bound TLRs (TLR9) recognize bacteria in the lungs. After bacterial recognition, TLR2 (with TLR1 or 6), TLR4 (in association with MD-2 and CD14), TLR5, and TLR9 recruit MyD88, whereas TLR2 and TLR4 recruit both MyD88 and Toll-IL-1R domain-containing adapter protein (TIRAP). All of these TLRs activate IL-1 receptor-associated kinase (IRAK) after MyD88 recruitment, followed by recruitment of tumor necrosis factor receptor–associated factor 6 (TRAF6), ultimately resulting in activation of the transcription factor nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs). MAPK activation, in turn, results in the induction of transcription factors AP-1 and c-fos. In addition, TLR3 and TLR4 recruit the adaptor TIR-domain–containing adapter-inducing IFN-β (TRIF), ultimately leading to IRF3-mediated IFN-α/β and inducible nitric oxide synthase (iNOS) production through the intermediate signaling molecule TRAM, which is the bridging adaptor for TRIF-mediated signaling. In addition, pulmonary bacterial pathogens release ligands during infection that are recognized by nucleotide-binding oligomerization domains (NODs) and activate subsequent signaling pathways leading to NF-кB activation. Furthermore, when stimulated by ligands, the NOD-like receptor proteins (NLRP) induce activation of effector caspase-1, which cleaves the pro forms of IL-1β and IL-18. In turn, cytokine activation results in differentiation of naive T cells into Th1, Th17, or regulatory T cells (Tregs), thereby leading to pulmonary host defense or, in the case of Treg accumulation, resulting in host pathology. A. baumannii = Acinetobacter baumannii; E. coli = Escherichia coli; F. tularensis = Francisella tularensis; H. influenzae = Haemophilus influenzae; K. pneumoniae = Klebsiella pneumoniae; L. pneumophila = Legionella pneumophila; M. tuberculosis = Mycobacterium tuberculosis; P. aeruginosa = Pseudomonas aeruginosa; S. pneumoniae = Streptococcus pneumoniae.
Other PRRs, particularly nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), also cooperate with TLRs to regulate host defense (5, 7). Importantly, few members of NLR family, such as NLRP12, NLRC3, and NLRP6, were also demonstrated to impair TLR signaling and thus NF-κB activation in mice against bacterial infection (7). However, their negative roles in TLR signaling in the context of lung infections are unclear. TLRs induce a range of events involved in antibacterial defense in the lung, including activation of transcription factors (e.g., NF-κB, mitogen-activated protein kinases [MAPKs], AP-1, and IRF-3) resulting in up-regulation of proinflammatory cytokines and chemokines as well as adhesion molecules important for recruitment and activation of phagocytes (Figure 1). Signaling through TLRs also induces DC maturation and production of IL-12 and transforming growth factor-β. The latter cytokine, together with IL-6, can stimulate the differentiation of Th0 to Th17 cells, whereas IL-12 alone can stimulate Th0 cells to Th1 cell differentiation, to generate effective adaptive immune responses (3, 5) (Figure 1). On the other hand, pathogens have developed strategies to avoid and/or antagonize TLR-mediated defense. Indeed, a complete understanding of the mechanisms underlying TLR-mediated immune evasion by pulmonary bacterial pathogens is critical to understand the pathogenesis of disease. Such knowledge will facilitate the design of therapeutic and/or prevention strategies against fatal lower respiratory tract infections. In this review, we discuss the function of specific TLRs and their signaling molecules in the pulmonary antibacterial response for extracellular as well as intracellular bacterial pathogens (Figure 1, Table 1). In addition, the potential evasive mechanisms used by bacteria to modulate TLR signaling in lung are discussed (Figure 2). Understanding the mechanisms underlying the evasion of TLR signaling is critical to design therapeutic and/or prevention strategies against bacterial infections in the lungs.
Table 1.
Role of Toll-like Receptors in Bacterial Lung Infection Using Gene-Deficient Mice
| Pneumonia Caused by | Phenotype* |
Adaptive Immune Response† | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Survival | Inflammation‡ | Neutrophil Influx§ | Lung Bacterial Burden | Bacterial Dissemination|| | ||||
| TLR | ||||||||
| TLR2 | Streptococcus pneumoniae | NA | ↓ | NA | NA | NA | ND | 8 |
| Legionella pneumophila | ↓ | NA | NA | ↑ | NA | 10 | ||
| Acinetobacter baumannii | NA | NA, IL-6 (↑) | NA | ↑ | NA | ND | 12 | |
| Mycobacterium tuberculosis (acute) | NA | ↓ | ND | NA | NA | NA on T-cell activation | 15 | |
| Mycobacterium tuberculosis (chronic) | ↓ | ↑ | ↑ | ↑ | ND | ↓ Treg accumulation | 16 | |
| Brucella melitensis | NA | ND | ND | ↑ | NA | ↓ IgG production | 11 | |
| Francisella tularensis | ↓ | ↓ | ND | ↑ | ↑ | ND | 13 | |
| Burkholderia pseudomallei | ↑ | ↓ | ND | ↓ | ↓ | ND | 14 | |
| TLR4 | Acinetobacter baumannii | ND | ↓ | ↓ | ↑ | ↑ | ND | 22 |
| Haemophilus influenzae | ND | ↓ | ↓ | ↑ | ND | ND | 20 | |
| Klebsiella pneumoniae | ↓ | ↓ | ND | ↑ | ↑ | ND | 19 | |
| Klebsiella pneumoniae | ND | ND | ND | ND | ND | Induces IL-17 production from both CD4+ and CD8+ T cells | 26 | |
| Streptococcus pneumoniae | ↓ | ↑ | ND | ↑ | NA | ND | 23 | |
| Mycobacterium tuberculosis (acute) | NA | NA | ND | NA | ND | ND | 15 | |
| Mycobacterium tuberculosis (chronic) | ↓ | ↑ | ↑ | ↑ | ↑ | ND | 25 | |
| TLR5 | Legionella pneumophila | ND | ↑ | ↓ | NA | ND | ND | 30 |
| TLR9 | Klebsiella pneumoniae | ↓ | ND | NA | ↑ | ↑ | ↓ DC accumulation and activation | 31 |
| Legionella pneumophila | ↓ | ND | NA | ↑ | NA | ↓ Accumulation and activation of DC and CD4+ T cells | 32 | |
| Staphylococcus aureus | ↑ | ND | NA | ↓ | ND | ND | 34 | |
| Pseudomonas aeruginosa | ↑ | ↓ | ↑ | ↓ | ND | ND | 33 | |
| TLR adaptors |
||||||||
| MyD88 | Escherichia coli | ND | ND | ↓ | ND | ND | ND | 74 |
| Klebsiella pneumoniae | ↓ | ↓ | ↓ | ↑ | ↑ | ND | 27 | |
| Legionella pneumophila | ↓ | ↓ | ↓ | ↑ | ↑ | ND | 10 | |
| Pseudomonas aeruginosa | ↓ | ↓ Early, ↑ late | ↓ | ↑ | ↑ | ND | 9 | |
| Staphylococcus aureus | NA | NA | ↓ | NA | NA | ND | 9 | |
| Burkholderia pseudomallei | ↓ | NA | ↓ | ↑ | ↑ | ND | 40 | |
| TIRAP/Mal | Klebsiella pneumoniae | ↓ | ↓ | ↓ | ↑ | ↑ | ND | 75 |
| Pseudomonas aeruginosa | NA | NA | NA | NA | NA | ND | 75 | |
| TRIF | Escherichia coli | NA | ND | ↓ | ↑ | ↑ | ND | 74 |
| Klebsiella pneumoniae | ↓ | ↓ | ↓ | ↑ | ↑ | ND | 27 | |
| Burkholderia pseudomallei | NA | ND | ND | ND | ND | ND | 40 | |
Definition of abbreviations: DC = dendritic cell; NA = not altered significantly; ND = not determined; TIRAP = Toll-IL-1R domain-containing adapter protein; TLR = Toll-like receptor; Treg = regulatory T cell; TRIF = TIR-domain–containing adapter-inducing IFN-β.
Phenotype was determined using gene-deficient (or mutant) mice after infection.
Adaptive immune response was determined using gene-deficient (or mutant) mice after infection.
Inflammation was determined in lungs based on different parameters after infection.
Neutrophil influx was determined in bronchoalveolar lavage fluid and/or lung parenchyma.
Bacterial dissemination was ascertained as bacterial presence or growth in blood, spleen, or liver.
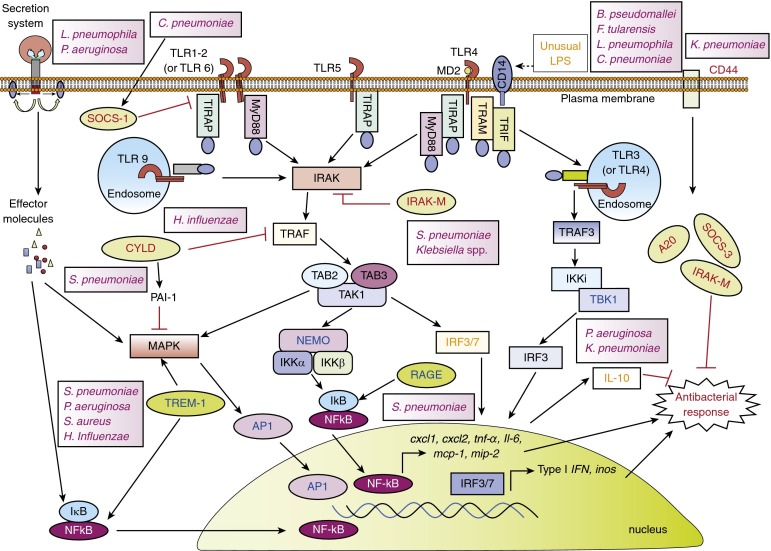
Figure 2.
Regulation of Toll-like receptor (TLR) signaling during bacterial lung infections. Negative regulators of TLRs (shown in red in pale green ellipses), IL-1 receptor-associated kinase-M (IRAK-M), suppressor of cytokine signaling 1 (SOCS-1), SOCS-3, cylindromatosis (CYLD), and A20, target different molecules within the TLR signaling pathway to inhibit their expression or activation. Positive regulators (shown in blue in darker green ellipses), triggering receptor expressed on myeloid cell 1 (TREM-1), receptor for advanced glycation end products (RAGE), secretory molecules of bacterial secretion system, amplify the TLR signaling response by activating nuclear factor (NF)-кB and/or mitogen-activated protein kinases (MAPKs). B. pseudomallei = Burkholderia pseudomallei; C. pneumoniae = Chlamydophila pneumoniae; F. tularensis = Francisella tularensis; H. influenzae = Haemophilus influenzae; K. pneumoniae = Klebsiella pneumoniae; L. pneumophila = Legionella pneumophila; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus; S. pneumoniae = Streptococcus pneumoniae.
Role of TLRs in Bacterial Lung Infections: Evidences from In Vivo Mouse Model
TLR2
TLR2 is a vital member of the TLR family that forms a heterodimer with either TLR1 or TLR6 to transmit signals (6). The TLR2 complex can recognize the molecular structures associated with gram-positive bacteria, gram-negative bacteria, mycoplasma, mycobacteria, and yeast, including lipoproteins, lipopeptides, β-glucan, glycoproteins, and zymosan, leading to the recruitment of the adaptors toll-IL receptor domain-containing adaptor protein (TIRAP) and MyD88 (Figure 1) (6). Although several molecular patterns associated with gram-positive bacteria can activate TLR2 signaling, it appears that TLR2 complex has a minor role in host defense against bacterial pathogens. For example, TLR2 signaling has been demonstrated to play a role in the inflammatory response but is dispensable for bacterial clearance as well as survival of mice infected with S. pneumoniae (8). Likewise, in Staphylococcus aureus–induced lung infection, survival and bacterial burden in lungs and other distant organs of MyD88 knockout mice were similar compared with wild-type (WT) mice, although the levels of inflammatory cytokines and chemokines were diminished in knockout compared with WT mice (9). Furthermore, TLR2 mediates only partial resistance to other pathogens that cause lung infections, including Legionella pneumophila (10), Brucella melitensis (11), and Acinetobacter baumannii (12), suggesting the importance of TLR2-independent pathways for the complete protection of the host. Moreover, although TLR2 confers an effective protective response against the lethal intracellular pathogen Francisella tularensis after aerosolized infection of mice (13), this receptor was found to impair host defense and survival during pulmonary infection with another intracellular bacterium, Burkholderia pseudomallei (14).
Despite having the ability to recognize multiple components of mycobacteria (e.g., lipoarabinomannan, lipopeptides), TLR2 does not play a major role in acute resistance to low-dose Mycobacterium tuberculosis pulmonary infection (15); however, it does play a significant role in protection against chronic M. tuberculosis infection by inducing recruitment of Foxp3+ regulatory T cells (Tregs) to the lung (16). TLR2 is also involved in the induction and maintenance of a protective Th17 response on infection with M. tuberculosis in mice. Furthermore, TLR2 induces expression of IL-23 and thus in the maintenance of Th17 cell responses during M. tuberculosis infection (17). Also, a potentially key role of TLR2 in the induction of the Th17 response in an experimental vaccine model of M. tuberculosis has been identified. Reinfection with virulent M. tuberculosis (strain H37Rv) after prior infection with H37Rv or a bacillus Calmette-Guérin strain containing the RD1 gene of M. tuberculosis, a region that also encodes the early secreted antigenic target protein 6 (a predominant antigen expressed by M. tuberculosis), was shown to enhance the Th17 response in a TLR2-dependent manner (18). It is well established that IL-17/IL-17R signaling is critical for the control of several infectious diseases of the lung, including lung infections caused by Klebsiella pneumoniae and M. tuberculosis (4).
TLR4
TLR4, one of the well-characterized TLRs, senses LPS of bacteria and induces pulmonary immunity to many gram-negative pathogens, including K. pneumoniae (19), Haemophilus influenzae (20), Bordetella pertussis (21), and A. baumannii (22) (Figure 1, Table 1). Although TLR4 plays an important role in the induction of host defense against gram-positive bacterial lung infections, discrepancies exist between studies that may be due to different doses, strains, and/or routes of infection used to induce bacterial lung infections in mice. For example, similar to TLR2, TLR4 has also been demonstrated to up-regulate inflammatory cytokines in response to S. pneumoniae and its toxin pneumolysin (23). TLR4 exerts a protective effect and improves host survival, based on the enhanced mortality rate observed in TLR4 knockout mice compared with WT mice. These protective effects were observed even after instillation of a high infectious dose (2 × 107 cfu/mouse) of S. pneumoniae via the nasopharynx (23). Although TLR4 was protective in terms of survival and bacterial clearance against low-dose (6,000 cfu/mouse) intranasal S. pneumoniae challenge, it was dispensable to protect mice when a higher dose (60,000 cfu/mouse) was used (24). Similar to TLR2, TLR4 was not critical for the control of an acute M. tuberculosis infection either at low or high doses (15), although this receptor has an important role in controlling the bacterial burden and dissemination and improving host outcomes during chronic infection (25).
In addition, TLR4 signaling was observed to be critical for in vivo IL-17 production by CD4+ T cells and exerted an important role in host defense against K. pneumoniae (26). In support of this, we demonstrated the requirement of both MyD88 and TRIF signaling for induction of IL-17A in the lungs to augment host defense against K. pneumoniae (27).
TLR5
TLR5 recognizes flagellin, the major protein moiety of bacterial flagella, and plays a key role in detecting invasive flagellated bacteria in the respiratory tract. Myeloid cells such as alveolar macrophages and neutrophils, and stromal cells such as epithelial cells of the respiratory tract, express TLR5 (28). TLR5 is crucial for enhanced clearance of Pseudomonas aeruginosa by alveolar macrophages (in vitro) and in vivo, an effect primarily mediated by IL-1β (29). TLR5 is modestly involved in neutrophil recruitment and cytokine/chemokine production in response to pulmonary L. pneumophila infection; however, TLR5 knockout mice exhibited no difference in bacterial burden compared with WT mice (30).
The host defense initiated via TLR5 activation in the lungs was observed not only against flagellated bacteria but also against nonflagellated bacteria such as S. pneumoniae (28). On exogenous administration of flagellin at the time of intranasal pneumococcal infection, resistance to infection, neutrophil infiltration, and the expression of IL-6, tumor necrosis factor-α (TNF-α), CXCL1, CXCL2, and CCL20 were enhanced (28), suggesting a potential value of flagellin as a therapeutic agent to control pulmonary infection.
TLR9
TLR9 senses unmethylated CpG motifs found in microbial DNA (6). TLR9 plays both host-protective and detrimental roles in response to bacterial lung infections. Unlike other TLRs that orchestrate neutrophil influx and activation in the lung during lower respiratory tract infections, TLR9 was found to invoke pulmonary defense by inducing accumulation and activation of DCs and macrophages. In this context, it was demonstrated that TLR9 activation induces the accumulation and maturation of DCs in addition to activating lung macrophages and T cells to produce an effective Th1 response for the control of lung infections caused by K. pneumoniae or L. pneumophila (31, 32). Because TLR9 is primarily expressed in DCs and B cells in mice, and to lesser extent in macrophages, these above studies (31, 32) may indicate the involvement of specific cell type–associated TLR9 signaling in pulmonary defense. Activation of TLR9 has been shown to prevent the shift of classical macrophage activation to the alternative pathway via facilitating the accumulation of DCs to lung (32). In contrast, a detrimental role of TLR9 during the pulmonary host response was observed during both P. aeruginosa and methicillin-resistant S. aureus pneumonia. For P. aeruginosa, signaling through TLR9 reduces production of IL-1β and nitric oxide (NO), impairing the ability of activated macrophages to clear bacteria (33). Additionally, during methicillin-resistant S. aureus pneumonia, deleterious type I IFN signaling and TNF-α up-regulation are induced downstream of TLR9 in DCs (34). Nonetheless, there is a paucity of information regarding whether the shift in roles, between host protective or detrimental, by TLR9 is a direct consequence of recognition of bacterial CpG DNA during infection or, rather, is mediated through indirect signaling events.
It is well known, however, that IFN-γ–producing CD4+ Th1 cells are critical for controlling pulmonary infections due to M. tuberculosis, and TLR9 may play a role in this process (4). Thus, TLR9, but not TLR2, contributes to the generation of Th1 cell responses in M. tuberculosis–infected mice via recruitment of IFN-γ–producing CD4+ T cells and production of IL-12 from DCs (35). Of note, IFN-γ is known to increase the bactericidal activity of monocytes/macrophages via several mechanisms, most importantly by increasing the level of reactive nitrogen species such as NO (36).
Cross-Talk between Signaling Downstream of TLRs and Other PRRs and Non-PRRs in Mice
Multiple, rather than single, TLRs and associated signaling molecules are required for full protection against bacterial pneumonia (6, 27, 35). Moreover, cooperative interactions between TLRs and other PRRs exist (Figure 1). For example, in addition to its role in TLR signaling, MyD88 is also involved downstream of cytokines such as IL-1 and IL-18, and, thus, it is believed that both TLR-dependent and -independent MyD88 signals cooperate to play a crucial role in the induction of immune response. In this regard, mice deficient in MyD88 or IL-1R1 exhibit similar mortality rates within 4 weeks of infection, as well as enhanced lung bacterial burden, compared with WT mice after low dose of intrapulmonary M. tuberculosis infection (37). Furthermore, MyD88 knockout mice display a more pronounced defect in IL-1β production compared with IL-1R knockout mice, suggesting the involvement of MyD88 signaling in both TLR-mediated and IL-1R–mediated IL-1β expression (37). Although several studies have established the major roles of both TLR-dependent and -independent MyD88 signaling in pulmonary defense (5), it is unclear how the TLR-independent MyD88 signaling axis is involved in promoting host defense responses.
In addition to the induction of IL-1β and IL-18 by TLRs, evidence suggests that the NLRs, particularly NOD1, NOD2, and the inflammasome complex (hetero-oligomeric structures formed by NLRs such as NLRC4 and NLRP3) directly or indirectly contribute to the production of these cytokines (7) (Figure 1). For instance, during S. pneumoniae pulmonary infection, IL-1β production was observed to be dependent on both NLRP3 and TLR2 (38). IL-18R and MyD88, but not TRIF, have also been shown to contribute to pulmonary defense, through induction of IFN-γ, during fatal B. pseudomallei infection (39, 40). From these studies, it is clear that MyD88 has redundant but physiologically important protective roles in both TLR and non-PRR signaling during the control of pulmonary bacterial infections (39).
Role of TLRs in Bacterial Lung Infection in Humans: Evidence from Genetic Variation Studies
Although the signaling pathways and effector responses initiated by TLRs in the host response against bacterial lung infections are much explored in mice, correlations between TLR family gene polymorphisms and susceptibility to bacterial lung infections in humans continue to emerge. However, few case-control or genetic association studies of TLR polymorphisms (e.g., single nucleotide polymorphisms [SNPs]) and bacterial infection susceptibility conducted in human subjects of different races and geographical areas have identified a protective role for TLRs (shown in Table 2). Moreover, some existing data indicate a potentially deleterious role for TLRs, similar to that observed in mouse models. Consistent with a minor role (if any) for TLR2 in pneumonia in mice, a TLR2 SNP (Arg753Gln) in humans was found not to affect susceptibility to S. aureus infections, including but not limited to pneumonia, in a large case-control study conducted in the UK (41); however, this SNP was observed to enhance the risk of developing tuberculosis among a Turkish population (42). Similarly, the TLR2 Arg677Trp SNP and insertion (I)/deletion (D) polymorphism (-196 to -174) were associated with tuberculosis in the Tunisian population, and white and African populations, respectively (43), underscoring the importance of TLR2 in the control of tuberculosis in humans.
Table 2.
Toll-like Receptor Polymorphism and Susceptibility to Bacterial Lung Infection in Humans
| Polymorphism | Pathogens Studied | Host Response (Pulmonary) to Infection | Reference | |
|---|---|---|---|---|
| TLRs | ||||
| TLR2 | Arg753Gln | Staphylococcus aureus | No impact | 41 |
| Arg753Gln | Mycobacterium tuberculosis | Susceptible | 42 | |
| TLR4 | D299G and T399I | Gram-negative bacteria | Susceptible | 45 |
| D299G and T399I | Legionella pneumophila | Resistance | 46 | |
| TLR5 | Stop codon SNPs (1174T and 1775G) | Legionella pneumophila | Susceptible | 47 |
| 1174C > T | Burkholderia pseudomallei | Resistance | 48 | |
| TLR6 | rs5743808 (359T > C) | Legionella pneumophila | Susceptible | 76 |
| TLR9 | rs352139 (A > G) | Mycobacterium tuberculosis | Susceptible | 77 |
| TLR signaling molecules |
||||
| MyD88 | In-frame MYD88 deletion or missense mutations | Streptococcus pneumoniae | Susceptible to pyogenic infections (ND) | 50 |
| Staphylococcus aureus | ||||
| Pseudomonas aeruginosa | ||||
| IRAK-4 | Deletion (82IdelT) or substitution (O293X) in IRAK-4 | Streptococcus pneumoniae | Susceptible to pyogenic infections (ND) | 49 |
| Staphylococcus aureus | ||||
| TRIF/Mal | ||||
| TIRAP S180L | Streptococcus pneumoniae | Protective to invasive pneumococcal disease and tuberculosis | 78 | |
| Mycobacterium tuberculosis | ||||
Definition of abbreviations: ND = not determined; SNP = single-nucleotide polymorphism; TIRAP = Toll-IL-1R domain-containing adapter protein; TLR = Toll-like receptor; TRIF = TIR-domain–containing adapter-inducing IFN-β.
Two common TLR4 polymorphisms (D299G and T399I), which are in linkage disequilibrium, are associated with LPS hyporesponsiveness (44) and enhanced susceptibility to sepsis due to gram-negative bacterial infections (45). Moreover, both polymorphisms have been shown to confer resistance to L. pneumophila pneumonia (46), suggesting the examples of balanced polymorphism where the D299G or T399I polymorphism has opposing (favorable and deleterious) effects depending on the environment, which may permit these SNPs to be retained at relatively high rates in the human genome. Another study demonstrated that a common stop codon polymorphism in the ligand-binding domain of TLR5 confers an inability to respond to flagellin and increased susceptibility to Legionella pneumonia (47). In contrast, a large case-control study conducted in the Thai population suggested that SNP TLR51174C > T is associated with reduced organ failure and improved survival of B. pseudomallei–infected patients and further suggested that such a protective effect among carriers of TLR51174C > T variant might be mediated via reduced production of IL-10 (48).
In addition to the association studies of TLR polymorphisms and disease susceptibility, available information regarding the impact of primary genetic deficiencies in TLR signaling molecules on disease outcomes underscores the critical role of TLRs in host defense. For example, similar to that seen in MyD88 or IL-1 receptor-associated kinase (IRAK)-4 knockout mice, a weaker immunological response and increased susceptibility to pyogenic infection caused by S. pneumoniae and S. aureus were observed in children with inherited MyD88 or IRAK-4 deficiencies (49, 50). However, whether such deficiencies also make children more vulnerable to pneumonia or pneumonia-associated complications such as sepsis is not known. Moreover, patients with IRAK-4 deficiency appear to improve with age, where other compensatory mechanisms, such as T-cell and B-cell responses, appear to provide patients with host defense against common microbial pathogens, including pulmonary bacteria (49), suggesting that the age of patients has a definitive role in phenotypic expression of IRAK-4 polymorphism.
The conflicting reports regarding the effect of various TLR polymorphisms on disease susceptibility likely relates to differences in the pathogens and populations studied as well as small sample sizes without statistical adjustments for multiple comparisons and validation by independent cohorts in some studies. Finally, in contrast to monogenic diseases, TLR polymorphisms likely interact with other genetic predispositions as well as environmental factors in a complex manner to either predispose or confer protection to disease (51). Future investigations will be required to define the mechanisms underlying these discrepant responses of TLRs to pulmonary infections in both humans and mice.
Methods Used by Pathogenic Pulmonary Bacteria to Circumvent TLR Signaling and Promote Infection in Mice and Humans
Although TLRs are key in generating protective host response against bacterial infections in the lung, their expression and function can be modulated by different extrinsic (i.e., bacterial origin) and intrinsic (i.e., induced/activated in the host by bacteria) regulators (Figure 2), which eventually give the pathogens the upper hand over the hostile environment of host and thus promote infection. Pulmonary bacteria exploit a plethora of built-in regulators, such as those of the bacterial secretion system, to modulate TLR signaling. In addition, the use of intrinsic regulators to modulate TLR responses varies among pathogens and may depend on a pathogen’s virulence strategy in the host. In either case, TLR signaling can be regulated to either provoke or down-regulate immune effects (52). In the former case, it may occur via over-activation of signaling molecules or transcription factors by bacterial effectors directly (53) or indirectly via up-regulation of positive regulators in the host, such as the Triggering Receptor Expressed on Myeloid cell 1 (TREM-1), an amplifier of TLR-mediated inflammation (52). Furthermore, microarray-based analyses have shown the direct involvement of the Dot/Icm type IV secretion system of L. pneumophila in the activation of NF-κB and MAPKs (53). The Dot/Icm system of L. pneumophila is known to translocate a large number of effectors that can modulate host cell functions (53). Through this secretion system, L. pneumophila may prolong the activation window of NF-κB and MAPKs leading to the inhibition of apoptosis, a critical host defense mechanism for the control of this pathogen (53); however, involvement of these phenomena during in vivo pulmonary L. pneumophila infection is less well understood. P. aeruginosa has been observed to use such hyperinflammation-mediated strategies, including the type III secretion system, for its survival and growth in a murine pneumonia model (54). This includes the promotion of unrestrained IL-18 production, which exacerbates acute lung inflammation and attenuates IL-17 production and IL-17–driven antimicrobial peptide production (54).
Studies have shown a significant correlation between TREM-1 up-regulation and poor disease outcome among patients with pneumonia caused by S. pneumoniae, S. aureus, P. aeruginosa, H. influenzae, or B. pseudomallei (55, 56). Similarly, the receptor for advanced glycation end products (RAGE), a TLR amplifier with a structure similar to TREM-1, is up-regulated in the lungs during S. pneumoniae pneumonia in mice and enhances the pulmonary inflammatory response resulting in reduced bacterial clearance and enhanced dissemination (57). Although the mechanisms underlying TREM-1– and RAGE-mediated dampening of antibacterial defense remain to be defined, it is possible that hyperinflammation caused by these inflammatory regulators may interfere with cell survival or bacterial killing mechanisms and thus hamper bacterial elimination.
It is believed that bacteria can use various strategies to down-regulate host TLR signaling. One common mechanism is by directly abrogating the expression or function of TLRs and/or TLR signaling molecules via negative regulators, whereas other strategies likely include: (1) evading the TLR recognition, and (2) inducing the production of antiinflammatory cytokines (e.g., IL-10).
Bacteria can induce negative regulators in the host, resulting in down-regulation of TLRs or their signaling molecules during infection. For example, expression of the suppressor of cytokine signaling 1 (SOCS-1), a negative regulator that targets the Mal/TIRAP adaptor for degradation, is induced by Chlamydophila pneumoniae in a STAT-1 and IFN-αβ–dependent manner in lungs and attenuates the host inflammatory response and bacterial clearance by interfering with secretion of IFN-γ and consequent NO production (58). Similarly, involvement of cylindromatosis (CYLD), a deubiquitinating enzyme that causes target molecule degradation, in host defense and survival against S. pneumoniae has been demonstrated and likely occurs via regulation of p38 MAPK activity (59). Furthermore, the early lethality induced by S. pneumoniae during pneumonia is believed to be caused by its toxin, pneumolysin, which may act through CYLD induction (59). In addition, CYLD has also been observed to impair inflammatory responses during nontypeable H. influenzae lung infections via inhibition of NF-κB activation caused by the deubiquitination of the upstream TRAF 6 and 7 molecules (60).
Rather than targeting TLR signaling molecules for degradation, some negative regulators inhibit the activity of these molecules, leading to impairment of subsequent downstream signal transduction and host defense. For example, signals from CD44 (a transmembrane receptor that can bind hyaluronic acid) have been shown to impair various components of host defense during gram-negative pneumonia (61), but not gram-positive pneumonia (62), by inducing expression of negative regulators (e.g., A20, IRAK-M etc.) (Figure 2). Similarly, the importance of single immunoglobulin IL-1 receptor-related molecule (SIGIRR), a transmembrane molecule that inhibits TLR signaling, in attenuating the antibacterial response was observed in pneumococcal pneumonia (63), whereas its deficiency proved beneficial for survival and bacterial clearance during P. aeruginosa–induced pneumonia (64), indicating the nonredundant but specific role of this negative regulator in lower respiratory tract infection. Additionally, S. pneumoniae and Klebsiella both exploit host IRAK-M, a regulator that inhibits the activity of IRAKs, to evade pulmonary immune responses (65, 66). Additionally, it has recently been shown that M. tuberculosis suppresses Th1 activation via DAP12-mediated induction of IRAK-M, which in turn induces IL-10 expression by antigen-presenting cells (67).
Some pulmonary bacteria, such as L. pneumophila, C. pneumoniae, and B. pseudomallei, possess unusual lipid A moieties in their LPS or express tetra-acylated LPS structure in vivo during infection (Francisella tularensis and Yersinia pestis) to evade recognition by host TLR4 (Figure 2). In fact, the decreased stimulatory nature of LPS from these pathogens is similar to the muted immune response seen during lung infections in TLR4- or TRIF-deficient mice (40, 68). Although these bacteria can trigger other TLRs (e.g., TLR5 and TLR2), failure to stimulate TLR4 signaling, and in particular, to activate TRIF, has a substantial effect on many host defense phenomena, such as induction of autophagy (69), neutrophil recruitment (27), and expression of inducible nitric oxide synthase (36).
It is well known that TLR stimulation produces IL-10 and thus can inhibit the production of key proinflammatory cytokines and chemokines. Accordingly, accumulating data suggest that the expression of IL-10 is detrimental to the host during bacterial pneumonia. In this regard, one study has shown that overexpression of IL-10 in the lung impaired the survival of mice in response to P. aeruginosa pneumonia (70), whereas neutralization of IL-10 with anti–IL-10 serum improved survival of mice with K. pneumoniae pneumonia (71). Furthermore, IL-10 expression correlated with decreased antibacterial responses, such as reduced cytokine/chemokine expression and bacterial clearance, in these studies (70, 71). Moreover, IL-10 up-regulation was observed to correlate with reduced IFN-γ expression, leading to increased bacterial burden and mortality in neutrophil-depleted mice with either L. pneumophila (72) or M. tuberculosis infections (73), suggesting a pivotal negative role of IL-10 in neutrophil recruitment and reversion of the Th2 response back to a Th1 response.
Conclusions
Although bacterial lung infections continue to be a major global public health threat, the immunopathogenesis of bacterial lung infections remains poorly understood. It is now appreciated that TLRs not only trigger host innate responses but also regulate specific adaptive immune responses. TLRs are critical receptors in the induction of protective pulmonary immune responses in humans and mice. Although the detrimental roles played by TLRs during bacterial pneumonia have only recently been uncovered, it is now clear that the host/pathogen interaction in the lung dictates whether activation of TLRs will be protective or detrimental to the host. Thus, it is imperative that we broaden our knowledge on the regulation of TLR-mediated immune responses to lung infection. Generally, pathogens are believed to exploit a variety of regulators to modulate TLR signaling and interfere with host responses, but controversies and discrepancies still exist regarding the cellular and molecular mechanisms. As our understanding of the interplay between TLR signaling and bacterial pneumonia grows, the ultimate goal of applying this knowledge to the development of better therapeutic options for the treatment of bacterial pneumonia may be realized. Host-targeted immunotherapies are a promising new strategy to fight infectious diseases because of the emergence of antibiotic resistance and the necessity to include pathogen evolution in vaccines.
Footnotes
Supported by Flight Attendant Medical Research Award CIA-113043 and National Institutes of Health grant R01-HL 091958 (S.J.).
Originally Published in Press as DOI: 10.1164/rccm.201406-1101PP on July 17, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mizgerd JP. Lung infection–a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The United Nations Children’s Fund (UNICEF)/World Health OrganizationWHO), Geneva. Pneumonia: the forgotten killer of children. 2006 [accessed 2014 May 5]. Available from: http://whqlibdoc.who.int/publications/2006/9280640489_eng.pdf
- 3.Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 4.Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol. 2012;24:424–430. doi: 10.1016/j.coi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balamayooran T, Balamayooran G, Jeyaseelan S. Review: Toll-like receptors and NOD-like receptors in pulmonary antibacterial immunity. Innate Immun. 2010;16:201–210. doi: 10.1177/1753425910366058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Chaput C, Sander LE, Suttorp N, Opitz B. NOD-like receptors in lung diseases. Front Immunol. 2013;4:393. doi: 10.3389/fimmu.2013.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knapp S, Wieland CW, van ‘t Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 9.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- 10.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 11.Pei J, Ding X, Fan Y, Rice-Ficht A, Ficht TA. Toll-like receptors are critical for clearance of Brucella and play different roles in development of adaptive immunity following aerosol challenge in mice. Front Cell Infect Microbiol. 2012;2:115. doi: 10.3389/fcimb.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CH, Kim DJ, Lee SJ, Jeong YJ, Kang MJ, Lee JY, Choi JA, Kwon SJ, Park JH, Park JH. Tolllike receptor 2 promotes bacterial clearance during the initial stage of pulmonary infection with Acinetobacter baumannii. Mol Med Rep. 2014;9:1410–1414. doi: 10.3892/mmr.2014.1966. [DOI] [PubMed] [Google Scholar]
- 13.Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–3662. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M, Florquin S, de Vos AF, et al. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis) PLoS Med. 2007;4:e248. doi: 10.1371/journal.pmed.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 16.McBride A, Konowich J, Salgame P. Host defense and recruitment of Foxp3(+) T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS Pathog. 2013;9:e1003397. doi: 10.1371/journal.ppat.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira-Coelho M, Cruz A, Carmona J, Sousa C, Ramos-Pereira D, Saraiva AL, Veldhoen M, Pedrosa J, Castro AG, Saraiva M. TLR2 deficiency by compromising p19 (IL-23) expression limits Th 17 cell responses to Mycobacterium tuberculosis. Int Immunol. 2011;23:89–96. doi: 10.1093/intimm/dxq459. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, Van Kaer L, Chattopadhyay D, Das G. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 2011;7:e1002378. doi: 10.1371/journal.ppat.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun. 2005;73:532–545. doi: 10.1128/IAI.73.1.532-545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol. 2002;168:810–815. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- 21.Moreno G, Errea A, Van Maele L, Roberts R, Leger H, Sirard JC, Benecke A, Rumbo M, Hozbor D. Toll-like receptor 4 orchestrates neutrophil recruitment into airways during the first hours of Bordetella pertussis infection. Microbes Infect. 2013;15:708–718. doi: 10.1016/j.micinf.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N, Akira S, van der Poll T. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med. 2006;173:122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- 23.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin S, van der Poll T. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, Bihl F, Ryffel B. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169:3155–3162. doi: 10.4049/jimmunol.169.6.3155. [DOI] [PubMed] [Google Scholar]
- 26.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz N, Van Maele L, Marques JM, Rial A, Sirard JC, Chabalgoity JA. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun. 2010;78:4226–4233. doi: 10.1128/IAI.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Descamps D, Le Gars M, Balloy V, Barbier D, Maschalidi S, Tohme M, Chignard M, Ramphal R, Manoury B, Sallenave JM. Toll-like receptor 5 (TLR5), IL-1beta secretion, and asparagine endopeptidase are critical factors for alveolar macrophage phagocytosis and bacterial killing. Proc Natl Acad Sci USA. 2012;109:1619–1624. doi: 10.1073/pnas.1108464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol. 2007;179:6981–6987. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- 31.Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, Zeng X, Moore TA, McMillan TR, Krieg AM, Akira S, Standiford TJ. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 32.Bhan U, Trujillo G, Lyn-Kew K, Newstead MW, Zeng X, Hogaboam CM, Krieg AM, Standiford TJ. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect Immun. 2008;76:2895–2904. doi: 10.1128/IAI.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benmohamed F, Medina M, Wu YZ, Maschalidi S, Jouvion G, Guillemot L, Chignard M, Manoury B, Touqui L. Toll-like receptor 9 deficiency protects mice against pseudomonas aeruginosa lung infection. PLoS ONE. 2014;9:e90466. doi: 10.1371/journal.pone.0090466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker D, Prince A. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol. 2012;189:4040–4046. doi: 10.4049/jimmunol.1201055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baral P, Utaisincharoen P. Involvement of signal regulatory protein alpha, a negative regulator of Toll-like receptor signaling, in impairing the MyD88-independent pathway and intracellular killing of Burkholderia pseudomallei-infected mouse macrophages. Infect Immun. 2012;80:4223–4231. doi: 10.1128/IAI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 38.Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, et al. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol. 2011;187:434–440. doi: 10.4049/jimmunol.1003143. [DOI] [PubMed] [Google Scholar]
- 39.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiersinga WJ, Wieland CW, Roelofs JJ, van der Poll T. MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS ONE. 2008;3:e3494. doi: 10.1371/journal.pone.0003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore CE, Segal S, Berendt AR, Hill AV, Day NP. Lack of association between Toll-like receptor 2 polymorphisms and susceptibility to severe disease caused by Staphylococcus aureus. Clin Diagn Lab Immunol. 2004;11:1194–1197. doi: 10.1128/CDLI.11.6.1194-1197.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogus AC, Yoldas B, Ozdemir T, Uguz A, Olcen S, Keser I, Coskun M, Cilli A, Yegin O. The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219–223. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 43.Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80:3343–3359. doi: 10.1128/IAI.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 45.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 46.Hawn TR, Verbon A, Janer M, Zhao LP, Beutler B, Aderem A. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires’ disease. Proc Natl Acad Sci USA. 2005;102:2487–2489. doi: 10.1073/pnas.0409831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to Legionnaires’ disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West TE, Chantratita N, Chierakul W, Limmathurotsakul D, Wuthiekanun V, Myers ND, Emond MJ, Wurfel MM, Hawn TR, Peacock SJ, et al. Impaired TLR5 functionality is associated with survival in melioidosis. J Immunol. 2013;190:3373–3379. doi: 10.4049/jimmunol.1202974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 50.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Netea MG, Wijmenga C, O’Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo ZQ, Vance RE. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 2011;7:e1001289. doi: 10.1371/journal.ppat.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, Grandjean T, Balloy V, Ryffel B, Dessein R, Chignard M, et al. Pseudomonas aeruginosa Type-3 Secretion System Dampens Host Defense by Exploiting the NLRC4-coupled Inflammasome. Am J Respir Crit Care Med. 2014;189:799–811. doi: 10.1164/rccm.201307-1358OC. [DOI] [PubMed] [Google Scholar]
- 55.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 56.Wiersinga WJ, Veer C, Wieland CW, Gibot S, Hooibrink B, Day NP, Peacock SJ, van der Poll T. Expression profile and function of triggering receptor expressed on myeloid cells-1 during melioidosis. J Infect Dis. 2007;196:1707–1716. doi: 10.1086/522141. [DOI] [PubMed] [Google Scholar]
- 57.van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, Bierhaus A, van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182:4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 58.Yang T, Stark P, Janik K, Wigzell H, Rottenberg ME. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J Immunol. 2008;180:4040–4049. doi: 10.4049/jimmunol.180.6.4040. [DOI] [PubMed] [Google Scholar]
- 59.Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, Xu H, Bourne P, Ha UH, Ishinaga H, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27:349–360. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Lim JH, Jono H, Koga T, Woo CH, Ishinaga H, Bourne P, Xu H, Ha UH, Xu H, Li JD. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenzae-induced inflammation in the middle ear and lung of mice. PLoS ONE. 2007;2:e1032. doi: 10.1371/journal.pone.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Windt GJ, Florquin S, de Vos AF, van’t Veer C, Queiroz KC, Liang J, Jiang D, Noble PW, van der Poll T. CD44 deficiency is associated with increased bacterial clearance but enhanced lung inflammation during Gram-negative pneumonia. Am J Pathol. 2010;177:2483–2494. doi: 10.2353/ajpath.2010.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;161:2219–2228. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blok DC, van Lieshout MH, Hoogendijk AJ, Florquin S, de Boer OJ, Garlanda C, Mantovani A, Van’t Veer C, de Vos AF, van der Poll T. Single immunoglobulin interleukin-1 receptor-related molecule impairs host defense during pneumonia and sepsis caused by Streptococcus pneumoniae. J Innate Immun. 2014;6:542–552. doi: 10.1159/000358239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, et al. Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection. Infect Immun. 2012;80:100–109. doi: 10.1128/IAI.05695-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoogerwerf JJ, van der Windt GJ, Blok DC, Hoogendijk AJ, De Vos AF, van ‘t Veer C, Florquin S, Kobayashi KS, Flavell RA, van der Poll T. Interleukin-1 receptor-associated kinase M-deficient mice demonstrate an improved host defense during Gram-negative pneumonia. Mol Med. 2012;18:1067–1075. doi: 10.2119/molmed.2011.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Windt GJ, Blok DC, Hoogerwerf JJ, Lammers AJ, de Vos AF, Van’t Veer C, Florquin S, Kobayashi KS, Flavell RA, van der Poll T. Interleukin 1 receptor-associated kinase m impairs host defense during pneumococcal pneumonia. J Infect Dis. 2012;205:1849–1857. doi: 10.1093/infdis/jis290. [DOI] [PubMed] [Google Scholar]
- 67.Jeyanathan M, McCormick S, Lai R, Afkhami S, Shaler CR, Horvath CN, Damjanovic D, Zganiacz A, Barra N, Ashkar A, et al. Pulmonary M. tuberculosis infection delays Th1 immunity via immunoadaptor DAP12-regulated IRAK-M and IL-10 expression in antigen-presenting cells. Mucosal Immunol. 2014;7:670–683. doi: 10.1038/mi.2013.86. [DOI] [PubMed] [Google Scholar]
- 68.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol. 2007;56:305–312. doi: 10.1099/jmm.0.46913-0. [DOI] [PubMed] [Google Scholar]
- 69.Jabir MS, Ritchie ND, Li D, Bayes HK, Tourlomousis P, Puleston D, Lupton A, Hopkins L, Simon AK, Bryant C, et al. Caspase-1 cleavage of the TLR adaptor TRIF inhibits autophagy and beta-interferon production during Pseudomonas aeruginosa infection. Cell Host Microbe. 2014;15:214–227. doi: 10.1016/j.chom.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L, Guo RF, Newstead MW, Standiford TJ, Macariola DR, Shanley TP. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am J Respir Cell Mol Biol. 2009;41:76–84. doi: 10.1165/rcmb.2008-0202OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 72.Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, Matsukawa A, Swanson MS, Yamaguchi K, Standiford TJ. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 73.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, O’Garra A. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–4087. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 75.Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, Worthen GS. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 76.Misch EA, Verbon A, Prins JM, Skerrett SJ, Hawn TRA. TLR6 polymorphism is associated with increased risk of Legionnaires’ disease. Genes Immun. 2013;14:420–426. doi: 10.1038/gene.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torres-Garcia D, Cruz-Lagunas A, Garcia-Sancho Figueroa MC, Fernandez-Plata R, Baez-Saldana R, Mendoza-Milla C, Barquera R, Carrera-Eusebio A, Ramirez-Bravo S, Campos L, et al. Variants in toll-like receptor 9 gene influence susceptibility to tuberculosis in a Mexican population. J Transl Med. 2013;11:220. doi: 10.1186/1479-5876-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, Frodsham AJ, Walley AJ, Kyrieleis O, Khan A, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]