Abstract
Rationale: Patients with severe asthma (SA) are less responsive to the beneficial effects of corticosteroid (CS) therapy, and relative CS insensitivity has been shown in airway smooth muscle cells (ASMC) from patients with SA.
Objectives: We investigated whether there was a defect in the actions of the glucocorticoid receptor (GR) underlying the ability of CS to suppress the inflammatory response in ASMC of patients with SA. ASMC from healthy subjects (n = 10) and subjects with severe (n = 8) and nonsevere asthma (N-SA; n = 8) were cultured from endobronchial biopsies.
Measurements and Main Results: GR expression in ASMC from SA and N-SA was reduced compared with that from healthy subjects by 49% (P < 0.01). Although baseline levels of nuclear GR were similar, GR nuclear translocation induced by dexamethasone (10−7 M) in SA was 60% of that measured in either healthy subjects or subjects with N-SA. Tumor necrosis factor (TNF)-α induced greater nuclear factor (NF)-κB (p65) mRNA expression in ASMC from subjects with SA (5.6- vs. 2.0-fold; P < 0.01), whereas baseline and TNF-α–induced nuclear translocation and dexamethasone-mediated suppression of p65 expression were similar between groups. Dexamethasone, although not modulating TNF-α–induced p65 nuclear translocation, attenuated p65 recruitment to the CCL11 promoter in the healthy and N-SA groups, but this suppressive effect was impaired in subjects with SA.
Conclusions: Decreased GR expression with impaired nuclear translocation in ASMC, associated with reduced dexamethasone-mediated attenuation of p65 recruitment to NF-κB–dependent gene promoters, may underlie CS insensitivity of severe asthma.
Keywords: airway smooth muscle, asthma, corticosteroid insensitivity, glucocorticoid receptor, nuclear translocation
At a Glance Commentary
Scientific Knowledge on the Subject
Expression of the glucocorticoid receptor (GR) in airway smooth muscle cells (ASMC) of patients with severe asthma and nonsevere asthma is reduced compared with that of healthy subjects. GR nuclear translocation induced by dexamethasone in ASMC of subjects with severe asthma is 40% lower than that in subjects with nonsevere asthma or healthy subjects. Dexamethasone attenuates nuclear factor-κB recruitment to the CCL11 promoter in ASMC of healthy subjects and patients with nonsevere asthma, but this effect is impaired in severe asthma.
What This Study Adds to the Field
Reduced expression of the glucocorticoid receptor with impaired nuclear translocation and subsequent inability to suppress recruitment of proinflammatory transcription factor to gene promoters contribute to corticosteroid insensitivity in airway smooth muscle of patients with severe asthma.
Asthma is a chronic disease characterized by airway inflammation, hyperresponsiveness, and remodelling (1). A proportion of patients with asthma do not achieve adequate asthma control, despite taking oral corticosteroids (CS) and β-adrenergic agonists leading to frequent hospital admissions and use of emergency services. These patients, referred to as having severe or refractory asthma, are relatively insensitive to the therapeutic benefits of CS as demonstrated in lung macrophages (2) and airway smooth muscle cells (3) (ASMC) by the lesser suppressive effect of dexamethasone on induced release of proinflammatory cytokines and on induced proliferation of ASMCs compared with cells from patients with nonsevere asthma or healthy subjects. In patients with severe asthma, ASM mass is increased in the airways (4, 5), contributing to increased thickening and narrowing of the airways (4) and bronchial hyperresponsiveness (6). ASMCs can synthesize cytokines and growth factors and express cell-surface molecules that allow them to interact with the extracellular matrix and inflammatory cells and may play a central role in orchestrating the inflammatory response within the bronchial wall (7, 8).
The antiinflammatory effects of CS are mediated through the glucocorticoid receptor (GR), a ligand-activated transcription factor that modulates both inflammatory and antiinflammatory gene expression. After corticosteroid binding to GR, GR dissociates from chaperone proteins and rapidly translocates into the nucleus, where GR either binds to specific glucocorticoid-responsive elements (GRE) on DNA to enhance transcription of antiinflammatory genes or represses transcription of proinflammatory genes by interaction with inflammatory transcription factors, such as nuclear factor (NF)-κB and activated protein-1 (AP-1). Changes in the phosphorylation pattern of GR as a consequence of its activation leads to many alterations of its function (9). Six serine residues have been identified as phosphorylation targets in the human GR (10), and phosphorylation of GR at serine 211 (Ser211) has been associated with ligand binding, nuclear translocation, and transcriptional activation (9).
NF-κB is of paramount importance in asthmatic inflammation (11). On cellular stimulation, NF-κB translocates to the nucleus and mediates gene transcription. NF-κB consists of hetero- or homodimers of the DNA-binding Rel family of proteins, of which the p65 subunit is ubiquitously expressed and confers transcriptional regulation (12). The NF-κB binding sites in the promoters of the proinflammatory genes such as CCL11 (eotaxin) and CXCL8 have been identified (13), and recruitment of the p65 subunit of NF-κB to the CCL11 promoter in lung epithelial cells is attenuated by dexamethasone (14). Moreover, we have shown that the suppressive effect of dexamethasone on TNF-α–induced CCL11 and CXCL8 release is impaired in ASMC of subjects with severe asthma compared with subjects with nonsevere asthma (3).
Insensitivity to CS may be attributed to a reduced ability of GR to bind to DNA or to an increase in the expression of proinflammatory transcription factors, such as NF-κB and AP-1 (15). CS insensitivity of ASMC from patients with severe asthma is associated with a greater p38 mitogen-activated protein kinase (MAPK) activation induced by TNF-α (3), similar to findings in alveolar macrophages from patients with severe asthma, wherein p38 MAPK inhibition reversed CS insensitivity (2, 16). In this study, we hypothesized that the regulation of GR and NF-κB (p65), in terms of their expression, nuclear translocation, and recruitment to gene promoters, is perturbed in ASMC of patients with severe asthma compared with those of patients with nonsevere asthma and healthy subjects. Some of the results of these studies have been previously reported in the form of an abstract (17).
Methods
More details are provided in the online supplement.
Subject Characteristics
Patients with severe asthma were on high-dose inhaled CS and sometimes on additional daily oral CS therapy to achieve a level of mild to moderate persistent asthma (18, 19) (Table 1). Patients with nonsevere asthma used inhaled beclomethasone (0–1,000 μg/d or equivalent) with good control of asthma. Current and ex-smokers of more than 5 pack-years were excluded. All patients gave informed consent to participate in this study approved by the local ethics committee. They underwent fiberoptic bronchoscopy, during which endobronchial biopsies were obtained.
Table 1.
Subject Characteristics
| Healthy Control Subjects | Nonsevere Asthma | Severe Asthma | |
|---|---|---|---|
| No. | 10 | 9 | 9 |
| Age, yr | 39.0 ± 11.3 | 31.8 ± 16 | 41.4 ± 12 |
| Sex, female/male | 5/5 | 4/5 | 5/4 |
| Duration of asthma, yr | N/A | 18.2 ± 3.8 | 25.7 ± 9.7* |
| Inhaled corticosteroid dose, μg BDP equivalent | N/A | 622 ± 290 | 2160 ± 466* |
| Atopy,† n | 3 | 6 | 5 |
| Receiving oral prednisolone, n | N/A | N/A | 6 |
| FEV1, L | 3.15 ± 1.860 | 2.96 ± 0.46 | 2.0 ± 0.47‡ |
| FEV1,% predicted | 91.0 ± 15.6 | 81.5 ± 8.3 | 61.59 ± 4.9§ |
| FEV1/FVC, % | 77.2 ± 3.9 | 75.0 ± 3.1 | 69.0 ± 5.9* |
| β-Agonist reversibility, %|| | N/A | 24.0 ± 3.5 | 31.0 ± 4.11* |
| PC20, mg/ml | >16 | 2.7 ± 0.104 | 0.331 ± 0.13 (4/9) |
Definition of abbreviations: BDP = beclomethasone dipropionate; N/A = not applicable; PC20 = provocative concentration of methacholine causing a 20% fall in FEV1.
P < 0.05 versus patients with nonsevere asthma.
Defined as positive skin prick tests to one or more common aeroallergens.
P < 0.01 versus patients with nonsevere asthma.
P < 0.001 versus patients with nonsevere asthma.
Measured as percent increase in FEV1 after 400 μg salbutamol.
ASMC Isolation, Culture, and Activation
Bronchial biopsies were cut into small pieces (<1 mm2) and transferred to 6-well culture plates. At confluence, cells were harvested and split into larger flasks at each passage. ASMC were identified by the characteristic “hill and valley” morphology and by their expression of calponin, smooth muscle α-actin, and myosin heavy chain in more than 95% of the cells (20). Cells were plated in 6-well culture plates for reverse transcription–quantitative polymerase chain reaction or in 75 cm2 flasks for Western blot and chromatin immunoprecipitation (ChIP assay). At 90% confluence, cells were serum starved for 24 hours and stimulated with dexamethasone and/or TNF-α for times as indicated. Cells were used at passage 4 or 5.
Determination of mRNA Expression
Total RNA was isolated using RNeasy Mini Kit (Qiagen, Crawley, UK) and reverse-transcribed with random primers and AMV reverse transcriptase (Promega, Southampton, UK) using manufacturer’s instructions. cDNA was quantified by reverse transcription–quantitative polymerase chain reaction (Rotor Gene 3000; Corbett Research, St. Neots, UK) using SYBR Green PCR Master Mix Reagent (Qiagen) and gene-specific primer sets for CCL11, p65, and 18S (Qiagen).
ChIP Assay
Cells were fixed in 1% formaldehyde for 10 minutes and DNA fragmented by sonication (5 × 10 s pulses). After adding ChIP dilution buffer, 4 μg of antibody was added to precleared chromatin solution overnight. Antibody/DNA complexes were captured, washed, eluted, and reverse cross-linked. Both the DNA and input fractions were purified by phenol/chloroform wash and ethanol precipitation. The precipitated DNA was resuspended and quantitative PCR was performed using primers for the CXCL11 promoter region.
Western Blotting
Forty micrograms of protein from each sample was loaded for electrophoresis. For protein from whole-cell lysates, the transfer membrane was incubated with a rabbit anti-phospho-GR antibody, followed by anti-rabbit-HRP antibody. Antibody-bound proteins were visualized by ECL or ECL plus (GE Healthcare, Little Chalfont, UK). The membrane was then reprobed with a rabbit anti-total GR antibody or with mouse anti-β-actin monoclonal antibody to control for protein loading. For proteins from cytoplasmic or nuclear extracts, the membrane was incubated with rabbit anti-GR p65 ant, and with α-tubulin or TATA-box binding protein (TBP) (to control for protein loading). Relevant band intensities were quantified by scanning densitometric analysis.
Statistical Analysis
Repeated measures analysis of variance with Dunnett multiple comparison test was used for intragroup analysis. Mann-Whitney test or Kruskal-Wallis test with Dunn multiple comparison was used to compare results between the groups. P less than 0.05 was taken as significant.
Results
Participant Characteristics
Subjects with severe asthma had a longer duration of asthma, used higher doses of inhaled CS, and had a lower FEV1 (% predicted) and increased obstruction (as determined by a reduced FEV1/FVC% ratio) compared with subjects with nonsevere asthma. Six out of eight patients with severe asthma were on a daily dose of oral prednisolone (Table 1).
Expression and Nuclear Translocation of GR
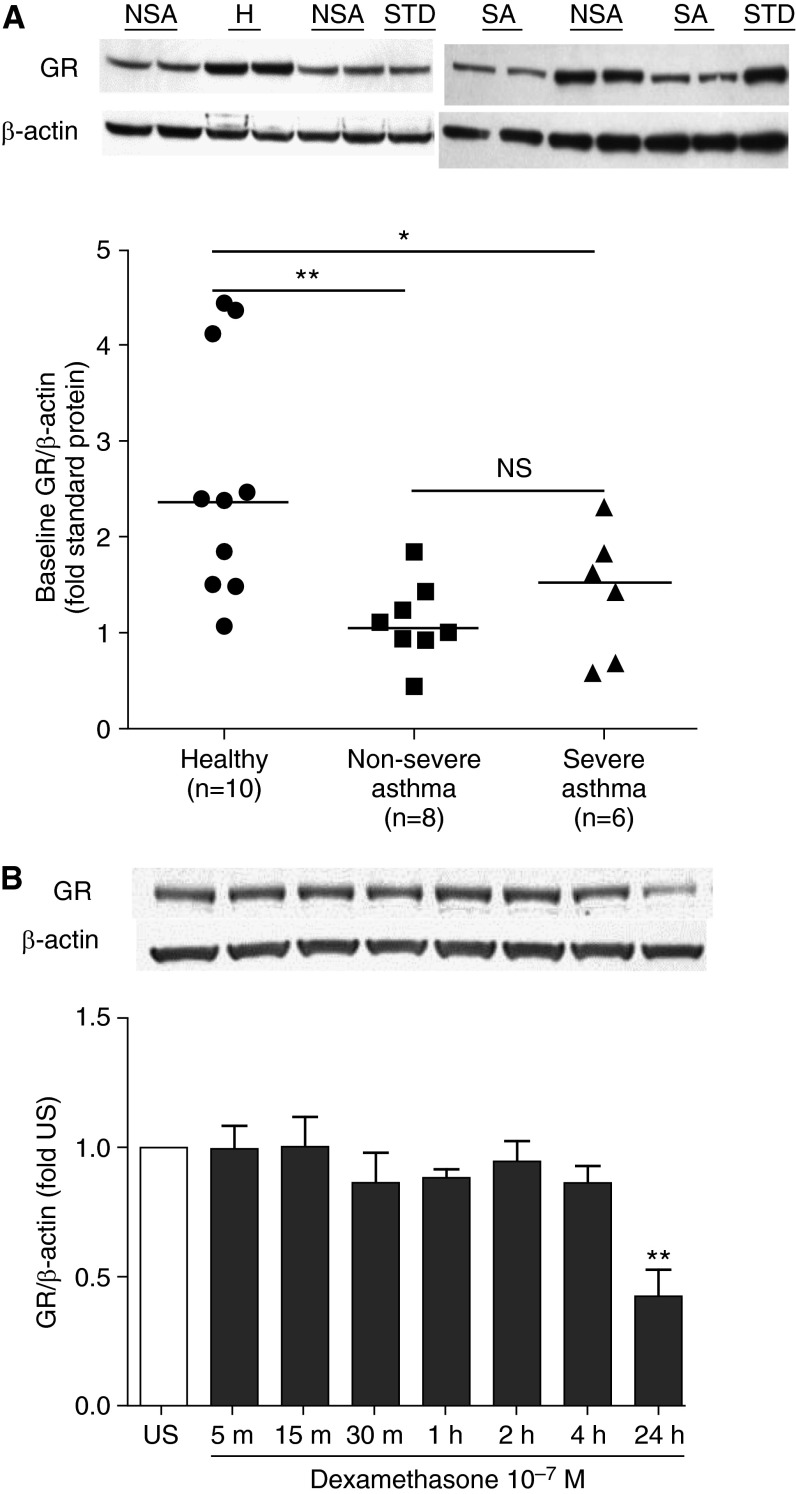
Baseline GR expression, measured by Western blot, was lower in ASMC of subjects with severe asthma (Kruskal-Wallis, post hoc test, P < 0.01) and nonsevere asthma (Kruskal-Wallis, post hoc test, P < 0.05) compared with healthy subjects, and there was no difference between nonsevere and severe asthma (Figure 1A). Dexamethasone-induced nuclear translocation of GR in ASMC was confirmed by Western blot (see Figure E1 in the online supplement), and dexamethasone did not influence GR expression up to 4 hours post-stimulation (Figure 1B). To compare nuclear translocation induced by dexamethasone in ASMC, cells were stimulated with dexamethasone (10−7 M) for 30 minutes to 4 hours. The quantity of nuclear GR at baseline was similar among ASMC of healthy subjects and patients with nonsevere and severe asthma (Figure 2A). In ASMC of healthy subjects and patients with nonsevere asthma, dexamethasone induced a threefold increase in nuclear GR abundance at 30 minutes, which was maintained at 1 hour post-stimulation, followed by a gradual decrease to 50% of induced levels at the 4-hour time point. In contrast, in ASMC of patients with severe asthma, nuclear GR was induced by less than twofold, which was significantly less than that observed in healthy subjects and patients with nonsevere asthma (Figure 2B).
Figure 1.
Comparison of glucocorticoid receptor (GR) expression at baseline and effect of dexamethasone on GR expression. (A) Baseline GR expression in airway smooth muscle cells (ASMC) of healthy subjects (H) and patients with nonsevere asthma (NSA) and severe asthma (SA). After serum starvation for 24 hours, GR and β-actin protein in whole-cell lysates from ASMC of healthy subjects and subjects with nonsevere and severe asthma were measured in duplicate along with a standard (STD) protein by Western blot followed by densitometric analysis. Representative blots are shown. Further analysis shows the comparison of baseline GR expression between the patients with nonsevere and severe asthma. Horizontal lines represent median. Kruskal-Wallis, post hoc test: *P < 0.05; **P < 0.01. NS = not significant. (B) Effect of dexamethasone on GR expression. ASMC were treated with dexamethasone (10−7 M) for times indicated. GR and β-actin protein in the whole-cell lysates were measured by Western blot followed by densitometric analysis. Bars represent mean ± SEM from ASMC of three healthy subjects. **P < 0.01 versus unstimulated (US).
Figure 2.
Impaired nuclear translocation of glucocorticoid receptor (GR) in airway smooth muscle cells (ASMC) of patients with severe asthma. (A) Nuclear GR expression at baseline. After serum starvation for 24 hours, GR and TATA-box binding protein (TBP) in nuclear extracts from ASMC of healthy subjects (H) and patients with nonsevere (NSA) and severe asthma (SA) were measured in duplicate along with a standard (STD) protein by Western blot followed by densitometric analysis; representative blot is shown. Horizontal lines represent median. (B) Dexamethasone-induced nuclear translocation of GR. ASMC of healthy subjects (circles, n = 8) and patients with nonsevere (squares, n = 8) and severe asthma (triangles, n = 8) were treated with dexamethasone (10−7 M) for times indicated. GR and TBP in the nuclear extracts, as well as a standard (STD) protein, were assessed by Western blot followed by densitometric analysis. Points represent mean ± SEM. *P < 0.05 versus healthy subjects. #P < 0.05, ##P < 0.01 versus patients with nonsevere asthma. US = unstimulated.
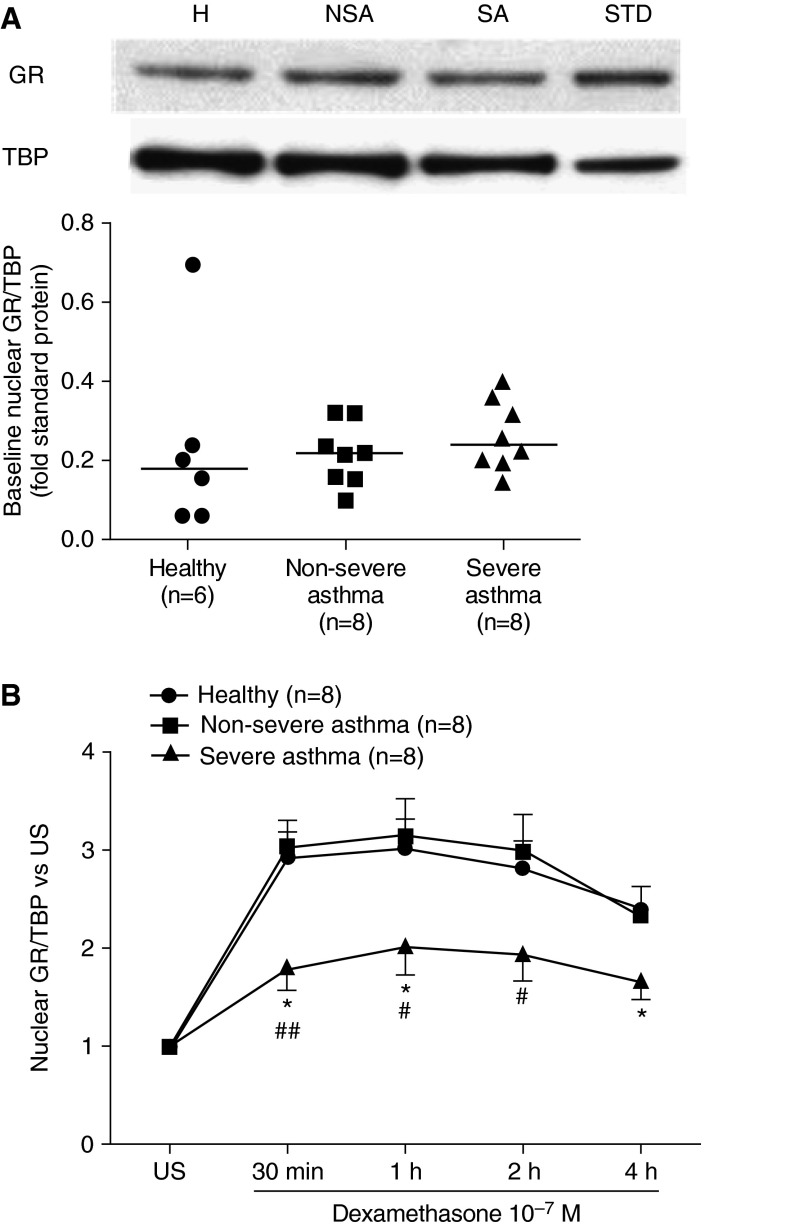
In order to translate our in vitro ASMC culture findings to those in the lung, we examined GR expression ex vivo in a limited number of biopsies from healthy subjects and subjects with nonsevere and severe asthma, using immunohistochemistry. We analyzed the relative percentage of GR-positive nuclei in airway smooth muscle bundles (Figures E2A–E2C). We show that, in support of our in vitro data, there are fewer GR-positive nuclei in biopsies samples from subjects with severe asthma compared with subjects with nonsevere asthma (Kruskal-Wallis, post hoc test, P < 0.05; Figure E2D) and normal subjects (Kruskal-Wallis, post hoc test, P < 0.05).
Phosphorylation of GR at Serine 211 by Dexamethasone
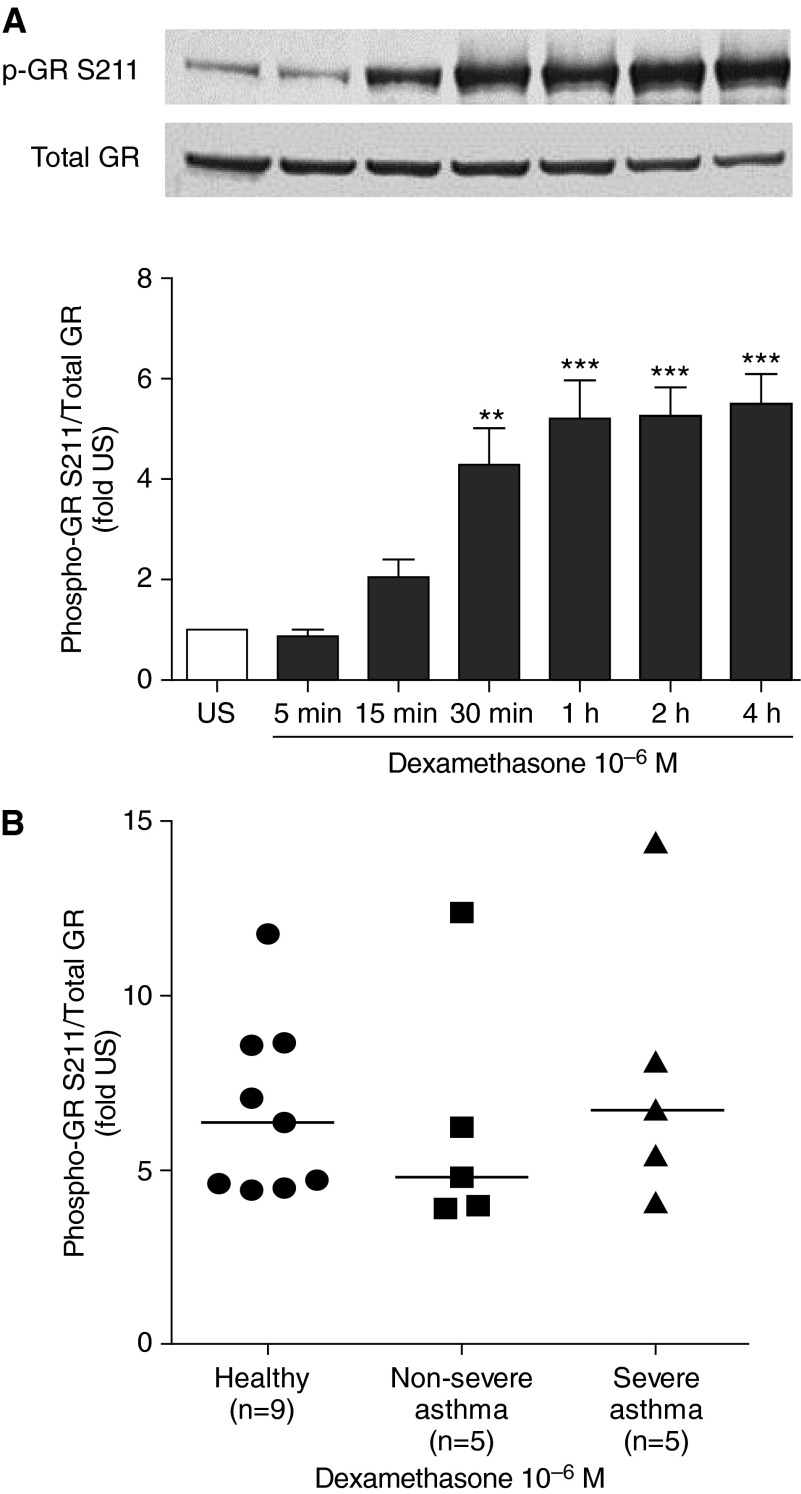
To investigate the effect of dexamethasone on phosphorylation of GR at the serine residue 211 (Ser211), ASMC of healthy subjects were stimulated with dexamethasone (10−6 M) for 5 minutes to 4 hours. GR phosphorylation was induced by dexamethasone at 30 minutes (P < 0.01), which further increased (5.2-fold) at 1 hour and was maintained at 4 hours (Figure 3A; P < 0.001). To compare dexamethasone-induced phosphorylation of GR at Ser211 between groups, cells were stimulated with dexamethasone (10−6 M) for 2 hours. In ASMC of healthy subjects, dexamethasone induced a sixfold increase in GR phosphorylation at Ser211, and this induction was similar in ASMC from patients with severe and nonsevere asthma (Figures 3B and 3C).
Figure 3.
Comparison of dexamethasone-induced glucocorticoid receptor (GR) phosphorylation at serine 211. (A) Time-dependent, dexamethasone-induced, phosphorylation of GR Ser211. After serum starvation for 24 hours, airway smooth muscle cells (ASMC) of healthy subjects were treated with dexamethasone (10−6 M) for times indicated. A representative blot is shown. Bars represent mean ± SEM from three ASMC of healthy subjects. **P < 0.01, ***P < 0.001 versus unstimulated (US). (B) Comparison of GR Ser211 phosphorylation. ASMC of healthy subjects and patients with nonsevere and severe asthma were treated with dexamethasone (10−6 M) for 2 hours. Phosphorylated (Ser211) and total GR protein in whole-cell lysates were measured by Western blot followed by densitometric analysis. Horizontal lines represent median.
Nuclear Translocation of p65 and Effect of Dexamethasone
At 24 hours, TNF-α induced an approximately twofold increase in p65 mRNA expression in ASMC of healthy subjects and subjects with nonsevere asthma compared with 5.6-fold in severe asthma (Figure E3A; P < 0.01). TNF-α–induced nuclear translocation of p65 was also examined (Figure E3B). The total quantity of nuclear p65 (Figure E3C) and the time course of TNF-α–induced p65 nuclear abundance in ASMC of patients with nonsevere and severe asthma and healthy subjects (Figure E3D) was the same. In ASMC of healthy subjects, dexamethasone suppressed TNF-α–induced p65 protein expression by 21.3% at 24 hours (P < 0.05; Figure E4A), and similar effect was observed in ASMC of patients with nonsevere asthma (P < 0.001) and severe asthma (P < 0.01). TNF-α–induced p65 mRNA was inhibited in a concentration-dependent manner in all three groups (Figure E4B), with maximal suppression of ∼50% by dexamethasone at 10−7 and 10−6 M (P < 0.01, respectively). We also investigated the effect of dexamethasone on nuclear translocation of p65 induced by TNF-α in ASMC of healthy subjects (Figure E4C) and patients with nonsevere asthma (Figure E4D). TNF-α increased the nuclear abundance of p65, but this was not affected by dexamethasone.
Effect of Dexamethasone on p65 Recruitment to the CCL11 Promoter
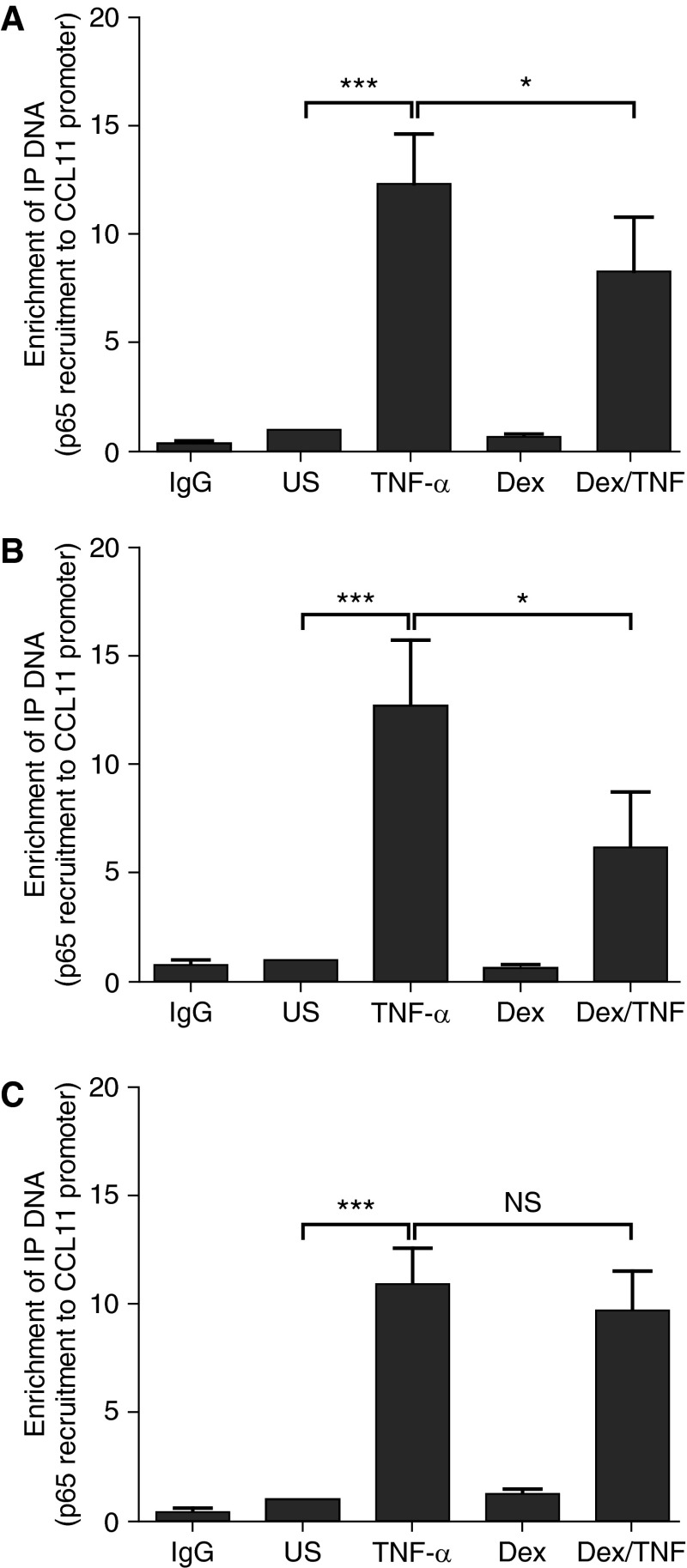
Having previously demonstrated that dexamethasone attenuates the recruitment of p65 to the fractalkine gene promoter in lung epithelial cells (21), we investigated the effect of dexamethasone on induced p65 recruitment to the gene promoter of CCL11 in ASMC. Cells were pretreated with dexamethasone (10−7 M) for 2 hours and then stimulated with TNF-α (10 ng/ml) for 1 hour. In ASMC of healthy subjects, TNF-α induced an 11.4-fold increase in the recruitment of p65 (P < 0.001), which was suppressed by dexamethasone by 52.7% (Figure 4A; P < 0.05). This effect was similar in ASMC of patients with nonsevere asthma, where dexamethasone suppressed TNF-α–induced p65 recruitment by 51.4% (Figure 4B). In contrast, the induced p65 recruitment to the gene promoter was not attenuated by dexamethasone in ASMC of severe asthma (Figure 4C). We also compared the effect of dexamethasone on p65 recruitment to the CCL11 (eotaxin) gene promoter in ASM cells from patients with severe asthma and nonsevere asthma. Our results show a significant difference in the percentage reduction in p65 recruitment mediated by dexamethasone between nonsevere asthma and severe asthma, (52 ± 12% vs. 13 ± 8%, respectively; P < 0.05).
Figure 4.
Impaired dexamethasone mediated attenuation of tumor necrosis factor (TNF)-α–induced p65 recruitment to the CCL11 promoter in airway smooth muscle cells (ASMC) of patients with severe asthma. After serum starvation for 24 hours, ASMC were pretreated with dexamethasone (10−7 M) for 2 hours and stimulated with TNF-α (10 ng/ml) for 1 hour. p65 recruitment to the CCL11 promoter in ASMC of (A) healthy subjects and (B) patients with nonsevere and (C) severe asthma was measured by chromatin immunoprecipitation assay. Bars represents mean ± SEM. Dex = dexamethasone; NS = not significant; US = unstimulated. *P < 0.05, ***P < 0.001.
Discussion
In light of our recent observation of CS insensitivity in ASM of patients with severe asthma (3), we report that GR expression in severe and nonsevere asthma is reduced compared with that in the healthy subjects. Furthermore, dexamethasone-induced GR nuclear translocation is attenuated in patients with severe asthma compared with that in healthy subjects or patients with nonsevere asthma. Baseline levels of nuclear GR were similar in the three groups. TNF-α induced greater p65 mRNA expression in patients with severe asthma, whereas baseline and TNF-α–induced nuclear translocation and dexamethasone-mediated suppression of p65 expression were similar in all groups. Dexamethasone, although not modulating TNF-α–induced p65 nuclear translocation, attenuated p65 recruitment to the CCL11 promoter in healthy subjects and patients with nonsevere asthma. However, this suppressive effect was impaired in severe asthma. Thus, decreased GR expression with impaired nuclear translocation in ASMC, associated with reduced dexamethasone-mediated attenuation of p65 recruitment to gene promoters, underlies the mechanism of corticosteroid insensitivity in severe asthma, and this may contribute to the chronic inflammation observed in this disease (see Figure 5).
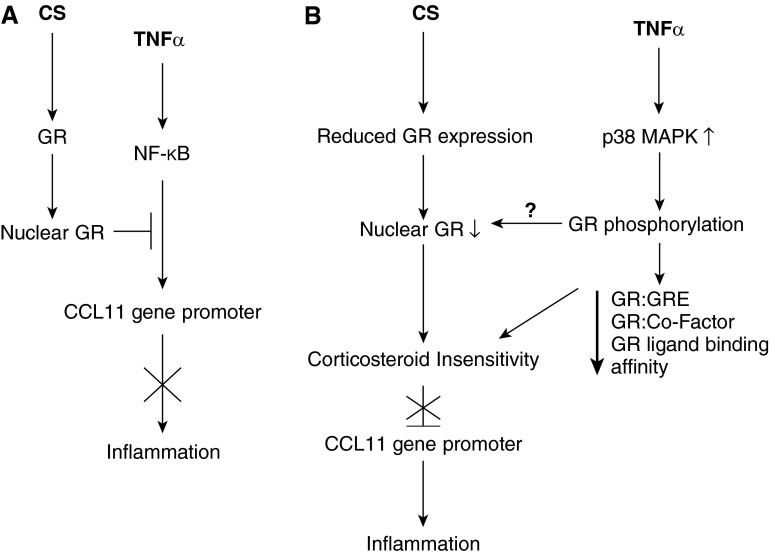
Figure 5.
Model of corticosteroid insensitivity in severe asthma. In nonsevere asthma (A) tumor necrosis factor (TNF)-α–induced inflammation is suppressed by corticosteroids (CS) through glucocorticoid receptor (GR)-mediated attenuation of nuclear factor (NF)-κB recruitment to the CCL11 promoter. In severe asthma (B), reduced GR expression and impaired nuclear translocation results in CS insensitivity and an inability to inhibit inflammation. p38 mitogen-activated protein kinase (MAPK)-mediated phosphorylation of GR may also affect GR nuclear translocation.
A reduction in GR may account for the failure of corticosteroid suppression of serum-induced ASMC proliferation in asthma (3, 22). In contrast, equivalent GR expression in ASMC of subjects with nonsevere and severe asthma is supported by reports of similar levels of mRNA expression of either GRα or GRβ in peripheral lymphocytes of patients with severe or moderate asthma (23) and similar protein and mRNA expression of both GRα and GRβ in peripheral blood mononuclear cells (PBMC) of subjects with steroid-dependent well-controlled asthma (24). The abundance of GR protein is reduced by dexamethasone at 24 hours post stimulation, indicative of a down-regulatory effect of glucocorticoids on GR expression. GR mRNA is negatively regulated by glucocorticoids (25), which could be attributed to their inhibitory effects at the GRE, AP-1, NF-κB, and cAMP response element-binding (CREB) regulatory motifs located in the promoter of GR (26). Alternatively, glucocorticoids may post-transcriptionally regulate the expression of GR, perhaps via destabilization of the GR mRNA (27). Ligand-activated GR protein could also be removed on prolonged exposure to glucocorticoids by the proteasome-ubiquitin degradation pathway (28).
Nuclear translocation of GR is the critical step in corticosteroid-mediated antiinflammatory effects. Based on our observation that total GR expression is not influenced by dexamethasone, we surmised that the increase in nuclear GR abundance is a direct consequence of GR nuclear translocation. However, the reduced dexamethasone-induced nuclear translocation of GR in ASMC of severe asthma, which extends the observation from peripheral blood mononuclear cells (29, 30) to airway resident cells, could underlie the mechanism of corticosteroid insensitivity in severe asthma.
Our study shows a reduction in GR expression in ASMC of patients with severe and nonsevere asthma, and yet CS insensitivity is only observed in the former. CS insensitivity is influenced by many factors, not only the number of GR but also by the affinity of GR and by interference with the glucocorticoid signaling pathways (31). The low-affinity state of GR is achieved through binding of HSP70, HSP40, and HSP70–HSP90 organizing protein (HOP) (32), whereas a switch to a high-affinity state occurs on binding of HSP90 and p23. A reduced HSP90:GR ratio has been reported in PBMC from patients with glucocorticoid-resistant asthma (33). Increased expression of the cochaperone FKB51, reported in PBMC of severe asthma, could also reduce ligand affinity of GR (34). Transcriptional activity of GR can also be impaired via direct interaction with cytokine-induced proinflammatory transcriptional factors such as NF-κB, which prevents GR:GRE binding, which is increased in severe asthma.
The defective GR nuclear translocation in CS-insensitive severe asthma may be partly attributed to hyperphosphorylation of GR (30), and this can also contribute to impaired effect of CS as a result of either decreased GR–GRE binding (35) or failure to suppress histone acetyltransferase activity induced by inflammatory stimuli (29). GR phosphorylation can be mediated by MAPK p38, c-Jun N-terminal kinase (JNK), or extracellular signal–regulated kinase (ERK) (31). We have reported heightened p38 MAPK activity in alveolar macrophages (2) and ASMCs (3) of patients with severe asthma, and, in addition, we have shown that inhibition of p38 MAPK leads to reversal of CS insensitivity in ASM and alveolar macrophages from patients with severe asthma (3, 16). This raises the possibility that p38 inhibition may improve corticosteroid responsiveness in severe asthma by reversal of defective nuclear translocation of GR, as demonstrated in IL2- and IL4-induced CS-resistant T cells (36). Phosphorylation of GR at serine 211 has been proposed as a marker for the transcriptional potential of GR (37). Absence of differential degrees of phosphorylation at Ser211 between the asthmatic groups indicates that this residue may not directly contribute to corticosteroid insensitivity in severe asthma.
TNF-α–induced expression of the p65 and p50 components of NF-κB is suppressed by dexamethasone in ASMC of healthy subjects at both protein and mRNA levels (38), which we now demonstrate in ASMC of patients with asthma. However, whereas corticosteroid insensitivity is displayed in ASMC of severe asthma, in terms of impaired suppression of the NF-κB–dependent genes such as CCL11 and CXCL8 (3), the suppressive effect of dexamethasone on p65 expression is similar between the normal and asthma groups. This suggests that reduced response to CS in severe asthma does not extend to impaired suppression of total NF-κB expression.
The inability of dexamethasone to inhibit induced p65 nuclear translocation is consistent with reports in the literature in both ASMC (14) and in human lung epithelial cells (21). It is also reported that dexamethasone does not attenuate either TNF-α–induced NF-κB DNA binding or NF-κB–mediated reporter activity in ASMC (39). However, TNF-α–induced p65 recruitment to the CCL11 promoter in AMSC from healthy subjects is attenuated by dexamethasone. This is associated with reduced acetylation of histone H4, resulting in condensation of chromatin and subsequent hindered access of p65 to DNA (14). However, this effect of dexamethasone is impaired in ASMC from subjects with severe asthma, and this suggests a possible mechanism by which CS fail to suppress NF-κB–medicated inflammatory genes, such as CCL11 and CXCL8, in ASMC from patients with severe asthma (3). These results provide a better mechanistic understanding of CS insensitivity observed in airway smooth muscle cells obtained from patients with severe asthma. We have shown an attenuation of the suppression of p65 recruitment by dexamethasone associated with a reduced translocation of GR from the cytoplasm to the nucleus in ASMC from subjects with severe asthma compared with subjects with nonsevere asthma. This observation is likely to explain the poor therapeutic effects of corticosteroid therapy in patients with severe asthma, where the magnitude of the reduction in p65 recruitment to proinflammatory gene promoters caused by CS determines their effects in controlling asthmatic inflammation.
Decreased GR expression with impaired nuclear translocation and subsequent inability to suppress p65 recruitment to the gene promoters contribute to the defective corticosteroid suppression of NF-κB–mediated chemokine expression in ASMC of subjects with severe asthma. These mechanisms may underlie CS insensitivity in severe asthma.
Acknowledgments
Acknowledgment
The authors thank Florence Chow and Sally Meah for the recruitment of patients.
Footnotes
Supported by Wellcome Trust grant 085935 (K.F.C.), Asthma UK grant 08/041 (K.F.C.), Chang Gung Memorial Hospital and College of Medicine grant CMRPG371781 (P.-J.C.), and by the Respiratory Disease Biomedical Research Unit at the Royal Brompton NHS Foundation Trust and Imperial College London. K.F.C. is a Senior Investigator of National Institute for Health Research, UK.
Author Contributions: P.-J.C. planned and performed all the experiments and wrote the first draft. C.M. supervised the laboratory studies and, with J.B., grew the smooth muscle cells from bronchial biopsies. N.S. performed additional experiments. J.Z. performed the immunohistochemistry. P.K.B. and K.F.C. devised the study, discussed the findings, and finalized the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201402-0314OC on November 20, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 3.Chang PJ, Bhavsar PK, Michaeloudes C, Khorasani N, Chung KF.Corticosteroid insensitivity of chemokine expression in airway smooth muscle of patients with severe asthma J Allergy Clin Immunol 2012130877–885.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 5.Macedo P, Hew M, Torrego A, Jouneau S, Oates T, Durham A, Chung KF. Inflammatory biomarkers in airways of patients with severe asthma compared with non-severe asthma. Clin Exp Allergy. 2009;39:1668–1676. doi: 10.1111/j.1365-2222.2009.03319.x. [DOI] [PubMed] [Google Scholar]
- 6.Begueret H, Berger P, Vernejoux JM, Dubuisson L, Marthan R, Tunon-de-Lara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305:L912–L933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–1032. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 9.Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007;21:2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 10.Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011;31:4663–4675. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adcock IM, Ito K, Barnes PJ. Glucocorticoids: effects on gene transcription. Proc Am Thorac Soc. 2004;1:247–254. doi: 10.1513/pats.200402-001MS. [DOI] [PubMed] [Google Scholar]
- 12.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-κ B and STAT6 in human airway epithelial cells. J Immunol. 1999;163:6876–6883. [PubMed] [Google Scholar]
- 14.Nie M, Knox AJ, Pang L. β2-Adrenoceptor agonists, like glucocorticoids, repress eotaxin gene transcription by selective inhibition of histone H4 acetylation. J Immunol. 2005;175:478–486. doi: 10.4049/jimmunol.175.1.478. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006;117:522–543. doi: 10.1016/j.jaci.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Bhavsar P, Khorasani N, Hew M, Johnson M, Chung KF. Effect of p38 MAPK inhibition on corticosteroid suppression of cytokine release in severe asthma. Eur Respir J. 2010;35:750–756. doi: 10.1183/09031936.00071309. [DOI] [PubMed] [Google Scholar]
- 17.Chang P-J, Baker J, Chung KF, Bhavsar PK. Corticosteroid insensitivity in severe asthma: impaired nuclear translocation of glucocorticoid receptor in airway smooth muscle cells. Eur Respir J. 2012;40:615s. doi: 10.1164/rccm.201402-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmon M, Liu YC, Mak JCW, Rousell J, Huang TJ, Hisada T, Nicklin PL, Chung KF. Contribution of upregulated airway endothelin-1 expression to airway smooth muscle and epithelial cell DNA synthesis after repeated allergen exposure of sensitized Brown-Norway rats. Am J Respir Cell Mol Biol. 2000;23:618–625. doi: 10.1165/ajrcmb.23.5.3909. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 20.Oltmanns U, Walters M, Sukkar M, Xie S, Issa R, Mitchell J, Johnson M, Chung KF. Fluticasone, but not salmeterol, reduces cigarette smoke-induced production of interleukin-8 in human airway smooth muscle. Pulm Pharmacol Ther. 2008;21:292–297. doi: 10.1016/j.pupt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-kappaB. FASEB J. 2008;22:1807–1816. doi: 10.1096/fj.07-094235. [DOI] [PubMed] [Google Scholar]
- 22.Roth M, Johnson PR, Borger P, Bihl MP, Rüdiger JJ, King GG, Ge Q, Hostettler K, Burgess JK, Black JL, et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med. 2004;351:560–574. doi: 10.1056/NEJMoa021660. [DOI] [PubMed] [Google Scholar]
- 23.Jakieła B, Bochenek G, Sanak M. Glucocorticoid receptor isoforms in steroid-dependent asthma. Pol Arch Med Wewn. 2010;120:214–222. [PubMed] [Google Scholar]
- 24.Gagliardo R, Chanez P, Vignola AM, Bousquet J, Vachier I, Godard P, Bonsignore G, Demoly P, Mathieu M. Glucocorticoid receptor alpha and beta in glucocorticoid dependent asthma. Am J Respir Crit Care Med. 2000;162:7–13. doi: 10.1164/ajrccm.162.1.9911032. [DOI] [PubMed] [Google Scholar]
- 25.Shimojo M, Hiroi N, Yakushiji F, Ueshiba H, Yamaguchi N, Miyachi Y. Differences in down-regulation of glucocorticoid receptor mRNA by cortisol, prednisolone and dexamethasone in HeLa cells. Endocr J. 1995;42:629–636. doi: 10.1507/endocrj.42.629. [DOI] [PubMed] [Google Scholar]
- 26.Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol. 2006;102:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Vedeckis WV, Ali M, Allen HR. Regulation of glucocorticoid receptor protein and mRNA levels. Cancer Res. 1989;49(8) Suppl:2295s–2302s. [PubMed] [Google Scholar]
- 28.Alarid ET. Lives and times of nuclear receptors. Mol Endocrinol. 2006;20:1972–1981. doi: 10.1210/me.2005-0481. [DOI] [PubMed] [Google Scholar]
- 29.Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–1108. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Mercado N, To Y, Kobayashi Y, Adcock IM, Barnes PJ, Ito K. p38 mitogen-activated protein kinase-γ inhibition by long-acting β2 adrenergic agonists reversed steroid insensitivity in severe asthma. Mol Pharmacol. 2011;80:1128–1135. doi: 10.1124/mol.111.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Grad I, Picard D. The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol. 2007;275:2–12. doi: 10.1016/j.mce.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Qian X, Zhu Y, Xu W, Lin Y. Glucocorticoid receptor and heat shock protein 90 in peripheral blood mononuclear cells from asthmatics. Chin Med J (Engl) 2001;114:1051–1054. [PubMed] [Google Scholar]
- 34.Chun E, Lee HS, Bang BR, Kim TW, Lee SH, Kim JH, Cho SH, Min KU, Kim YY, Park HW. Dexamethasone-induced FKBP51 expression in peripheral blood mononuclear cells could play a role in predicting the response of asthmatics to treatment with corticosteroids. J Clin Immunol. 2011;31:122–127. doi: 10.1007/s10875-010-9463-9. [DOI] [PubMed] [Google Scholar]
- 35.Adcock IM, Lane SJ, Brown CR, Peters MJ, Lee TH, Barnes PJ. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J Immunol. 1995;154:3500–3505. [PubMed] [Google Scholar]
- 36.Goleva E, Li LB, Leung DY. IFN-gamma reverses IL-2- and IL-4-mediated T-cell steroid resistance. Am J Respir Cell Mol Biol. 2009;40:223–230. doi: 10.1165/rcmb.2007-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck IME, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30:830–882. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of CD38 expression by tumor necrosis factor-alpha in human airway smooth muscle cells: role of NF-kappaB and sensitivity to glucocorticoids. FASEB J. 2006;20:1000–1002. doi: 10.1096/fj.05-4585fje. [DOI] [PubMed] [Google Scholar]
- 39.Amrani Y, Lazaar AL, Panettieri RA., Jr Up-regulation of ICAM-1 by cytokines in human tracheal smooth muscle cells involves an NF-κ B-dependent signaling pathway that is only partially sensitive to dexamethasone. J Immunol. 1999;163:2128–2134. [PubMed] [Google Scholar]