Abstract
Rationale: As overall survival improves, individuals with HIV infection become susceptible to other chronic diseases, including accelerated chronic obstructive pulmonary disease (COPD).
Objectives: To determine whether individuals with HIV-associated COPD exhibit dysregulated lung mucosal T-cell immunity compared with control subjects.
Methods: Using flow cytometry, we evaluated peripheral blood and lung mucosal T-cell immunity in 14 HIV+COPD+, 13 HIV+COPD−, and 7 HIV−COPD+ individuals.
Measurements and Main Results: HIV+COPD+ individuals demonstrated profound CD4+ T-cell depletion with reduced CD4/CD8 T-cell ratios in bronchoalveolar lavage–derived lung mononuclear cells, not observed in peripheral blood mononuclear cells, and diminished CD4+ T cell absolute numbers, compared with control subjects. Furthermore, HIV+COPD+ individuals demonstrated decreased pulmonary HIV-specific and staphylococcal enterotoxin B–reactive CD4+ memory responses, including loss of multifunctionality, compared with HIV+COPD− control subjects. In contrast, lung mucosal HIV-specific CD8+ T-cell responses were preserved. Lung CD4+ T cells from HIV+COPD+ individuals expressed increased surface Fas death receptor (CD95) and programmed death-1, but similar bronchoalveolar lavage viral loads as control subjects. However, programmed death-1 expression inversely correlated with HIV-specific lung CD4+IFN-γ+ T-cell responses, suggesting functional exhaustion. Moreover, lung CD4+ T cells from HIV+COPD+ patients demonstrated increased basal and HIV antigen-induced expression of the early apoptosis marker annexin V compared with control subjects, which was significantly attenuated with anti-Fas blockade. Lastly, lung mucosal, but not blood, CD4+/CD8+ ratios from HIV+ patients significantly correlated with the FEV1, but not in HIV−COPD+ patients.
Conclusions: Together, our results provide evidence for profound lung mucosal CD4+ T-cell depletion via a Fas-dependent activation-induced cell death mechanism, along with impaired HIV-specific CD4+ immunity as immunologic features of HIV-associated COPD.
Keywords: HIV, T cells, COPD, apoptosis, immune activation
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) is a major cause of death worldwide. Certain HIV-infected individuals are at risk for accelerated development of COPD, though the mechanisms for this are poorly understood. Specifically, little is known regarding lung mucosal T-cell populations and function, and interactions with HIV antigens in HIV-associated COPD. Understanding these mechanisms may lead to targeted therapies in this disease.
What This Study Adds to the Field
Our study provides novel insights into the T-cell immunity features of HIV-associated COPD. We show that HIV+COPD+ patients demonstrate profound lung mucosal CD4+ T-cell depletion and impaired HIV-specific CD4+ immunity. Lung CD4+ T cells demonstrated increased expression of Fas death receptor (CD95) and PD-1, and undergo increased activation-induced cell death. We show that the lung CD4+:CD8+ T-cell ratio, but not the blood ratio, significantly correlates with the FEV1 in our HIV+ cohort at risk for COPD. Together, our data demonstrate that lung mucosal CD4+ depletion occurs through a Fas-dependent activation-induced cell death mechanism, along with impaired HIV-specific CD4+ T-cell immunity as immune features in HIV-associated COPD.
Although advancement of antiretroviral therapies (ART) has led to improved survival in HIV disease, evidence suggests these individuals are susceptible to premature development of other chronic diseases. In this regard, select HIV-infected individuals who smoke cigarettes develop accelerated chronic obstructive pulmonary disease (COPD) through mechanisms that are incompletely understood (1–4). However, the roles of HIV and host immunity in HIV-associated COPD remain poorly understood. An earlier autopsy study from HIV-infected patients with COPD demonstrated increased HIV RNA in areas of emphysema, whereas only rare HIV-infected cells were evident in normal lung (5). Recently, we demonstrated high HIV plasma viral loads and advanced peripheral CD4+ lymphopenia correlated with accelerated decline in FEV1 compared with patients without advanced HIV disease or uninfected control subjects at risk (6). Additionally, previous studies reported CD8+ alveolitis in HIV disease but not in association with obstructive lung disease (7–9). Collectively, these data suggest potential roles for HIV and host immunity contributing to lung pathology. Indeed, polyinosinic/polycytidylic acid treatment-induced inflammation, a synthetic analog of viral dsRNA, along with cigarette smoke exposure resulted in increased murine emphysema pathology (10). Furthermore, overexpression of IFN-γ was shown to drive increased emphysema in smoke-exposed mice (11). Thus, the host response to persistent viral infection, along with chronic cigarette smoke exposure, may together be a potent stimulus for HIV-associated COPD.
Profound CD4+ T-cell depletion is the hallmark of progressive HIV infection. Although CD4+ depletion strikingly occurs in the gastrointestinal tract during early HIV-1 infection (12), subsequent studies found CD4+ T-cell depletion in the lung mucosa was proportionate to the periphery (13), with HIV-specific CD4+ memory T cells enriched in the bronchoalveolar space compared with the periphery in healthy HIV-infected individuals. Another cardinal feature of HIV disease progression is immune activation, with elevated levels of proinflammatory cytokines and chemokines produced during the acute and chronic phases of infection (14, 15). A consequence of HIV-induced immune activation is apoptosis of CD4+, CD8+, and B lymphocytes (16). Additionally, evidence suggests proinflammatory cytokines are increased in HIV-uninfected COPD patients and that cigarette smoke is proinflammatory (17, 18). Thus, HIV-infected individuals who smoke may be at higher risk for pulmonary and systemic inflammation that potentiates HIV-associated COPD.
Therefore, we hypothesized increased dysregulation of lung mucosal immunity in HIV-associated COPD. Herein, our studies in an HIV-infected inner-city cohort show profound lung mucosal CD4+ T-cell depletion occurs during HIV-associated COPD and that HIV-specific CD4+, but not CD8+, lung mucosal T-cell immunity is impaired. Additionally, we show higher levels of surface expression of Fas death receptor (CD95) and programed death (PD)-1 in lung mucosal CD4+ T cells from HIV+COPD+ patients correlates with impaired CD4+ function and that Fas-dependent activation-induced cell death (AICD) occurs in response to HIV antigen. Moreover, a decreased CD4/CD8 T-cell ratio in the lung mucosa, but not the blood, correlates with the FEV1, further supporting lung CD4+ depletion is an immune feature of HIV-associated COPD.
Methods
Subjects and Tissue Samples
Participants were recruited from two potential sources: the AIDS Linked to the IntraVenous Experience (ALIVE) study and the Johns Hopkins HIV Care Clinic. The ALIVE study, a longitudinal cohort of persons with a history of injecting drugs with or at risk for HIV infection followed in Baltimore, Maryland since 1988 (19), collects spirometric, clinical, and laboratory data at regular 6-month intervals. The Johns Hopkins HIV Clinic provides primary care for approximately 3,500 patients with HIV in the Baltimore area. Participants were screened at scheduled study or clinic visits, and considered eligible if they were willing to undergo spirometry and bronchoscopy, and had no history of pneumonia or worsening of breathing status requiring steroids or antibiotics in the prior 6 weeks. COPD was defined as a post-bronchodilator FEV1/FVC ratio less than 0.70 (20). Subjects underwent bronchoalveolar lavage (BAL) using a standard protocol with instillation of 180 ml sterile normal saline in the right middle lobe. Study subjects had blood drawn on the same day.
Cell Preparation, Culture, and Antigen Stimulation
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by density gradient centrifugation using Ficoll-Paque (GE Healthcare, Piscataway, NJ). Lung mononuclear cells (LMNC) were obtained via BAL centrifugation. Cells were cryopreserved in liquid nitrogen storage. Pooled overlapping 15-mer peptides for Gag and Pol were obtained from the National Institutes of Health AIDS Reagent Program. Cells were cultured in round-bottom tissue culture tubes (Sarstedt) with and without HIV peptides or control staphylococcal enterotoxin B (SEB; Toxin Technology) at 1 μg/ml for 6 hours at 37°C.
Flow Cytometry
Stimulations for intracellular cytokine staining (ICS) were performed for 6 hours at 37°C and brefeldin-A (10 μg/ml; Sigma) added for the final 4 hours of culture, followed by Cytofix/Cytoperm (BD Biosciences). For CD107a ICS (anti–CD107a-Pacific Blue), monensin (5 μg/ml), and brefeldin-A were added at the beginning of culture. Following restimulation cells were surface-stained with anti–CD3-AlexaFluor700, anti–CD4-allophycocyanin-Cy7, anti–CD8-violet 500, anti-CD95 fluorescein isothiocyanate, and anti–PD-1 phycoerythrin. Live/Dead Fixable Blue Dead Cell Stain was used for gating on viable cells (Invitrogen). ICS was performed using anti–IFN-γ-allophycocyanin, anti–tumor necrosis factor (TNF)-α-PE-cyanine7, anti–MIP-1β-phycoerythrin, anti–IL-2–fluorescein isothiocyanate, and anti-CD107a Pacific Blue. For apoptosis studies cells were stained with anti–annexin V-Brilliant Violet-450 using either anti-Fas IgG1 (clone-ZB4; neutralizing) or anti-Fas IgM (clone- CH11 activating) at 10 μg/ml plate-bound for 12 hours at 37°C or isotype control Abs (Millipore). Antibodies were purchased from BD Biosciences unless otherwise noted. Cell fluorescence was analyzed using a FACS LSR Fortessa cytometer (BD Biosciences) and FlowJo software (Tree Star).
Statistical Analysis
Distributions of all measured variables were performed using nonparametric testing. Mean cytokine production was compared using Mann-Whitney and Wilcoxon signed rank analysis using GraphPad Prism (La Jolla, CA). The multifunctional analysis and presentation of distributions was performed using SPICE software (version 5.1), downloaded from http://exon.niaid.nih.gov/spice (21). A P value of less than 0.05 was used to determine statistical significance. For additional details, see the online supplement.
Results
Markedly Decreased CD4+/CD8+ Lung T-Cell Ratio and Lung Mucosal CD4+ T-Cell Numbers in HIV-associated COPD
We evaluated T-cell immunity in an inner-city cohort of 27 HIV-infected individuals comprised of 14 HIV+COPD+, 13 HIV+COPD−, and 7 HIV−COPD+ subjects, whose clinical characteristics are shown (Table 1). Despite similar cigarette smoke exposure, HIV+COPD+ individuals demonstrated significantly reduced FEV1% predicted values and FEV1/FVC ratios compared with HIV+COPD− control subjects. Notably, there were no significant differences between plasma HIV viral loads, antiretroviral usage, or peripheral CD4+ T-cell counts between the HIV+ groups.
Table 1.
Clinical Characteristics of Study Cohort
| HIV+COPD− (n = 13) | HIV+COPD+ (n = 14) | HIV−COPD+ (n = 7) | |
|---|---|---|---|
| Age | 48.2 (10.5) | 51.2 (7.8) | 50.6 (7.3) |
| African American, n (%) | 11 (85) | 14 (100) | 7 (100) |
| Male, n (%) | 10 (77) | 12 (86) | 4 (57) |
| Current smoker, n (%) | 9 (69) | 9 (64) | 9 (100) |
| Pack-years smoked, median (IQR) | 8.45 (2.2–22.5) | 13.4 (7.2–30) | 18.7 (13.6–35.9) |
| FEV1/FVC ratio | 0.78 (0.04) | 0.56 (0.10)* | 0.63 (0.05)* |
| FEV1 | |||
| Absolute, L | 2.84 (0.63) | 1.92 (0.76)† | 2.00 (0.63)† |
| % Predicted | 89.5 (13) | 62.6 (20)* | 78.0 (20) |
| Plasma viral load | |||
| Undetectable, n (%) | 9 (69) | 10 (71) | |
| Copies/ml, median (IQR)‡ | 30,779 (1,717–58,122) | 18,492 (7,552–40,252) | — |
| Antiretroviral use, n (%) | 9 (69) | 11 (79) | — |
| Blood CD4 | |||
| Less than 200, n (%) | 2 (15) | 3 (21) | — |
| Cells/cm3, median (IQR) | 485 (427–551) | 352 (293–609) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IQR = interquartile range.
All values mean (SD) unless otherwise indicated.
P value versus HIV+COPD− participants: *P < 0.001, †P < 0.01.
Among detectable viral load.
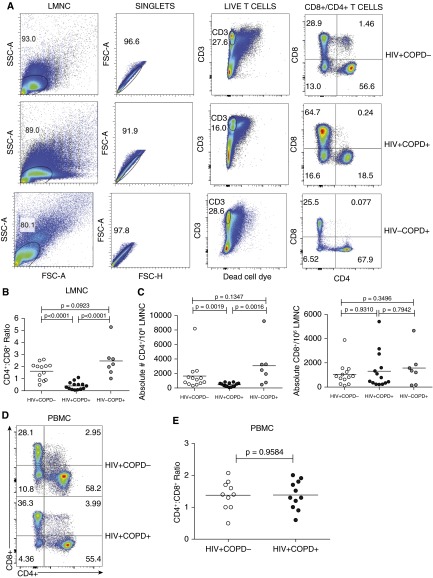
We evaluated BAL-derived LMNC and PBMC CD4+/CD8+ T-cell ratios from HIV+COPD+, HIV+COPD−, or HIV−COPD+ patients using flow cytometry. LMNC CD4+/CD8+ ratios were significantly decreased in HIV+COPD+ versus HIV+COPD− and HIV−COPD+ individuals (Figures 1A and 1B). In contrast, there was no difference in PBMC CD4+/CD8+ ratios between HIV+ groups (Figures 1D and 1E).
Figure 1.
Marked depletion of lung mucosal CD4+ T cells in HIV-associated chronic obstructive pulmonary disease (COPD). (A) Representative flow cytometric plots of gating strategy to analyze the numbers of lung mononuclear cells (LMNC) CD8+ or CD4+ T cells from HIV+COPD−, HIV+COPD+, and HIV−COPD+ patients. Gating was on LMNC cells with doublet exclusion, live/CD3+ T cells, and flow plot numbers indicate frequencies of CD4+ or CD8+ T-cell populations. Plots are representative of 34 LMNC analyzed from individuals during chronic HIV infection (14 COPD+, 13 COPD−, and 7 HIV−COPD+). (B) Pooled data showing the CD4+/CD8+ ratio of LMNC from 13 HIV+COPD− (white circles), 14 HIV+COPD+ (black circles), and 7 HIV−COPD+ (gray circles) patients. (C) Pooled data showing the absolute numbers of CD4+/106 LMNC T cells from 13 HIV+COPD− (white circles), 14 HIV+COPD+ (black circles), and 7 HIV−COPD+ (gray circles) patients. (D) Representative flow cytometric plots of peripheral blood mononuclear cells (PBMC) CD8+ or CD4+ T cells from cohort. (E) Pooled data showing the CD4+/CD8+ ratio of PBMC T cells from 11 HIV+COPD+ (black circles) and 10 COPD− (white circles) patients. Values represent mean ± SEM. P values were calculated using the Wilcoxon signed rank test or the Mann-Whitney t test.
Next, we determined whether the absolute number of lung mucosal CD4+ T cells differed. We observed a significant reduction in LMNC CD4+ T-cell absolute numbers in HIV+COPD+ patients, but no difference in absolute CD8+ T cells (Figure 1C). Interestingly, decreased absolute BAL CD4+ T-cell numbers/volume were detected despite significantly lower total BAL volume yields in HIV+COPD+ subjects (see Figures E1A and E1B in the online supplement). Together, these data demonstrate a marked decrease in BAL CD4+/CD8+ ratios, and a quantitative reduction of lung CD4+ T cells during HIV-associated COPD, not seen in HIV-negative COPD.
Diminished Lung Mucosal HIV-Specific CD4+ T-Cell Memory in HIV-associated COPD
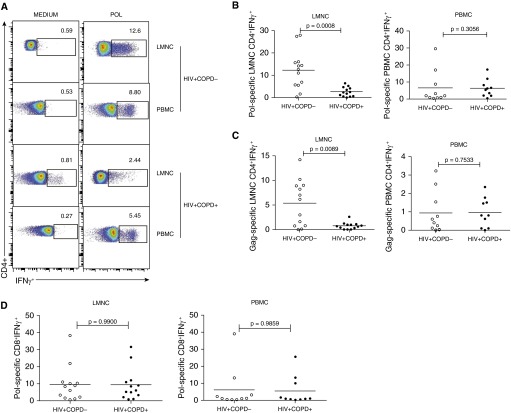
Functional viral-specific T-cell memory is critical for viral control at mucosal sites. We hypothesized HIV-specific functional T-cell responses from LMNC or PBMC were dysregulated in HIV+COPD+ versus HIV+COPD− individuals. For this, cells from each tissue compartment were cultured in the presence or absence of pooled peptides for the major HIV antigens Pol or Gag, and IFN-γ+ T-cell responses determined using ICS and flow cytometry (22, 23). Pol- and Gag-specific CD4+IFN-γ+ T-cell frequencies in LMNC were diminished in HIV+COPD+ patients compared with HIV+COPD− patients (Figures 2A–2C), whereas HIV-specific CD4+IFN-γ+ T-cell responses in PBMC did not significantly differ. In contrast, HIV-specific CD8+IFN-γ+ T-cell responses were similar in both compartments (Figure 2D). Together, our results demonstrate impaired HIV-specific CD4+IFN-γ+ T-cell responses in LMNC from HIV+COPD+ individuals versus control subjects.
Figure 2.
Lung mucosal HIV-specific CD4+IFNγ+ T-cell memory responses are reduced in HIV-associated chronic obstructive pulmonary disease (COPD). (A) Representative flow cytometric plots of the CD4+IFN-γ+ lung mononuclear cells (LMNC) and peripheral blood mononuclear cells (PBMC) from HIV+COPD− and HIV+COPD+ patients cultured in the presence or absence of Pol HIV peptides, followed by intracellular cytokine staining as detailed in the Methods section. Flow plot numbers indicate HIV-specific IFNγ+ frequencies of populations, with gating on CD3+CD4+ T cells. Plots are representative of LMNC analyzed from 26 subjects, 13 COPD− and 13 COPD+ patients during chronic HIV chronic infection, following 6 hours in vitro restimulation with pooled overlapping 15-mer Pol peptides. (B) Pooled data showing cumulative frequencies of Pol-specific CD3+CD4+IFN-γ+ in LMNC (left) versus PBMC (right). (C) Cumulative frequencies of Gag-specific LMNC CD3+CD4+ + (left) and Gag-specific PBMC CD3+CD4+IFN-γ+ (right), following in vitro restimulation with pooled overlapping 15-mer Gag peptides. (D) Pooled data showing cumulative frequencies of lung Pol-specific CD3+CD8+IFNγ+ in LMNC (left) and blood PBMC (right). Lines represent mean values. P values were calculated using the Wilcoxon signed rank test or the Mann-Whitney t test.
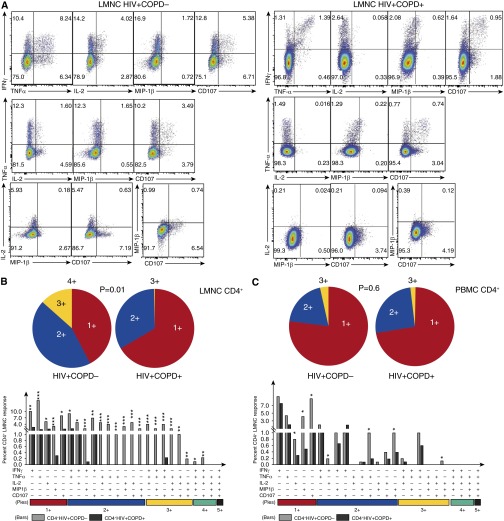
Next, we evaluated the multifunctional capacity of effector function in HIV-specific CD4+ memory T cells in LMNC and PBMC for IFN-γ, TNF-α, IL-2, CD107 mobilization, and MIP-1β. Lung HIV-specific CD4+ T cells from a representative HIV+COPD+ subject exhibit diminished multifunctional capacity compared with an HIV+COPD− individual (Figure 3A). Using Boolean analysis, we determined the percentage of individual multifunctional responses between HIV+COPD+ and HIV+COPD− individuals for LMNC and PBMC and found that LMNC from HIV+COPD+ demonstrated decreased frequencies of Pol- and Gag-specific (P = 0.03; data not shown) CD4+ T cells producing 2+, 3+, or 4+ cytokines and chemokines in contrast to CD4+ T cells from HIV+COPD− individuals (Figure 3B).
Figure 3.
Loss of multifunctional HIV-specific CD4+ T-cell memory in the lung mucosa in HIV-associated chronic obstructive pulmonary disease (COPD). (A) Representative multifunctional flow cytometric plots of lung mononuclear cells (LMNC) from HIV+COPD− (left) versus HIV+COPD+ (right), following restimulation with Pol peptides showing multifunctional subset frequencies of CD4+ T cells producing IFN-γ, tumor necrosis factor (TNF)-α, IL-2, MIP-1β, and CD107 during chronic HIV infection. (B) Individual pie charts of LMNC CD4+ T cell reflecting the percentage of Pol-specific CD4+ T cells from HIV+COPD− (left pie chart) versus HIV+COPD+ (right pie chart) patients producing one, two, three, or four functional responses. (C) Individual pie charts of peripheral blood mononuclear cells (PBMC) CD4+ T cells, reflecting the same cohort responses as in B. Using Boolean analysis, the percentage of total for individual multifunctional subset responses for HIV+COPD− (gray bars) and HIV+COPD+ (black bars) are shown in the bar graph for each of the multifunctional subsets (B and C). Significant differences when comparing mean frequencies of Pol-specific single and multifunctional responses are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. All P values were determined by the Kruskal-Wallis one-way analysis of variance or Wilcoxon signed rank test.
As a control, we compared IFN-γ+ and multifunctional SEB-reactive lung CD4+ T-cell responses between the groups, and observed significantly reduced responses in HIV+COPD+ patients compared with control subjects (see Figures E2A and E2B). In contrast, HIV-specific multifunctional CD4+ memory was similar in PBMC between both groups for Pol-specific responses (Figure 2C) and Gag-specific responses (P = 0.98; data not shown). Additionally, HIV-specific multifunctional CD8+ memory from LMNC (see Figure E3) and PBMC (data not shown) were similar between the groups. An intercompartmental comparison of HIV-specific CD4+ memory showed increased multifunction in the LMNC more than PBMC from the HIV+COPD− (P = 0.03), but not the HIV+COPD+ group (P = 0.8; data not shown). As an additional control for the cytokine milieu, we measured neat BAL supernatants for TNF-α and the antiinflammatory cytokine IL-10 using ELISA. However, TNF-α was not detected and IL-10 levels in only 3 of 21 HIV+COPD+ subjects and there were no significant differences between groups (data not shown). Together, these data show both quantitative and qualitative impairment in HIV-specific/SEB-reactive multifunctional CD4+ T-cell memory restricted to the lung mucosa during HIV-associated COPD.
Lung Mucosal CD4+ T Cells Express Increased Surface Fas (CD95) and PD-1, and Undergo Increased Fas-Dependent AICD in HIV-associated COPD
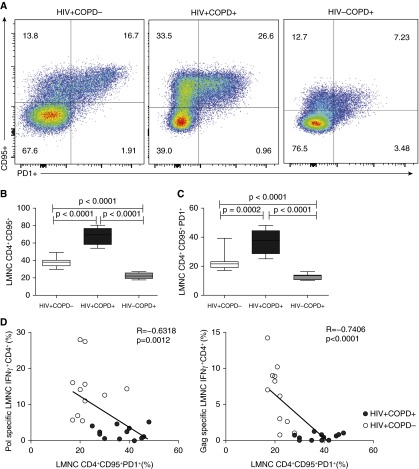
To understand the mechanisms regulating lung mucosal CD4+ T-cell depletion in HIV+COPD+ individuals, we evaluated surface expression of CD95 (Fas) and PD-1. We detected increased surface expression of PD-1 and/or CD95 in LMNC CD4+ T cells from HIV+COPD+ compared with HIV+COPD− individuals (Figures 4A–4C). Unexpectedly, most CD4+PD-1+ T cells coexpressed CD95, with a higher percentage of double-positive cells in lung CD4+ T cells from HIV+COPD+ patients. Notably, lung CD4+ T cells from HIV−COPD+ patients demonstrated significantly reduced frequencies of CD95+ and CD95+PD-1+ double-positive cells compared with HIV-infected groups. Additionally, we observed more than 98% of lung CD4+CD95+ T cells were CD28− and CTLA4− and did not differ between the HIV+ groups (P = 0.35 data not shown). We also compared BAL HIV viral loads with CD4+CD95+PD-1+ expression and did not find a relationship (P = 0.4; data not shown). However, comparing LMNC HIV-specific CD4+IFN-γ+ responses with CD95+PD-1+ expression revealed a significant inverse correlation (Figure 4D).
Figure 4.
HIV+COPD+ patients have a significantly higher surface expression of CD95+ and/or programed death (PD)-1+ on lung mucosal CD4+ T cells. (A) Representative flow cytometric plots of the lung mononuclear cells (LMNC) CD4+CD95+PD-1+ for HIV+COPD− (left) versus HIV+COPD+ (middle), and HIV−COPD+ (right) patient. (B) Pooled data showing cumulative frequencies of LMNC CD4+CD95+ for 11 HIV+COPD−, 12 HIV+COPD+, and 7 HIV−COPD+ patients. (C) Pooled data showing cumulative frequencies of LMNC CD4+CD95+PD-1+ for 11 HIV+COPD− patients (white bars), 12 HIV+COPD+ (black bars), and 7 HIV−COPD+ (gray bars) patients. Values represent mean ± SEM, and P values were calculated using the Mann-Whitney t test. (D) Scatterplot analysis of LMNC Pol- and Gag-specific CD4+IFNγ+ responses and CD4+CD95+PD-1+ frequencies. Values are for 12 HIV+COPD+ (black circles) and 11 HIV+COPD− (white circles) individuals in the cohort. Analysis was performed using the Spearman rho test.
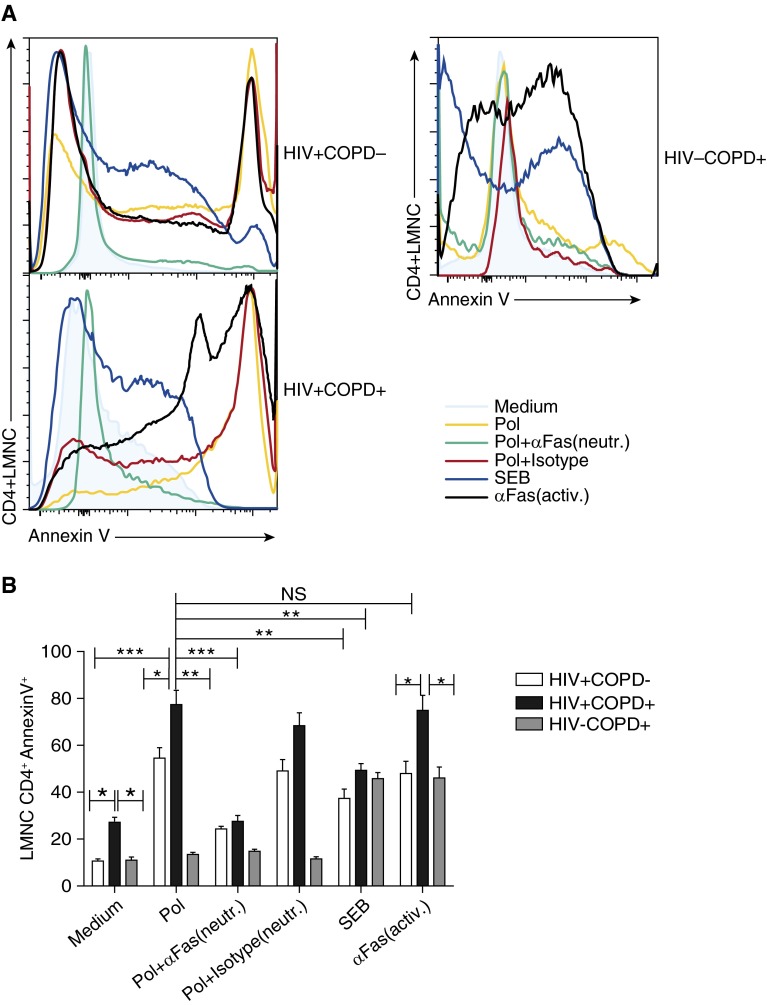
We next determined whether increased Fas expression resulted in apoptosis using the early apoptotic marker annexin V in LMNC CD4+ T cells. Lung CD4+ T cells from HIV+COPD+ patients demonstrated increased basal levels of annexin V in medium alone compared with control subjects, which increased significantly higher following in vitro restimulation with HIV peptides or anti-Fas activating Ab compared with HIV+COPD− subjects (Figures 5A and 5B). Blockade of Fas/Fas ligand (FasL) interactions using an anti-Fas neutralizing Ab significantly reduced annexin V expression in antigen-activated LMNC CD4+ T cells from HIV+COPD+ and HIV+COPD− individuals compared with isotype control Ab. As expected, CD4+ cells from HIV−COPD+ patients did not express annexin V in response to HIV antigens, and all three groups showed similar induction in response to SEB. Collectively, these data show increased expression of Fas/PD-1 in lung mucosal CD4+ T cells in HIV-associated COPD along with increased Fas-mediated AICD, as one mechanism for reduced lung mucosal CD4+ T-cell numbers in HIV-associated COPD.
Figure 5.
CD95/Fas regulate increased activation-induced cell death in lung mucosal HIV-specific CD4+ T cells in HIV-associated chronic obstructive pulmonary disease (COPD). (A) Representative histograms depicting the annexin V positivity on lung mononuclear cells (LMNC) CD4+ from HIV+COPD− (upper left), HIV+COPD+ (lower left), and HIV−COPD+ (right) donors. LMNCs were cultured in the presence or absence of plate-bound anti-Fas antibodies that are either neutralizing (ZB4) or activating (CH11) at 10 μg/ml, or an isotype control (IgG for neutralizing clone shown), with/without restimulation with Pol peptides or staphylococcal enterotoxin B (SEB), as described in the Methods section. (B) Pooled data showing LMNC annexin V+ subsets from gating on live CD3+CD4+ T cells from four HIV+COPD−, five HIV+COPD+, and seven HIV−COPD+ patients. Cumulative mean frequencies ± SEM of LMNC CD4+ annexinV+ cells from HIV+COPD− (white bars), HIV+COPD+ (black bars), and HIV−COPD+ (gray bars) patients. *P < 0.05, **P < 0.003, ***P < 0.0002, calculated using the Wilcoxon signed rank test or the Mann-Whitney t test.
Next we asked whether differences in HIV lung viral burden might contribute to differences in LMNC CD4+ T cells between HIV+ patients. Although most (71%) patients in our cohort were receiving ART (Table 1), three subjects in both the HIV+COPD+ and HIV+COPD− groups were not receiving ART, and measurement of their HIV RNA viral loads in BAL supernatants did not reveal differences (P = 0.7; see Figure E4). Furthermore, BAL HIV viral loads were similar between HIV+COPD+ and HIV+COPD− patients receiving ART (P = 0.44; see Figure E4), whereas comparison of BAL HIV viral loads between HIV+COPD+ patients either receiving or not receiving ART showed a reduction in viral loads, although this did not reach statistical significance (P = 0.096; see Figure E4). Thus, our data do not indicate significant differences in BAL HIV viral load between HIV+COPD+ and HIV+COPD− patients in our cohort to account for differences in lung mucosal CD4+ T cells. Nonetheless, these data do suggest ART can effectively reduce lung HIV viral loads in individuals with HIV-associated COPD.
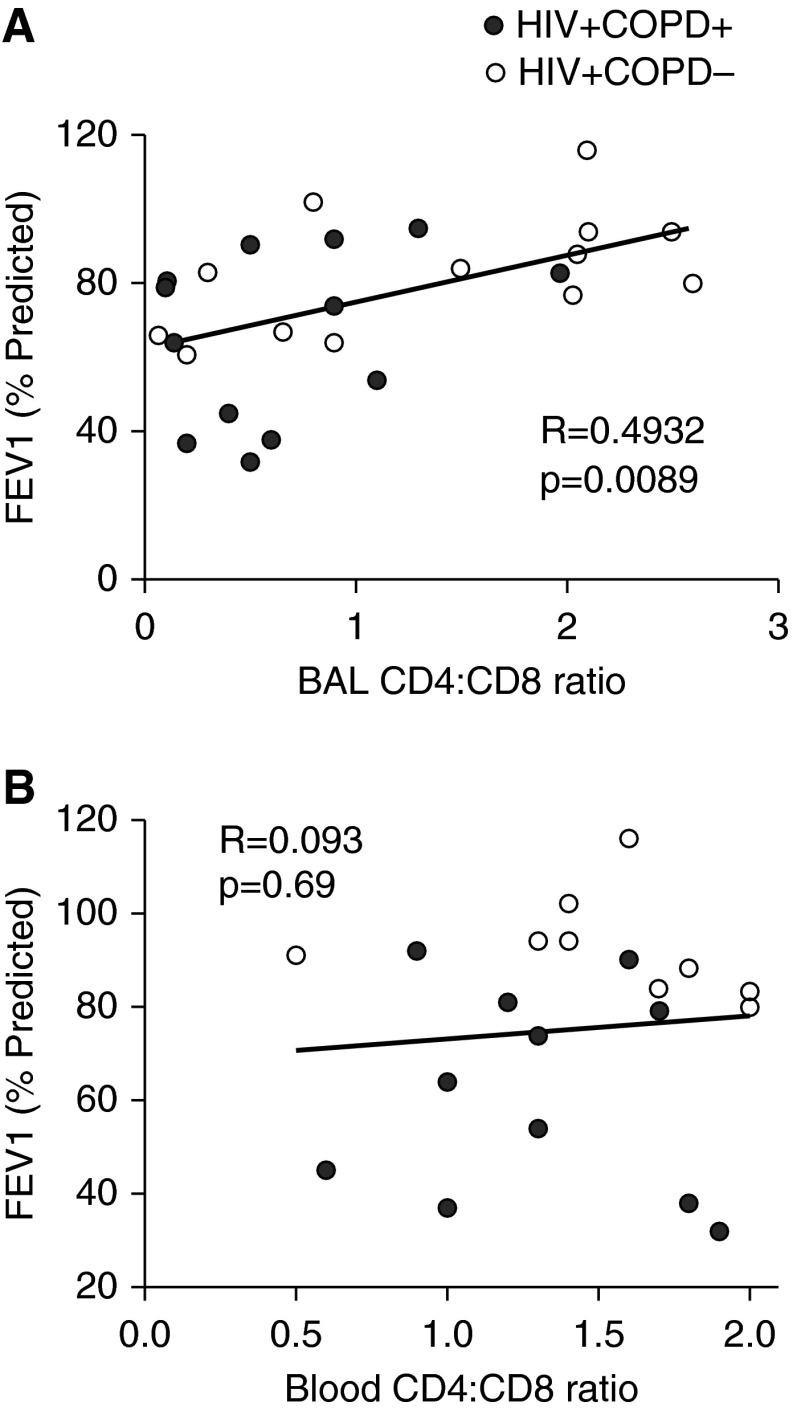
Decreased Lung Mucosal CD4+/CD8+ T-Cell Ratio Correlates with HIV-associated COPD and the FEV1 in HIV-associated COPD
We hypothesized that the LMNC CD4+/CD8+ T-cell ratio, being reduced in HIV-associated COPD, would exhibit a relationship with the FEV1. To test this, we performed a scatterplot analysis of the LMNC CD4+/CD8+ T-cell ratio and the FEV1% predicted, and found that these parameters were significantly correlated (Figure 6A). Similar results were found plotting the LMNC CD4+/CD8+ T-cell ratio and FEV1 absolute values (P = 0.046; r = 0.44) from our cohort patients. In contrast, we did not find a relationship between PBMC CD4+/CD8+ T-cell ratio and the FEV1% predicted (Figure 6B), or the LMNC CD4+/CD8+ ratio and FEV1% predicted in HIV−COPD+ patients (P = 0.18 and r = −0.56; data not shown). Thus, the lung mucosal CD4+/CD8+ T-cell ratio correlates with the FEV1 in HIV-associated COPD but not HIV-negative COPD.
Figure 6.
The bronchoalveolar lavage (BAL) but not peripheral blood mononuclear cells CD4+/CD8+ ratio correlates with the FEV1% predicted. Scatterplot analysis of the (A) lung mononuclear cells CD4+/CD8+ ratio and the FEV1% predicted values for 14 HIV+COPD+ (black circles) and 13 HIV+COPD− (white circles) individuals in the cohort and (B) peripheral blood mononuclear cells (n = 21 total). Analysis was performed using the Spearman rho test. COPD = chronic obstructive pulmonary disease.
Discussion
Herein, we report for the first time a quantitative depletion of lung mucosal CD4+ T cells, along with qualitative impairment in lung HIV-specific CD4+ T-cell immunity, occurs in HIV-associated COPD. Moreover, we show that LMNC CD4+/CD8+ T-cell ratios correlate with FEV1 values in our cohort of HIV+COPD+ and HIV+COPD− individuals, showing CD4+ T-cell dysregulation as an immune feature in HIV-associated COPD. Notably, the HIV+COPD− and the HIV−COPD+ individuals in our cohort had preserved, normal LMNC CD4+/CD8+ T-cell ratios of approximately 1–2.5:1 (24, 25), in striking contrast to reduced ratios observed in HIV+COPD+ individuals. These findings are consistent with a previous study showing a relative preservation of CD4+ T cells in the lung mucosa of HIV-infected individuals compared with the massive CD4+ T-cell depletion that occurs in the gut mucosa during the acute phase of HIV infection (13). However, our data demonstrate certain individuals are at increased risk for progressive lung mucosal CD4+ T-cell depletion disproportionately higher compared with peripheral CD4+ T-cell depletion and this correlates with the presence of HIV-associated COPD. Finally, our data from HIV-negative COPD+ control subjects indicate CD4+ T-cell depletion in HIV-associated COPD is not caused by the COPD itself.
CD4+ T cells demonstrate significant heterogeneity in function (26–28). In addition to depletion of lung mucosal CD4+ T cells, we observed a quantitative and qualitative impairment in lung HIV-specific CD4+ T-cell responses in HIV-associated COPD. Our findings of diminished frequencies of lung mucosa Pol- and Gag-specific CD4+IFN-γ+ T-cell memory responses in HIV+COPD+ individuals, but not in the PBMC, was somewhat surprising and not predicted by CD4+ T-cell depletion alone, because we anticipated reduced HIV-specific CD4+ T-cell immunity commensurate with CD4+ depletion. However, our findings may reflect preferential HIV-specific CD4+ T-cell depletion previously demonstrated (29). Additionally, our findings of increased PD-1 expression on CD4+ T cells inversely correlating with HIV-specific CD4+IFN-γ+ responses are consistent with lung CD4+ T-cell exhaustion, although this did not correlate with BAL viral loads (30). In contrast, HIV+COPD− individuals demonstrated increased lung mucosal HIV-specific CD4+ memory responses relative to PBMC as previously reported (13). Furthermore, we found impairment extended to CD4+ multifunctional memory, with increased single-positive CD4+IFN-γ+ T cells in HIV-associated COPD. Indeed, previous studies have shown loss of CD4+ multifunctional cells is correlated with HIV progression (31, 32). Although our data did not show increased HIV RNA viral loads in the BAL fluid from HIV+COPD+ subjects, it is possible that low levels of viral replication in the lung are caused by impaired CD4+ HIV-specific immunity. To this end, we recently demonstrated an important role for CD4+ T cells in lung mucosal viral control during chronic cytomegalovirus infection (28). Moreover, we found impaired CD4+ SEB-reactive T-cell responses in HIV+COPD+ patients compared with control subjects indicating more global effector dysfunction. Collectively, our data show quantitative and qualitative impairment of lung mucosal CD4+ T-cell immunity that occurs during HIV-associated COPD.
CD8+ T cells have been implicated in the pathogenesis of non-HIV COPD through unclear mechanisms (33–35), although recent evidence suggests increased cytotoxic potential with disease severity (36). Additionally, earlier reports of CD8+ T-cell alveolitis in HIV disease led investigators to speculate whether CD8+ T cells play a pathogenic role in HIV-associated COPD (1, 2). Indeed, enhanced IFN-γ in combination with cigarette smoke exposure (11), or in the presence of viral pathogen-associated molecular patterns, such as polyinosinic/polycytidylic acid or influenza infection, result in increased pulmonary inflammation and emphysema in mice, suggesting viral pathogen-associated molecular patterns and the host response are integral in the emphysema development (10). Our studies unexpectedly showed lung HIV-specific CD8+ responses are similar in HIV+COPD+ versus HIV+COPD− individuals, apparently contradicting the CD8+ alveolitis hypothesis for HIV-associated COPD. However, loss of lung mucosal CD4+ T cells might result in a differential impact of preserved CD8+ T-cell responses in HIV+COPD+ individuals. For example, progressive CD4+ regulatory T-cell loss might lead to unopposed CD8+ T-cell inflammation resulting in pulmonary pathology. Indeed, increased CD8+ T-cell activation was previously associated with HIV disease progression and shortened survival (37). Therefore, further studies are needed to determine whether CD8+ T cells play an important role in HIV-associated COPD.
Immune activation is a hallmark of chronic HIV infection, with apoptosis being a physiologic consequence of this process (16). We find lung mucosal CD4+ T cells express increased levels of surface PD-1 and/or CD95/Fas from HIV+COPD+ individuals with increased spontaneous apoptosis by higher basal annexin V expression compared with control subjects. Our data are consistent with a study showing PD-1 and CD95/Fas coexpression in blood CD8+ T cells in HIV infection, further supporting PD-1 as a preapoptotic factor in T cells (38). Furthermore, our studies show anti-Fas neutralization blocks enhanced apoptosis following in vitro restimulation with HIV antigen, demonstrating Fas-dependent apoptosis as a mechanistic pathway for lung CD4+ T-cell apoptosis in HIV-associated COPD. However, although the Fas/FasL-pathway was previously shown to be important in HIV infection (39, 40), it is plausible other pathways play a role in lung CD4+ T-cell depletion (41). Furthermore, although we detected similar HIV viral load levels in the BAL between groups, low levels of HIV antigen may be an important factor driving AICD in lung CD4+ T cells, because earlier studies in blood and lymph node showed CD4+ apoptosis correlated with lymphocyte immune activation, although interestingly, not with viral burden (42, 43). Taken together, our data demonstrate that Fas-dependent AICD in response to HIV is an important mechanism driving lung mucosal CD4+ T-cell depletion in HIV-associated COPD and not a consequence of COPD.
There are several caveats to our studies. First, because the entire cohort of patients was actively smoking cigarettes, it is possible this factor differentially contributed to immune activation and dysregulation among patients. However, this characteristic of the ALIVE cohort does allow for isolation of the effects of HIV infection independent of smoking. Although the characteristics of this cohort may not represent the “standard” COPD patient, the presence of unique characteristics in the HIV-infected and HIV-uninfected groups removes these traits as potential confounders in our comparisons. As well, this cohort is highly representative of the population at risk for COPD and HIV: lower-income African Americans with high smoking and injection drug use history. Second, although we detected similar BAL HIV viral loads between groups, our data suggest efficacy of ART in reducing lung viral loads in HIV+COPD+. Although these findings need to be validated in more patients, one could argue implementing ART therapy in current HIV-infected smokers is the most conservative clinical management strategy, in addition to smoking cessation, because our data suggest lung viral replication would exacerbate AICD in CD4+ T cells. Third, although our data indicate lung mucosal CD4+ correlates with the FEV1, we do not yet know whether this is causal in driving disease, because this was a cross-sectional study. Nonetheless, it is plausible given our previous study correlating markers of advanced HIV disease with accelerated decline in pulmonary function in a larger prospective cohort (6). We did not collect data on inhaled corticosteroid use, which may impact the observed associations. Furthermore, although the diffusing capacity was not measured in this study, its impairment has been shown in HIV disease (44). Alternatively, other opportunistic infections beyond HIV itself may contribute to lung disease in the setting of lung mucosal CD4+ depletion in these patients (45). Therefore, longitudinal studies are needed to further elucidate the role of CD4+ T-cell depletion in the pathogenesis of HIV-associated COPD.
In conclusion, we report profound lung mucosal CD4+ T-cell depletion, with reduction in absolute CD4+ numbers and the BAL CD4+/CD8+ ratio, along with impaired CD4+ T-cell mucosal immunity in HIV-associated COPD. We also find increased expression of the CD95/Fas and PD-1, and apoptosis in mucosal CD4+ T cells from HIV+COPD+ patients, and demonstrate Fas-dependent AICD in response to HIV antigen as a mechanism for lung mucosal CD4+ apoptosis. Finally, we show the lung CD4+/CD8+ ratio correlates with the FEV1 in HIV-infected smokers. An improved understanding of the underlying mechanisms that contribute to HIV-associated COPD may lead to targeted interventions to prevent this important complication of chronic HIV infection.
Acknowledgments
Acknowledgment
The authors thank Dr. Mark Connors and Dr. Stephen Migueles for their valuable input.
Footnotes
Supported by National Institutes of Health grants HL090483 and HL121814.
Author Contributions: I.P., M.B.D., and L.G., acquisition of the data or the analysis and interpretation of such information, writing the article, or substantial involvement in its revision prior to submission. T.C., acquisition of the data or the analysis and interpretation of such information. C.A.M., R.A.W., J.E.C., and G.D.K., involvement in the conception, hypotheses delineation, and design of the study. J.F.M., acquisition of the data or the analysis and interpretation of such information; writing the article or substantial involvement in its revision prior to submission; involvement in the conception, hypotheses delineation, and design of the study.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201407-1226OC on August 19, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 2.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 3.Gingo MR, Morris A, Crothers K. Human immunodeficiency virus-associated obstructive lung diseases. Clin Chest Med. 2013;34:273–282. doi: 10.1016/j.ccm.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2:583–592. doi: 10.1016/S2213-2600(14)70017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yearsley MM, Diaz PT, Knoell D, Nuovo GJ. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol. 2005;14:48–52. doi: 10.1097/01.pas.0000142168.72253.11. [DOI] [PubMed] [Google Scholar]
- 6.Drummond MB, Merlo CA, Astemborski J, Marshall MM, Kisalu A, McDyer JF, Mehta SH, Brown RH, Wise RA, Kirk GD. The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. AIDS. 2013;27:1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plata F, Autran B, Martins LP, Wain-Hobson S, Raphael M, Mayaud C, Denis M, Guillon JM, Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987;328:348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- 8.Twigg HL, Soliman DM, Day RB, Knox KS, Anderson RJ, Wilkes DS, Schnizlein-Bick CT. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;159:1439–1444. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 9.Agostini C, Facco M, Siviero M, Carollo D, Galvan S, Cattelan AM, Zambello R, Trentin L, Semenzato G. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am J Respir Crit Care Med. 2000;162:1466–1473. doi: 10.1164/ajrccm.162.4.2003130. [DOI] [PubMed] [Google Scholar]
- 10.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, Hill BJ, Hage CA, Brahmi Z, Khoruts A, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Zaman T, Stone D, Mefford M, Morgello S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon ML, Piacentini M. New insights on the role of apoptosis and autophagy in HIV pathogenesis. Apoptosis. 2009;14:501–508. doi: 10.1007/s10495-009-0314-1. [DOI] [PubMed] [Google Scholar]
- 17.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26:142–153. doi: 10.1055/s-2005-869535. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, Solomon L, Polk BF. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 20.Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–57. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol. 2003;77:10900–10909. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Committee TBCGS. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141:S169–S202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 25.Pipeling MR, West EE, Osborne CM, Whitlock AB, Dropulic LK, Willett MH, Forman M, Valsamakis A, Orens JB, Moller DR, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 27.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 28.Akulian JA, Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. High-quality CMV-specific CD4+ memory is enriched in the lung allograft and is associated with mucosal viral control. Am J Transplantation. 2013;13:146–156. doi: 10.1111/j.1600-6143.2012.04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilton JC, Luskin MR, Johnson AJ, Manion M, Hallahan CW, Metcalf JA, McLaughlin M, Davey RT, Jr, Connors M. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J Virol. 2007;81:2713–2725. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 34.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T- lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–826. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 35.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 36.Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, Polak TJ, Sonstein J, Todt JC, Ames TM, et al. Cytotoxic potential of lung CD8(+) T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol. 2010;184:6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 38.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, et al. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gougeon ML, Lecoeur H, Boudet F, Ledru E, Marzabal S, Boullier S, et al. Lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to Fas/Apo-1 (CD95)-induced apoptosis and preservation of a T helper 1 phenotype. J Immunol. 1997;158:2964–2976. [PubMed] [Google Scholar]
- 40.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Oliveira Pinto LM, Garcia S, Lecoeur H, Rapp C, Gougeon ML. Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1)- and TNFR2- mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood. 2002;99:1666–1675. doi: 10.1182/blood.v99.5.1666. [DOI] [PubMed] [Google Scholar]
- 42.Muro-Cacho CA, Pantaleo G, Fauci AS. Analysis of apoptosis in lymph nodes of HIV- infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 43.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 44.Crothers K, McGinnis K, Kleerup E, Wongtrakool C, Hoo GS, Kim J, Sharafkhaneh A, Huang L, Luo Z, Thompson B, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64:271–278. doi: 10.1097/QAI.0b013e3182a9215a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]