Abstract
Background
Cyclophosphamide (CYP) is used to treat a wide range of human tumors. However, the mutagenic effect of CYP is still the primary limitation for wider applications to treat a variety of human malignancies. It has been reported that CYP entrapped in liposomes reduces non-specific toxicity and enhances anticancer effects in animal systems.
Methods
In the present experiment, mice were injected with 50 mg/kg free CYP or encapsulated in liposomes to compare their ability to induce mutagenic damages including chromosomal aberrations, changes in Sister Chromatid Exchange (SCEs) frequencies, and in Mitotic Index (MI), as well as in cell cycle kinetics.
Results
Both forms of CYP induced an increase in chromosomal aberrations and SCEs at the different sampling time. On the contrary, a decrease in mitotic index and delay in cell cycle kinetics was observed at all stages of the experiment.
Conclusion
Encapsulation of CYP increased its mutagenicity, especially at a longer sampling time. This may due to interaction of liposomes with cells which is mainly through endocytosis or fusion resulting in accumulation of drug inside the cell causing chromosomal damage. Further evaluation of possible toxicity of encapsulation drugs in healthy tissue is needed.
Keywords: Cyclophosphamide, Liposome-encapsulated, Genotoxicity, Sister Chromatid Exchanges, Mice
Introduction
The Cyclophosphamide (CYP) belongs to class of oxazaphosphorines and it is an alkylating agent extensively used as an anticancer chemotherapeutic agent for childhood [1] and adult malignancies [2, 3] and other benign diseases [4]. It produces highly active carbonium ion, which reacts with the extremely electron-rich centers of nucleic acids and proteins.
CYP has been extensively tested to induce dominant lethal mutation, mononuclei, DNA damage and generation of free radicals or Reactive Oxygen Species (ROS) in vivo as well. Free radicals due to their high chemical reactivity induce cellular damage in a number of ways [5]. The most deleterious affects of CYP free radicals in vivo were genotoxic activities including DNA damages, chromosome aberrations, sister chromatid exchanges, and gene mutations, which can lead to a number of pathological conditions including cancer [6, 7].
Management and treatment for cancer cases involve invariable usage of antineoplastic agents. These agents are toxic to rapidly proliferating cells and therefore kill neoplastic tissue. However, because of their low therapeutic index, they can damage proliferating normal cells as well. Thus, long term usage of antineoplastic agents is a compromise with many destructive and untoward effects and so they are the subject of increasing concern [5]. Monitoring mutagenic potential of anticancer agents will help to minimize immediate harmful effects on the genetic materials and also to create another cancer in patients undergoing chemotherapy.
The use of carrier system, which can improve specificity in delivery of therapeutic drugs, has been investigated in a number of clinical trials; in particular, liposomes have been studied as carriers of a variety of antineoplastic drugs including cyclophosphamide and doxorubicin [8]. It has been demonstrated in animals that liposome-encapsulated anticancer drugs are far less toxic than their unencapsulated ones [9]. In addition, when they were administered intravenously, liposomes concentrate primarily in organs rich in reticuloendothelial cells. Therefore, liposomal delivery of antineoplastic agents may enhance some of their effects by targeting the drug away from healthy tissue or by reducing the dose needed to achieve a cytotoxic effect on tumor cells.
The purpose of the present study is to evaluate the chromosomal damages, changes in Sister Chromatid Exchange (SCEs) frequencies, in Mitotic Index (MI) and in cell cycle kinetics induced by Cyclophosphamide (CYP) encapsulated in liposomes in compare to the free drug in vivo mammalian system.
Materials and Methods
Chemicals
Cyclophosphamide (CYP) (vial containing 500 mg cyclo-phosphamide) was purchased in form of powder from Baxter Healthcare Corporation (Deerfield, IL 60015, USA). While 5'-bromo-2-deoxyuridine (Br dU) and colchicine were obtained from Sigma-Aldrich Chimie (Saint-Quentin Fallavier, France). All other chemicals used in the present study were analytical grade.
Animals
Fourty adult male Swiss mice, weighed from 25-30 gm were purchased from the Biological Supply Center, Theodore Bilharz Research Institute (TBRI, Cairo, Egypt). The Housing was at 25-28°C with light from 8:00 to 20:00 with free access to water. Mice were housed in stainless-steel cages in a pathogen-free centre belonged to the University Laboratory Animal Research Facility. The animals did not take any antibiotics, vitamins, and insecticides except a standard commercial diet.
Liposome Preparation and Cyclophosphamide Encapsulation
Liposomes used in the present work were multilamellar vesicles. These liposomes were composed of Hydrogenated Soy Phosphatidylcholin (HSPC) with cholesterol and polyethylene glycol (1.5:1.0:0.1), which were prepared by hydration method [10, 11]. The lipids were mixed in chloroform and the solvent was removed under reduced pressure. Multilamellar vesicles were formed by vigorous shaking of lipid film in an aqueous solution of 250 mM ammonium sulfate at 55°C, and then the preparation was treated by freeze-thaw for 5 times.
The resultant large multilamellar vesicles were sonicated for 2 hours under continuous stream of nitrogen to prevent any lipid oxidation. The resultant liposomes after sonication were found to have an average diameter of 150 nm as measured by quasi elastic light scattering apparatus. Unentrapped ammonium sulfate was removed by gel filtration through Sephadex (G-75) equilibrated with 20 mM HEPES buffer containing 0.9% NaCl at pH 7.4 and osmolarity of 290 mOs.
CYP was encapsulated by the ammonium sulfate gradient method [12] as follows: CYP powder was added to the liposome suspension described above at concentration of 1mg CYP/10 μmol phospholipids in 1 ml buffered saline solution. Liposome-CYP mixture was incubated in a water bath for one hour at 55°C. After incubation, unentrapped CYP was removed by passing through Sephadex (G-75) gel filtration column. The final concentration of CYP encapsulated into the liposomal formulations was estimated by Bicinconinic Acid (BCA) method following protocol of Masood et al [13] with some modification. The amount of drug entrapped inside the liposomes was measured by a Perkin Elmer UV-vis spectrometer using 470 nm as an excitation wavelength and 592 nm as emission wavelength, after adding Triton X100.
Grouping and Sampling
Animals were classified into four groups as follow: the first group (G1) remained as a control group, and was intra-peritoneal (i.p.) injected with physiological saline solution (NaCl, 0.9%). The second (G2) was injected i.p. once with CYP at dose 50 mg/kg body weight [14]. The third group (G3) was injected i.p. once with the CYP encapsulated in liposomes in a volume equivalent to 50 mg/kg body weight. The fourth group (G4) was injected with empty liposomes in the same volume used with group three. All animals in these groups were implanted with BrdU tablets before killing.
Subcutaneous implantation of BrdU tablets was carried out 21 hours before scarifying the animals and was conducted in compliance with the protocol of Allen et al [15] but with some modifications.
After 24 hours post-injection with the saline or empty liposomes ten mice (five animals for each group) were sacrificed by cervical dislocation. While other 30 animals of group 2 and 3 were sacrificed at each of 3 sampling times, 24, 48 and 72 hours post treatment (five animals at each sampling time).
Preparation of the Mice Bone Marrow Cells
Bone marrow cell preparations for the analysis of chromosomal aberrations, SCEs, mitotic indices and cell cycle kinetics were conducted by the colchicine-hypotonic technique. After completion of the treatment period, five animals from each group were scarified by cervical dislocation. Colchicine was given at the dose of 4 mg/kg body weight intraperitoneally at 22, 46 or 70 hours prior to sacrificing the animals. The bone marrow smears of animals in each group were prepared according to Preston et al [16]. For each group, slides were stained according to the modified fluorescence plus Giemsa technique described by Conner et al [17]. Slides were stained in 50 μg/ml of Hoechst 33258 dye for 15 minutes (protected from light). Then slides were rinsed in distilled water and layered with Mc Livian's buffer before subjected to UV light for 45 minutes at 50°C. Finally, these slides were re-rinsed in distilled water, and then immersed in 4% Giemsa dye for 7 minutes.
Chromosomal Analysis
For each group, slides were analyzed for chromosomal aberrations, SCEs, mitotic indices and cell cycle kinetics. Fifty metaphases per animal were examined microscopically for chromosomal aberrations while frequencies of SCEs were recorded in 25cells/animal. The mitotic index was obtained by counting the number of mitotic cells in 1000 cells/animal. Cell cycle analysis was studied by calculating the Replicative Index (RI) [18] a derived index that reflects the relative contribution of each cell cycle to the sample population in 100 consecutive metaphase cells/ individual; the number of first (M1), second (M2) and third (M3) or subsequent divisions was determined and RI was calculated as follows:
Formula 1
Analysis of cell cycle kinetics was also studied in terms of hours by calculating the Average Generation Time (AGT) as follows [19]:
Formula 2
Statistical Analysis
Statistical analyses for the difference in the mean number of chromosomal aberrations, SCEs, mitotic indices and cell cycle kinetics amongst groups were obtained by using Student-t-test (P < 0.05 was considered significant).
Results
Tables 1 and 2 show the frequencies of chromosomal aberrations, SCEs, mitotic indices and cell cycle kinetics observed in different stages of the experiment. The results of the present study did not indicate any significant difference in frequency of chromosomal abnormalities, SCEs, mitotic indices and cell cycle kinetics between the negative control group (G1) and group (G3) of animals treated with empty liposomes.
Table 1.
Chromosomal aberrations and Sister Chromatid Exchanges (SCEs) in bone marrow cells of mice treated with empty liposomes or Cyclophosphamide (CYP) or CYP encapsulated in liposomes
| Groups | Sampling time (h) | Number of metaphases analyzed | Cells with chromatid breakage (%) | Cells with centric fusions (%) | Cells with centromeric attenuation (%) | Cells with end to end association (%) | Total a Aberrations (%) | SCEs / Cell a |
|---|---|---|---|---|---|---|---|---|
| G1 (Negative Control) | 24 | 250 | 2.20 | 0.20 | - | - | 2.40 + 1.496 | 4.20 + 0.374 |
| G2 (Empty lipo.) | 24 | 250 | 2.00 | - | - | - | 2.00 + 1.265 | 4.60 + 0.400 |
| G3 (CYP) | 24 | 250 | 44.40 | 6.40 | 1.20 | 3.20 | 55.20 + 6.997 | 6.85 + 0.374 |
| 48 | 250 | 28.60 | 3.40 | 0.40 | 1.20 | 33.60 + 4.271 | 7.15 + 0.489 | |
| 72 | 250 | 21.60 | 2.40 | - | 0.80 | 24.80 + 4.118 | 6.80 + 0.245 | |
| G4 (CYP+ Lipo.) | 24 | 250 | 37.20 | 3.40 | - | 3.40 | 44.00 + 4.382 | 8.05 + 0.548 |
| 48 | 250 | 34.80 | 1.80 | 1.40 | 4.40 | 42.40 + 4.964 | 7.70 + 0.245 | |
| 72 | 250 | 28.00 | 3.20 | 2.00 | 3.60 | 36.80 + 4.118 | 8.80 + 0.244 |
Values represent mean + S.E. of five animals
Empty lipo. = Empty liposomes
CYP+ Lipo. = CYP encapsulated in liposomes
Table 2.
Incidence of aberrant cells %, mitotic index, replication index and average generation time of mice bone marrow cells treated with cyclophosphamide free or encapsulated in liposomes
| Groups | Sampling time (h) | Incidence of aberrant cells a (%) | Mitotic index a | Replication index a | Average generation time a |
|---|---|---|---|---|---|
| G1 (Negative Control) | 24 | 2.40 + 0.244 | 85.84 + 0.748 | 1.76 + 0.158 | 11.23 + 0.268 |
| G2 (Empty lipo.) | 24 | 2.00 + 0.447 | 83.29 + 0.836 | 1.73 + 0.144 | 11.89 + 0.259 |
| G3 (CYP) | 24 | 38.00 + 1.363 | 46.47 + 0.860 | 1.47 + 0.181 | 14.25 + 1.183 |
| 48 | 22.40 + 0.836 | 56.94+ 0.927 | 1.54 + 0.170 | 13.80 + 0.780 | |
| 72 | 17.20 + 1.816 | 50.66 + 0.583 | 1.65 + 0.037 | 12.20 + 0.200 | |
| G4 (CYP+ Lipo.) | 24 | 32.60 + 1.113 | 42.10 + 0.871 | 1.63 + 0.153 | 12.58 + 0.245 |
| 48 | 30.00 + 0.663 | 36.16 + 0.707 | 1.54 + 0.046 | 12.82 + 0.244 | |
| 72 | 25.40 + 1.593 | 39.10 + 0.663 | 1.46 + 0.025 | 14.20 + 0.374 |
Values represent mean + S.E. of five animals
The cytogenetic results which are illustrated in Tables 1and 2 reveal that when the CYP is given at a single dose of 50 mg/kg body weight free or encapsulated in liposomes, it can cause a high incidence of chromosomal aberrations, SCEs and average generation time in Swiss albino mice. The mitotic index and replication index were decreased in different stages of the experiment indicating bone marrow cytotoxicity.
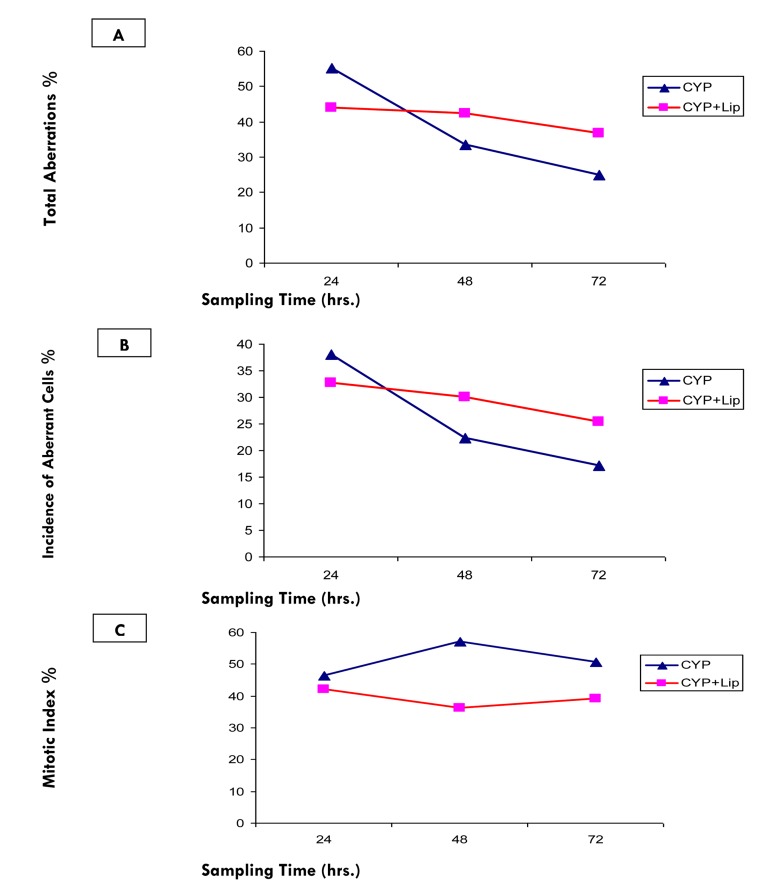
Figure 1 shows a significant elevation (at P< 0.05) in frequencies of total aberration and incidence of aberrant cells in bone marrow cells of animals with free CYP compared to those treated with encapsulated CYP at sampling time 24 hours. However, these elevations were significantly decreased at 48 and 72 hours after treatment with free CYP compared to animals treated with encapsulated CYP (Tables 1, 2 and 3).
Figure 1.
Relationship between sampling times and (A) Total aberrations %, (B) Incidence of aberrant cell % and (C) Mitotic index % after treatment with CYP free or encapsulated in liposomes
Table 3.
Significance of difference between empty liposomes, Cyclophosphamide (CYP) and CYP encapsulated in liposomes
| CYP-24 | CYP-48 | CYP-72 | CYP + Liposomes-24 | CYP + Liposomes-48 | CYP + Liposomes-72 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAC | SCE | MI | AGT | IAC | SCE | MI | AGT | IAC | SCE | MI | AGT | IAC | SCE | MI | AGT | IAC | SCE | MI | AGT | IAC | SCE | MI | AGT | |
| Negative Control | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| Empty liposomes | a | a | a | a | a | a | a | a | a | a | a | b | a | a | a | b | a | a | a | a | a | a | a | a |
| CYP-24 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CYP-48 | a | b | a | b | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CYP-72 | a | b | a | a | a | b | a | a | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CYP + Liposomes-24 | a | b | a | a | a | b | a | b | a | a | a | b | - | - | - | - | - | - | - | - | - | - | - | - |
| CYP + Liposomes-48 | a | b | a | a | a | b | a | b | a | a | a | b | b | b | a | b | - | - | - | - | - | - | - | - |
| CYP + Liposomes-72 | a | a | a | b | a | a | a | b | a | a | a | a | a | b | b | a | a | b | a | a | - | - | - | - |
a = significantly different at P˂ 0.05
b = non-significantly different at P˂ 0.05
IAC: Incidence of Aberrant Cells
SCE: Sister Chromatid Exchange
MI : Mitotic Index
AGT: Average Generation Time
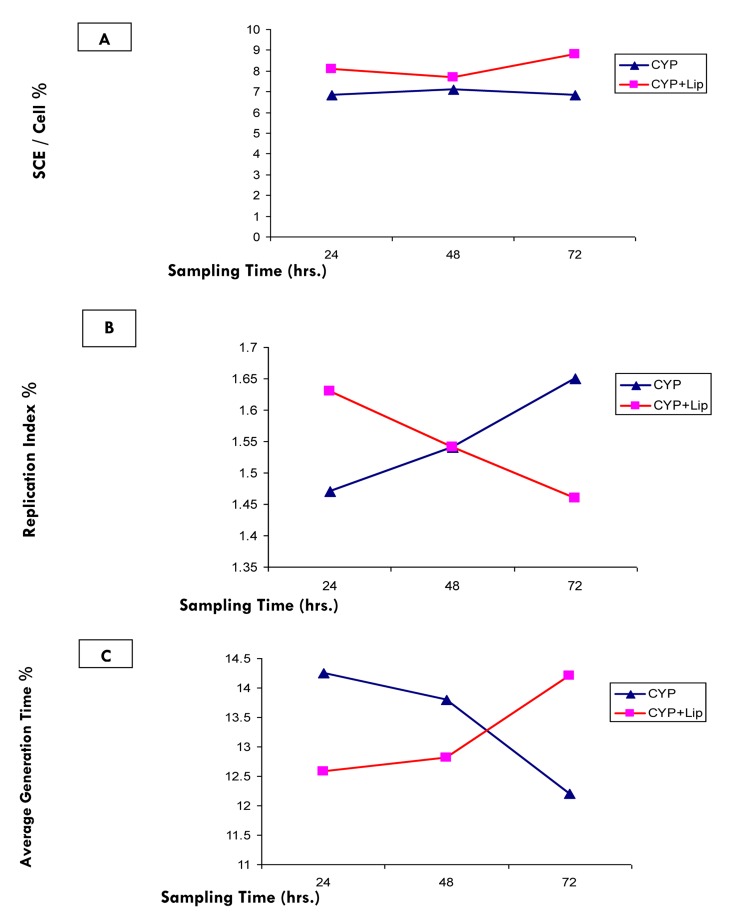
Tables 1 and 3 indicate a general significant elevation in frequency of SCEs in bone marrow cells of different treated groups. While the frequencies of SCEs in animals treated with the encapsulated CYP (G4) were significantly increased at all sampling times (24, 48 and 72 hours) compared to the free CYP (G3) treated groups (Figure 2).
Figure 2.
Relationship between sampling times and (A) SCEs / Cell %, (B) Replication index %and (C) Average generation time % after treatment with CYP free or encapsulated in liposomes
Statistically significant decrease in mitotic activity, which is indicated by decreased mitotic index of bone marrow cells of animals treated with CYP free or encapsulated was recorded at all stages of the experiment (Tables 2 and 3). The observed decrease in mitotic index of bone marrow cells after treatment with encapsulated CYP was found to be more drastic rather than induced by free CYP at different sampling times (Figure 1).
Also treatment with free or encapsulated CYP caused significant delay in cell cycle kinetics indicated by a significant decrease in the Replication Indices (RI) or a significant increase in the Average Generation Times (ATG) at all stages of this experiment (Tables 2 and 3). The data obtained for RI and ATG (calculated in hours) was used to determine the relationship between increased sampling times and changes in cell cycle kinetics (Figure 2).
Discussion
Cyclophosphamide (CYP) as one of the widely used anti-tumor agents creates cross-links and strand breaks in DNA of many cells like germ cells [20]. Such commonly used anticancer agents fail to distinguish normal cells from cancerous cells, so it kills normal proliferating cells as well. In fact use of most available anticancer drugs including CYP for killing cancer cells is a compromise between necessity and undesirable toxicity to normal cells.
Some studies have shown intraperitoneal administration of CYP can cause an increase in chromosomal aberrations and Sister Chromatid Exchanges (SCEs) as well as decrease in mitotic index [21, 7]. It has been reported that CYP and its metabolites induce oxidative stress and react with electron rich areas of the susceptible molecules such as nucleic acids and proteins. Therefore CYP targets rapidly dividing cells causing disruption of cell growth, mitotic activity and functions via alkylation of DNA at the N7 position of guanine [21, 22]. Liposomes-encapsulated anticancer drugs appear to represent an increasingly useful method for delivery of chemotherapeutic agents [11] reducing their nonspecific toxicity and enhance their anticancer effect [23].
The above mentioned results of our study indicated that animals treated with single dose of free CYP at 24, 48 and 72 hours sampling times showed several times increase in frequency of aberrant cells, SCEs and decrease in the mitotic index. This is in compliance with previous investigations which reported the ability of CYP to produce chromosome aberrations and SCEs [24- 26].
The most serious and frequent complication of CYP chemotherapy is suppression of the immune system, immunological dysregulation, and increasing intracellular amount of reactive oxygen species and glutathione depletion; such compounds can exert clastogenic effects, especially by acting as spindle inhibitors, thereby causing c-anaphasis (abnormal mitosis) and consequently aneuploidy and/or polyploidy [27]. So it is reasonable to assume that liposome encapsulation of cancer chemotherapy agents aim to down-regulate the mutagenic effect of such anticancer alkylating agents.
However, the unexpected result obtained from the present work was a higher frequency of chromosomal aberrations and SCEs after treatment with encapsulated CYP in liposomes in compared to the free drug. Reduction of non-specific toxicity of CYP without affecting its anti-tumor activity is considered the main advantage of liposome encapsulated CYP [5, 8, 11]. In addition, slow elimination of liposomes from blood circulation accompanies with low quantity of encapsulated drug entering healthy tissues. Entering low concentrations of CYP to different tissues including bone marrow is expected to cause less damage to the genetic material rather than expected with free CYP which is eliminated from blood much faster, hence it is expected to enter much more into different tissues [28].
It has been reported that liposomes and cells interact in different ways [29]. Some liposomes are ingested by the process of endocytosis and then degraded in lysosomes which release the liposome's contents into the cytoplasm. Also liposome contents may enter cytoplasm directly if liposomes fuse with cell membrane.
Conclusion
It is possible to conclude that the higher effect of CYP encapsulated in liposomes may be attributed to the accumulation of high concentrations of the released drug inside cells, not in tissues as a whole, where it can directly affect cell content. Also development of nontoxic biodegradable sustained release systems for CYP represents a significant advance in cancer chemotherapy. However, further evaluation of possible toxicity in healthy tissues is needed.
Acknowledgments
This study was performed in the Zoology Department Lab, Faculty of Science, Beni-Suef University.
Footnotes
Conflicts of Interest
There is no conflict of interest for this study.
Authors' Contribution
The Author contributed to the study design, cytogenetically part including chromosomal aberration assay, sister chromatid exchanges assay, and wrote the manuscript, while the biophysical part (Liposomes preparation) was done by Biophysical Department, Faculty of Science, Cairo University.
REFERENCES
- 1.Thomson AB, Critchley HO, kelnar C, Wallace WH. Late reproductive sequelae following treatment of childhood cancer and options for fertility preservation. Best Pract Res Clin Endocrinol Metab. 2002;16(2):311–34. doi: 10.1053/beem.2002.0200. [DOI] [PubMed] [Google Scholar]
- 2.Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999;5(8):555–60. [PubMed] [Google Scholar]
- 3.Triphaty DN, Jena GB. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicol. 2008;248(2-3):96–103. doi: 10.1016/j.tox.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez Borroto JI, Creus A, Marcos R, Molla R, Zapatero J. The Mutagenic Potential of the Furylethylene Derivative 2-Furyl-1-nitroethene in the Mouse Bone Marrow Micronucleus Test. Toxicol Scien. 2003;72(2):359–62. doi: 10.1093/toxsci/kfg038. [DOI] [PubMed] [Google Scholar]
- 5.Arif K, Ejaj A, Maroof A, Azmat AK, Arun C, Fatima N, et al. Protective effect of liposomal formulation of tuftsin (a naturally occurring tetrapeptide) against cyclophosphamide-induced genotoxicity and oxidative stress in mice. Indian Journal of Biochemistry and Biophysics. 2009;46(1):45–52. [PubMed] [Google Scholar]
- 6.Anderson D, Bishop JB, Garner RC, Ostrosky-Wegman P, Selby PB. Cyclophosphamide. Review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res. 1995;330(1-2):115–81. doi: 10.1016/0027-5107(95)00039-l. [DOI] [PubMed] [Google Scholar]
- 7.Abdella E. Bacterial lipopolysaccharides pretreatment protects against mutagenic and immunosuppressor effects of cyclophosphamide in mice. Iran J Cancer Prev. 2008;4(1):155–65. [Google Scholar]
- 8.Valero V, Buzdar AU, Theriault RL, Azarnia N, Fonseca GA, Willey J, et al. Phase II Trial of Liposome-Encapsulated Doxorubicin, Cyclophosphamide, and Fluorouracil as First-Line Therapy in Patients With Metastatic Breast Cancer. Journal of Clinical Oncology. 1999;17(5):1425–34. doi: 10.1200/JCO.1999.17.5.1425. [DOI] [PubMed] [Google Scholar]
- 9.Creaven PJ, Cowens JW, Ginsberg R, Gabizon A, Peretz T. Clinical studies with liposomal doxorubicin. J Liposome Res. 1990;1:481–90. [Google Scholar]
- 10.Gaber MH, Hong K, Huang SK, Papahadjopoulos D. Thermosensitive sterically stabilized liposomes: Formatulation and in vitro studies on mechanism of doxorubicin release by bovin serum and human plasma. Pharmaceutical Research . 1995;12(10):1–10. doi: 10.1023/a:1016206631006. [DOI] [PubMed] [Google Scholar]
- 11.Papagiannaros A, Hatziantoniou S, Lelong-Rebel H, Papaioannou GT, Dimas K, Demetzos C. Antitumor Activity of Doxorubicin Encapsulated in Hexadecylphosphocholine (HePC) Liposomes against Human Xenografts on Scid Mice. In Vivo. 2006;20(1):129–35. [PubMed] [Google Scholar]
- 12.Haran G, Ghon R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphiphiles. Biochim Biophysi Acta. 1993;1151(2):201–15. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 13.Masood AK, Faisal SM, Haque W, Owais M. Immunomodulator tuftsin augments anti-fungal activity of amphotericin B against experimental murine candidiasis. J Drug Target. 2002;10(3):185–92. doi: 10.1080/10611860290022615. [DOI] [PubMed] [Google Scholar]
- 14.Pandurangarao VL, Blazina S, Bherje R. Cadmium chloride strongly enhances cyclophosphamide-induced chromosome aberrations in mouse bone marrow cells. Environ Molecul Mutagenesis. 1997;29(Suppl. 28):19–23. [Google Scholar]
- 15.Allen JW, Shuler CF, Latt SA. Bromodeoxyuridine tablet methodology for in vivo studies of DNA synthesis. Somatic Cell Genet. 1978;4(4):393–405. doi: 10.1007/BF01538862. [DOI] [PubMed] [Google Scholar]
- 16.Preston RJ, Dean BJ, Galloway S, Holden H, Mcfee AF, Shelby M. Mammalian in vivo cytogenetic assays-analysis of chromosomal aberrations in bone marrow cells. Mut Res. 1987;189(2):157–65. doi: 10.1016/0165-1218(87)90021-8. [DOI] [PubMed] [Google Scholar]
- 17.Conner MK, Boggs SS, Turner JH. Comparisons of in vivo BrdU labeling methods and spontaneous sister chromatid exchange frequencies in regenerating murine liver and bone marrow cells. Chromosome (Beri.). 1978;68(4):303–11. doi: 10.1007/BF00327165. [DOI] [PubMed] [Google Scholar]
- 18.Schneider EL, Lewis JC. Ageing on SCE: effect of ageing environment on SCE induction and cell cycle kinetics in Ehrlich ascites tumor cells. Mech Ageing Dev. 1981;17(4):327–30. doi: 10.1016/0047-6374(81)90051-8. [DOI] [PubMed] [Google Scholar]
- 19.Tice RR, Ivett JL. Cytogenetic analysis of bone marrow damage. In: Toxicology of, edited by., editors. Irons. New York: Reven Press; 1985. [Google Scholar]
- 20.Codrington AM, Hales BF, Robaire B. Spermiogenic Germ Cell Phase-Specific DNA Damage Following Cyclophosphamide Exposure. Journal of Andrology. 2004;25(3):354–62. doi: 10.1002/j.1939-4640.2004.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 21.Murata M, Suzuki T, Midorikawa K, Oikawa S, Kawanishi S. Oxidative DNA damage induced by a hydroperoxide derivative of cyclophosphamide. Free Radical Biology and Medicine . 2004;37(6 ):793–802. doi: 10.1016/j.freeradbiomed.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Barton TS, Wyrobek AJ, Hill FS, Robaire B, Hales BF. Numerical chromosomal abnormalities in rat epididymal spermatozoa following chronic cyclophosphamide exposure. Biol Reprod. 2003;69(4):1150–7. doi: 10.1095/biolreprod.103.016261. [DOI] [PubMed] [Google Scholar]
- 23.McLoon LK, Wirtschafter JD. Direct Injection of Liposome-Encapsulated Doxorubicin Optimizes Chemomyectomy in Rabbit Eyelid. Investigative Ophthalmology & Visual Science. 1999;40(11):2561–7. [PubMed] [Google Scholar]
- 24.Reimer DL, Singh SM. Cyclophosphamide-induced in vivo sister chromatid exchanges (SCE) in Mus musculus. Quantitative Genetic Analysis, Genetics. 1983;105(1):169–79. doi: 10.1093/genetics/105.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pariani S, Buscaglia M, Piantanida M, Simoni G. Cyclophosphamide increases the frequency of sister chromatid exchange in direct preparations of human chorionic villi in the absence of supplementary enzymatic activation systems. J Med Genet. 1992;29(2):109–11. doi: 10.1136/jmg.29.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valadares MC, Pereira ER, Benfica PL, Paula JR. Assessment of mutagenic and antimutagenic effects of Punica granatum in mice. Brazilian Journal of Pharmaceutical Sciences. 2010;46(1):121–7. [Google Scholar]
- 27.Oh MS, Chang MS, Park W, Huh Y, Bae H, Ahn D. Yukmijihwangtang protects against cyclophosphamide-induced reproductive toxicity. Reprod. Toxicol. 2007;24(3-4):365–70. doi: 10.1016/j.reprotox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Gabizon A, Goren D, Fuks Z, Meshorer A, Barenhalz Y. Superior therapeutic activity of liposomes-associated adriamycin in a murine metastatic tumor model. Br J Cancer. 1985;51(5):681–9. doi: 10.1038/bjc.1985.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasic DD. Synthetic lipid microsphere serves as multipurpose vehicles for the delivery of drugs, genetic material and cosmetics. American Scientist. 1992;80(1):20–31. [Google Scholar]