Abstract
Alcohol consumption exhibits diverse effects on different types of immune cells. NKT cells are a unique T cell population and play important immunoregulatory roles in different types of immune responses. The effects of chronic alcohol consumption on NKT cells remain to be elucidated. Using a mouse model of chronic alcohol consumption, we found that alcohol increases the percentage of NKT cells, especially iNKT cells in the thymus and liver, but not in the spleen or blood. Alcohol consumption decreases the percentage of NK1.1− iNKT cells in the total iNKT cell population in all of the tissues and organs examined. In the thymus, alcohol consumption increases the number of NK1.1+CD44hi mature iNKT cells, but does not alter the number of NK1.1− immature iNKT cells. A BrdU incorporation assay shows that alcohol consumption increases the proliferation of thymic NK1.1− iNKT cells, especially the NK1.1− CD44lo stage I iNKT cells. The percentage of NKG2A+ iNKT cells increases in all of the tissues and organs examined; whereas, CXCR3+ iNKT cells only increases in the thymus of alcohol-consuming mice. Chronic alcohol consumption increases the percentage of IFN-γ-producing iNKT cells and increases the blood concentration of IFN-γ and IL-12 after in vivo α-galactosylceramide (αGalCer) stimulation. Consistent with the increased cytokine production, in vivo activation of iNKT cells also enhances the activation of dendritic cells (DC) and NK, B and T cells in the alcohol-consuming mice. Taken together the data indicate that chronic alcohol consumption enhances iNKT cell maturation and activation, which favors the Th1 immune response.
Keywords: Alcohol, NKT cells, proliferation, Chemokines, Th1 response
INTRODUCTION
NKT cells are a unique population of T cells, and most of these cells express the NK cell receptor, NK1.1. For that reason, they are called NKT cells. Unlike conventional αβ T cells, which recognize peptide antigens presented by MHC I and MHC II molecules, NKT cells only recognize lipid antigens presented by the non-classic MHC I molecule, CD1d. Thus, NKT cells are CD1-restricted T cells. The major population of NKT cells expresses an invariant T cell receptor (TCR) alpha chain, which is Vα14Jα18 in mice and Vα24Jα18 in humans. These NKT cells are designated as invariant NKT (iNKT) or type I NKT cells. Type II NKT cells comprise the remainder of the NKT cells, and they exhibit diverse TCR (Bendelac et al., 2007).
Although NKT cells are a small population of lymphocytes, they comprise many functionally distinct subsets (Godfrey et al.). One of the unique features for iNKT cells is that they exhibit a broad cytokine profile. They can produce Th1 cytokines such as IFN-γ and TNF-α; Th2 cytokines including IL-4, IL-10, and IL-13; and the Th17 cytokines, (Coquet et al., 2008; Watarai et al., 2012). Due to the broad spectrum of cytokines produced, these cells play important roles in the regulation of immune responses associated with antitumor immunity, autoimmune diseases, and liver injury (Hong et al., 2001; Jahng et al., 2001; Crowe et al., 2005; Terabe and Berzofsky, 2008; Park et al., 2009; Jin et al., 2011; Kumar, 2013; Wen et al., 2013). iNKT cells are more like immunoregulatory cells than immune effector cells. Once activated, they quickly produce large amount of cytokines, chemokines, and effector molecules, which further shape the downstream innate and adaptive immune responses. Consequently, these cells act more like innate immune cells than adaptive immune cells, and they form a bridge between innate and adaptive immunity (Brennan et al., 2013).
Like conventional T cells, iNKT cells develop in the thymus (Benlagha et al., 2002). Based on the expression of the cell surface markers, NK1.1 and CD44, the development of iNKT cells in the thymus can be divided into three developmental stages: stage I: NK1.1−CD44lo, stage II: NK1.1−CD44hi, and stage III: NK1.1+CD44hi. The stage I and II iNKT cells are immature iNKT cells and the stage III are mature iNKT cells. iNKT cells egress from the thymus to the periphery at stage II where they further mature into stage III in the peripheral organs (Benlagha et al., 2002; Pellicci et al., 2002). Some of the iNKT cells stay in the thymus and mature into stage III cells (Benlagha et al., 2002; Berzins et al., 2006). iNKT cells distribute into many lymphoid and non-lymphoid organs and tissues including the spleen, lymph nodes (LN), bone marrow (BM), lungs, and liver. Around 10–30% of liver lymphocytes are NKT cells. Most of the iNKT cells in the lymph nodes are NK1.1− (Brennan et al., 2013).
The development and maturation of iNKT cells require the activation of TCR stimulated by endogenous lipids. It still remains unknown what specific mammalian endogenous lipids work as self-antigens to control iNKT cell development and activation, although several mammalian lipids were identified as agonists of iNKT cells. Ganglioside GD3 produced by some cancer cells can modulate iNKT cell activation (Wu et al., 2003; Webb et al., 2012).
Isoglobotrihexosylceramide (iGB3) can activate iNKT cells and was proposed as mammalian endogenous self-antigen that governs iNKT cell development (Zhou et al., 2004). Further research indicated that iGB3 is not crucial for iNKT cell development and maturation (Porubsky et al., 2007; Porubsky et al., 2012). β-D-glucopyranosylceramide, a glycosphingolipid, was identified as a potent endogenous agonist of iNKT cells in mouse and human (Brennan et al., 2011). The production of this lipid was up-regulated during the microbe infection (Brennan et al., 2011). Recently, thymic peroxisome-derived ether-bounded mono-alkyl glycerophosphates lipids were found to play important roles in iNKT cell development and maturation as well as the control of peripheral iNKT cell numbers (Facciotti et al., 2012). These plasmalogen lipids could be the natural ligands of iNKT cell receptors that control iNKT cell development and maturation in vivo.
One marine sponge-derived lipid, α-galactosylceramide (αGalCer), is a potent agonist of iNKT cell TCR (Kawano et al., 1997). Studies on iNKT cell biological function have heavily relied on the activation of these cells through αGalCer stimulation. Once activated by αGalCer, iNKT cells rapidly produce a large amount and a broad spectrum of cytokines. These cytokines subsequently activate other immune cells such as dendritic cells (DC), NK, T, and B cells. The balance between the Th1 and Th2 cytokines produced by iNKT cells determines the downstream immune response. If iNKT cells produce Th1 dominant cytokines, they will activate DC to produce IL-12, which, in turn, will activate NK, NKT, and T cells to produce IFN-γ, and induce Th1 immune responses (Fujii et al., 2003). If iNKT cells produce Th2 dominant cytokines, such as IL-4 and IL-10, they will induce DC to produce IL-10, which inhibits Th1 immune responses, and enhances Th2 immune responses (Kojo et al., 2005). Activated iNKT cells produce IFN-γ, which triggers the MHC/NKG2A inhibitory signaling pathway to prevent further activation of iNKT cells (Ota et al., 2005). Repeat activation of iNKT cells with αGalCer leads to iNKT cell anergy (Chang et al., 2008; Parekh et al., 2009). The anergic iNKT cells temporarily lose the function to produce IFN-γ, but still retain the ability to produce IL-4. As a result, anergic iNKT cells exhibit a Th2-dominant cytokine profile (Parekh et al., 2005).
A large body of research indicates, convincingly, that chronic alcohol consumption increases the incidence of infectious diseases, cancer, and liver injury (Takada and Tsutsumi, 1995; Nelson and Kolls, 2002; Horie et al., 2003; Miller et al., 2011; Nelson et al., 2013). Deterioration of the immune system likely plays a key role in these illnesses in alcoholics. The effects of chronic alcohol consumption on NKT cells are largely unexplored as compared to the intensive studies on the effects of alcohol on other immune cells. Although it is reported that alcohol consumption increases NKT cells in the liver (Minagawa et al., 2004), the underlying mechanism is not known. Using a murine chronic alcohol consumption model that does not induce liver injury, we found that alcohol not only increases iNKT cells in the liver, but also in the thymus. Alcohol consumption enhances immature iNKT cell proliferation and maturation in the thymus, and increases IFN-γ-producing iNKT cells. In vivo activation of iNKT cells induces a Th1-dominant immune response.
MATERIALS AND METHODS
Animals and alcohol administration
Female C57BL/6 mice, at 6–7 weeks of age, were purchased from Charles River laboratories (Wilmington, MA). Breeders of IFN-γ knockout (KO) mice with a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). The KO mice were bred and maintained in the Wegner Hall Vivarium, College of Pharmacy, Washington State University, which is accredited by the American Association for Assessment and Accreditation of Laboratory Animal Care. Only female offspring were used in experiments. Mice in experiments were single-housed in plastic cages with microfilter tops and allowed free access to Rodent Lab chow 5001 and sterilized Milli-Q water. Mice were randomly divided into two groups after one week of acclimation to the new environment. One group was provided 20% w/v alcohol (Everclear, St. Louis, MO) as the sole drinking fluid, while the other group continued to be given Milli-Q water as a control. Both groups were allowed free access to chow. Mice were used in experiments after 3–6 months of alcohol consumption, which is a time frame when the immune responses are relatively stable (Zhang and Meadows, 2008). In this model, mice consume at least 30% of their caloric intake from alcohol, the blood concentration of alcohol is around 0.03%, and no liver injury is observed in the alcohol-consuming mice (Blank et al., 1991). All of the experimental protocols were approved by the Institutional Animal Care and Use Committee at Washington State University.
Antibodies and reagents
The following anti-mouse monoclonal antibodies used in the experiments were conjugated with PE, FITC, PerCP, PE-Cy5.5, or allophycocyanin (APC) and were purchased from BioLegend (San Diego, CA). These include: anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD11c (N418), anti-CD19 (6D5), anti-CD44 (IM7), anti-CD69 (H1.2F3), anti-CD80 (16-10A1), anti-CD86 (GL-1), anti-NK1.1 (PK136), anti-NKG2A (16A11), anti-CCR6 (29-2L17), anti-CXCR3 (CXCR3-173), anti-CXCR4 (2B11/CXCR4), anti-IFN-γ (XGM1.2), anti-IL-4 (11B11). Anti-mouse CXCR6-PerCP (Clone 221002) was purchased from R & D systems (Minneapolis, MN). BrdU was purchased from Sigma (St. Louis, MO). Anti-BrdU antibody was purchased from eBiosciences (San Diego, CA). Mouse CD1d/PBS57-tetramer was synthesized by NIH tetramer facility (Atlanta, GA). Fixation and permeabilization wash buffer for intracellular staining was purchased from BioLegend (San Diego, CA).
Cell isolation and phenotyping
The isolation of bone marrow (BM) cells, splenocytes, thymocytes, and peripheral blood lymphocytes (PBL) followed the methods established in our laboratory as reported previously (Zhang et al., 2012). Mechanical disruption-based intrahepatic immune cell isolation followed the methods of Blom et al. (Blom et al., 2009). Multi-color flow cytometry-based cell phenotype analysis followed our previously reported methods (Zhang et al., 2012). CellQuest software (BD Biosciences, San Jose, CA) was used to analyze the data.
BrdU in vivo incorporation assay
Each mouse was injected i.p. with 2 mg of BrdU in 200 µl of sterilized PBS. Mice were euthanized 3 hr after BrdU injection. BM cells, splenocytes, thymocytes, and liver leukocytes were isolated as described above, and then suspended in PBS + 0.1% BSA. An appropriate amount of isolated cells from each organ was incubated with anti-CD16/32 antibody on ice for 5 min to block Fc receptors, followed by incubation with either anti-CD3-PerCP/CD1d-PBS57-tetramer-PE or anti-NK1.1-APC/anti-CD44-PE-Cy5.5/CD1d-PBS57-tetramer-PE at room temperature for 30 min. Cells were washed twice with 200 µl of FACS buffer (PBS + 0.1% BSA +0.1% NaN3), resuspended in 100 µl of fixation buffer, and incubated on ice for 30 min. The fixed cells were washed twice with 200 µl of permeabilization wash buffer. The cells were incubated with anti-BrdU antibody on ice for 30 min and washed twice with permeabilization wash buffer. BrdU positive cells were analyzed by flow cytometry as described above.
In vivo activation of iNKT cells by αGalCer
αGalCer was dissolved into DMSO at 1 mg/ml and stored at −20°C as a stock solution. Each mouse was injected i.p. with 4 µg of αGalCer in 200 µl of sterilized PBS. Mice were euthanized at 2 hr, 12 hr, and 24 hr after αGalCer injection. Plasma was prepared for the measurement of IL-12, IL-4 and IFN-γ production. PBL and splenocytes were isolated for the analysis of NK cell, T cell, B cell, and DC activation or intracellular cytokine staining.
Cytokine intracellular staining
IFN-γ-producing NK cells in aGalCer stimulated mice were determined by intracellular staining. For in vivo activation, mice were injected i.p. with 4 µg of αGalCer in 200 µl of sterilized PBS. At the indicated time points after αGalCer injection, splenocytes were isolated and used for cytokine intracellular staining. Freshly isolated splenocytes were incubated in RPMI 1640 medium at 37°C in a 5% CO2 incubator for 4 hr. The culture medium was supplemented with 10% FBS, 1% penicillin and 5 µg/ml Brefeldin A. After incubation, cells were collected and incubated with anti-CD16 on ice for 5 min, followed by cell surface staining with anti-CD3-PE and anti-NK1.1-PerCP for 30 min. After surface staining, cells were washed twice with FACS buffer then fixed with Cytofix/Cytoperm buffer on ice for 30 min. Next, cells were washed twice with washing buffer and stained with anti-IFN-γ-FITC for 30 min. Cytokine-producing cells were analyzed by flow cytometry using CellQuest software.
ELISA
Mouse DuoSet IFN-γ (DY485), IL-4 (DY404) ELISA kits from R & D Systems and mouse IL-12 (p70) ELISA MAX Deluxe kits from BioLegend were used to measure the concentration of IFN-γ, IL-4 and IL-12 in the plasma. The experimental procedure followed the manufacturers’ protocol.
Statistical analysis
All experiments were repeated twice with similar results. The data in the figure are from one representative experiment. Microsoft Excel and GraphPad Prism5 software were used to analyze the data. Two-tailed Student’s t-test was used to determine differences between the water-drinking and alcohol-consuming groups. Differences are considered significant when the p-value is less than 0.05.
RESULTS
Chronic alcohol consumption increases NKT cells, especially iNKT cells, in the thymus and liver but not in the spleen, or PBL
To study the effects of chronic alcohol consumption on NKT cells, we first determined if the distribution of NKT cells among different organs or tissues changed as a function of alcohol consumption. Changes in conventional NKT cells (CD3+NK1.1+) in specific organs are shown in Fig. 1A–E. Changes in iNKT cells (CD3+CD1d/PBS57-tetramer+) in the various organs are shown in Fig. 1F–J. The results indicate that chronic alcohol consumption increases the percentage of conventional NKT cells in the liver and thymus (Fig. 1 B, 1E) and not in the PBL, or spleen (Fig.1C, 1D). iNKT cells exhibit the same pattern with significant increases in these cells in the liver and thymus (Fig. 1G, 1J), and not in the PBL, or spleen (Fig. 1H, 1I). Consistent with the percentage changes, chronic alcohol consumption increases the number of iNKT cells in the liver and thymus (Figs. 1K, 1N), and not in the blood, or spleen (Figs. 1L, 1M). These results suggest that the effects of chronic alcohol consumption on iNKT cell distribution are organ specific.
Fig. 1.
Effects of chronic alcohol consumption on distribution of CD3+NK1.1+ conventional NKT cells and CD3+CD1d/PBS57-tetramer+ iNKT cells in different organs or tissues. (A) Representative dot plot showing CD3+NK1.1+ (black dots in gated region R3) conventional NKT cells in liver lymphocytes of alcohol-consuming mice, the number in the plot is the percentage of gated NKT cells in liver lymphocytes. (B–E) Percentage of CD3+NK1.1+ NKT cells in the indicated organs or tissues. (F) Representative dot plot showing CD3+CD1d/PBS57-tetramer+ iNKT cells (black dots in the gated region R2) in liver lymphocytes of alcohol-consuming mice, the number in the plot is the percentage of gated iNKT cells in liver lymphocytes. (G–J) Percentage of iNKT cells in the indicated organs or tissues. (K–N) Number of iNKT cells in the indicated organs or 700 µl of blood. Experiments were repeated twice. Water=water-drinking mice. EtOH= alcohol-consuming mice. Values are mean ± SD for groups containing 7–10 mice. **p<0.01, ***p<0.001.
Chronic alcohol consumption decreases the proportion of NK1.1− iNKT cells in liver, PBL, spleen, and thymus
NKT cells are generated in the thymus, and either stay in the thymus, or migrate to peripheral organs for further maturation. We next determined if chronic alcohol consumption affected iNKT cell maturation. NK1.1 is used as a marker to distinguish immature from mature iNKT cells (Benlagha et al., 2002; Pellicci et al., 2002). Immature iNKT cells are NK1.1− and mature iNKT cells are NK1.1+. We found that chronic alcohol consumption significantly decreases the proportion of NK1.1− iNKT cells within the iNKT population in all of the organs examined (Fig. 2). Thus, chronic alcohol consumption also increases the proportion of mature (NK1.1+) iNKT cells in these organs. We found further that chronic alcohol consumption does not significantly decreases the number of immature iNKT cells in the examined organs except for PBL, in which immature iNKT cells are significantly lower compared to water-drinking controls (Fig. 2D). Chronic alcohol consumption significantly increases the number of mature iNKT cells in the liver, BM and thymus, and there is a trend toward increasing mature iNKT cells in the spleen (Fig. 2E). The number of mature iNKT cells is not different in the PBL. These findings suggest that chronic alcohol consumption enhances iNKT cell maturation.
Fig. 2.
Chronic alcohol consumption decreases the percentage of NK1.1− iNKT cells. (A) Dot plot showing the CD3+CD1d/PBS57-tetramer+ iNKT cells (gated region R2) in splenocytes of alcohol-consuming mice. The number in the plot is the percentage of iNKT cells in splenocytes. (B) Dot plots showing NK1.1+ iNKT cells (upper right quadrant) and NK1.1− iNKT cells (lower right quadrant) in gated splenic iNKT cells. The numbers in the upper right quadrants and lower right quadrants are the percentage of NK1.1+ iNKT cells and NK1.1− iNKT cells in total iNKT cells, respectively. The upper panel is from water-drinking mice (Water) and the lower panel is from alcohol-consuming mice (EtOH). (C) Percentage of NK1.1− iNKT cells in the total iNKT cells in the indicated organs or tissues. (D) Number of NK1.1− iNKT cells in the indicated organs or tissues. (E) Number of NK1.1+ iNKT cells in the indicated organs or tissues. (F) Gated CD1d/PBS57-tetramer+ iNKT cells (gated region R2) in the thymocytes of water-drinking mice. The number is the percentage of gated tetramer+ iNKT cells in thymocytes. (G–I) Percentage of the indicated subsets of iNKT cells in the total thymic iNKT cells. (J) NK1.1− CD44lo stage I (lower left quadrant), NK1.1−CD44hi stage II (lower right quadrant), and NK1.1+CD44hi stage III iNKT cells (upper right quadrant) in the gated thymic iNKT cells. The numbers are the percentage of each stage of iNKT cells in the total iNKT cells. (K–M) Number of the indicated subsets of iNKT cells in the thymus. Experiments were repeated twice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD for groups containing 7–10 mice. *p<0.05, **p<0.01.
We next sought to determine if chronic alcohol consumption affects the status of iNKT development and maturation in the thymus. We found that chronic alcohol consumption decreased the percentage of immature iNKT cells in stage I (NK1.1−CD44lo) (Fig. 2G) and stage II (NK1.1−CD44hi) (Fig. 2H), and slightly increased the proportion of mature iNKT cells in stage III (NK1.1+CD44hi) (Fig. 2I) in the total thymic iNKT cells. While, chronic alcohol consumption significantly increased the number of total iNKT cells in the thymus (Fig. 1N), the number of stage I and stage II iNKT cells is not different between water-drinking and alcohol-consuming mice (Fig. 2K, 2L). However, the number of iNKT cells in stage III increased 1.6-fold in the alcohol-consuming mice compared to their water-drinking counterparts (Fig. 2M). These results further support the concept that chronic alcohol consumption enhances iNKT cell maturation in the thymus.
Chronic alcohol consumption enhances stage I iNKT cell proliferation in the thymus
We conducted a short-term BrdU assay to determine if the increase in iNKT cells in the thymus and liver was due to enhanced proliferation of these cells. Chronic alcohol consumption did not significantly alter the percentage of BrdU+ iNKT cells in the spleen and liver (Fig. 3A–C), suggesting that chronic alcohol consumption does not alter the proliferation of iNKT cells in these organs. In the thymus, the percentage of BrdU+ iNKT cells within the total iNKT cell population and NK1.1+ iNKT cells are not significantly different between the water-drinking and alcohol-consuming groups (Fig. 3F). However, the percentage of BrdU+ iNKT cells in the NK1.1− cell population is increased significantly in alcohol-consuming mice compared to water-drinking mice (Fig. 3D–F). Further analysis indicated that the increased BrdU+NK1.1− iNKT cells are CD44lo cells (Fig. 3G–I), suggesting that chronic alcohol consumption mainly enhances stage I iNKT cell proliferation in the thymus.
Fig. 3.
Chronic alcohol consumption does not alter the proliferation of iNKT cells in liver and spleen, but increases NK1.1−CD44lo iNKT cell proliferation in the thymus. Mice consuming alcohol or water for 3 mo were treated with BrdU for 3 hr. BrdU+ iNKT cells were determined by flow cytometry. (A) Dot plot showing BrdU+ cells (right upper and lower quadrants) in liver lymphocytes. Numbers are the percentage of the indicated cells in the total liver lymphocytes. Upper left quadrant: CD1d/PBS57-tetramer+BrdU− cells; upper right quadrant: CD1d/PBS57-tetramer+BrdU+ proliferating iNKT cells; lower left quadrant: CD1d/PBS57-tetramer−BrdU− cells; lower right quadrant: CD1d/PBS57-tetramer+BrdU+ cells. (B) Histogram showing BrdU+ iNKT cells (M1) in gated liver iNKT cells. Number is the percentage of BrdU+ iNKT cells in liver total iNKT cells. (C) Percentage of BrdU+ cells in iNKT cells in the liver and spleen. (D) Dot plot showing NK1.1− iNKT cells (in the gated region R4) in the gated thymic iNKT cells. Numbers are the percentage of the gated iNKT cells in the total iNKT cells. (E) Dot plots showing BrdU+ iNKT cells (upper right quadrants) and BrdU− iNKT cells (upper left quadrants) in the gated NK1.1− iNKT cells. Numbers are the percentage of BrdU− and BrdU+ iNKT cells in the gated iNKT cells. (F) Percentage of BrdU+ cells in the thymic NK1.1− iNKT cells (NK1.1−), NK1.1+ iNKT cells (NK1.1+), and total iNKT cells (iNKT). (G) Dot plot showing gated NK1.1− CD44lo (gated region R5), NK1.1−CD44hi (gated region R6) and NK1.1+CD44hi iNKT (gated region R3) cells in the gated thymic iNKT cells. Numbers are the percentage of the gated iNKT cells in total iNKT cells. (H) Dot plots showing BrdU+ cells (upper right quadrants) and BrdU− cells (upper left quadrants) in the gated NK1.1−CD44lo iNKT cells. Numbers are the percentage of BrdU− and BrdU+ iNKT cells in the gated iNKT cells. (I) Percentage of BrdU+ cells in the NK1.1−CD44lo and NK1.1−CD44hi thymic iNKT cells. Experiments were repeated twice. Each group contained 5–7 mice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD. * p<0.05, **p<0.01.
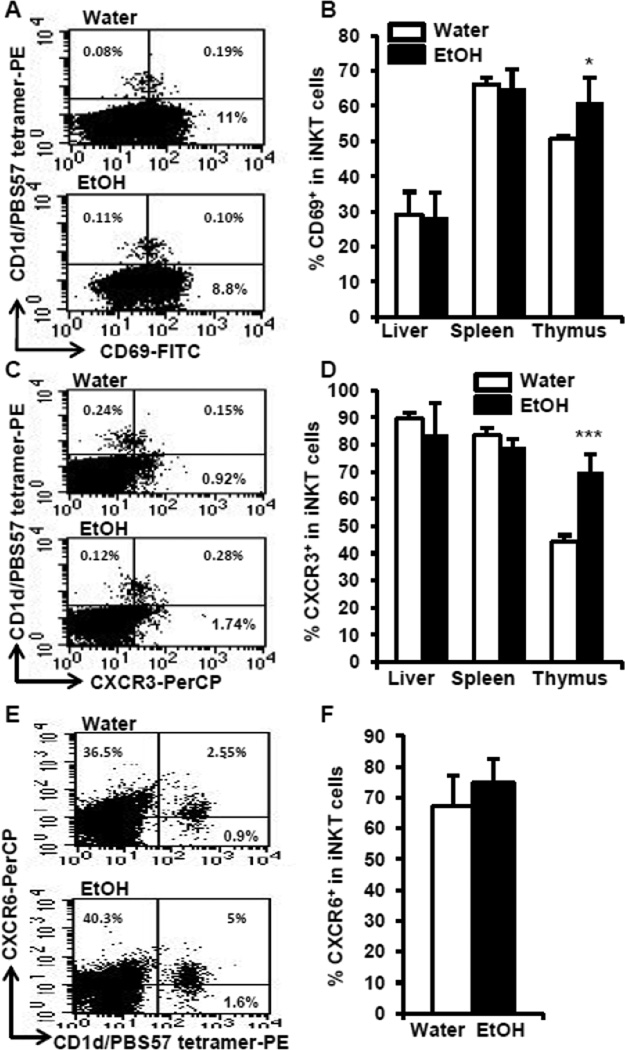
Chronic alcohol consumption increases CD69+ iNKT cells in the thymus and alters the expression of chemokine receptor CXCR3 on iNKT cells in an organ specific manner
iNKT cells become activated upon proliferation. If chronic alcohol consumption enhances iNKT cell proliferation, this also should be reflected in expression of CD69, an iNKT cell activation marker. Thus, we examined CD69+ iNKT cells in the thymus, spleen, and liver. The results indicate that chronic alcohol consumption increases the percentage of CD69+ iNKT cells in the thymus, but not in the liver or spleen (Fig. 4A, 4B). These results further indicate that chronic alcohol consumption activates iNKT cells in the thymus, but not in the spleen or liver.
Fig. 4.
Chronic alcohol consumption increases CD69+ iNKT cells and CXCR3+ iNKT cells in the thymus. (A) Dot plots showing CD69+ iNKT (upper right quadrants) cells in the thymus. (B) Percentage of CD69+ iNKT in the total iNKT cells in liver, spleen and thymus. (C) Dot plot showing CXCR3+ iNKT cells (upper right quadrant) in the thymus. (D) Percentage of CXCR3+ iNKT cells in total iNKT cells in liver, spleen and thymus. (E) Dot plot showing CXCR6+ iNKT cells (upper right quadrants) in the gated liver lymphocytes. (F) Percentage of CXCR6+ iNKT cells in total liver iNKT cells. Numbers in the dot plots are the percentage of the indicated cells in the total lymphocytes. Experiments were repeated twice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD for groups containing 7–10 mice. *p<0.05, ***p<0.001.
The retention of iNKT cells in the thymus is controlled through signaling via the chemokine receptor, CXCR3, and its ligands (Drennan et al., 2009). The CXCR6 signaling pathway plays a key role in the accumulation and activation of iNKT cells in the liver (Germanov et al., 2008). Since alcohol consumption increases iNKT cells in the thymus and liver, we next sought to determine if the expression of these chemokine receptors on iNKT cells is altered by alcohol consumption. We found that alcohol consumption significantly increases CXCR3+ iNKT cells in the thymus, but not in the spleen or liver (Figs. 4C, 4D). Alcohol consumption does not alter the expression of CXCR6+ iNKT cells in the liver (Figs. 4E, 4F). These results suggest that the increase of iNKT cells in the thymus could result from the enhanced retention associated with the increased CXCR3+ iNKT cells. The increase of iNKT cells in the liver is unlikely associated with the CXCR6 signaling pathway in the alcohol-consuming mice.
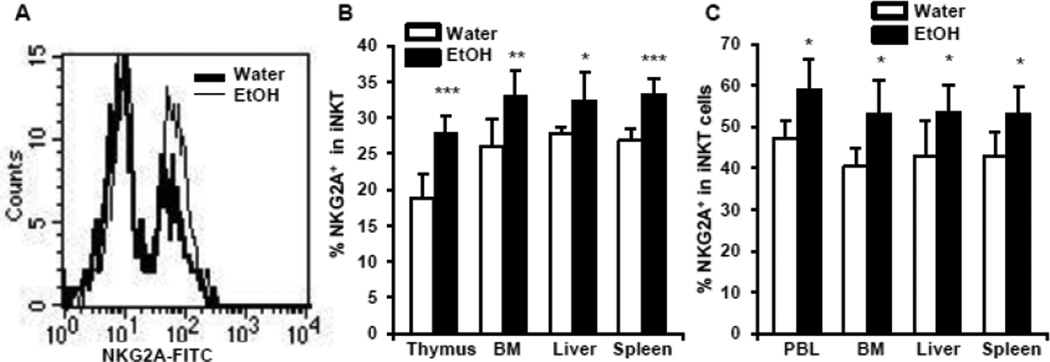
Chronic alcohol consumption increases NKG2A+ iNKT cells, which is independent from the IFN-γ signaling pathway
Besides the expression of TCR and the common NK cell receptor, NK1.1, NKT cells also express a group of NK cell receptors including the Ly49 family of receptors and NKG2A. These receptors play important roles in the regulation of iNKT cell activation (Ota et al., 2005; Kawamura et al., 2009). The results in Fig. 5A and 5B indicate that chronic alcohol consumption significantly increases the expression of NKG2A on iNKT cells in the thymus, spleen, blood and liver. However, alcohol consumption does not alter the expression of Ly49A, C, G2, D, or H on iNKT cells (data not shown).
Fig. 5.
Effects of chronic alcohol consumption on the expression of NKG2A in iNKT cells from wild type mice and IFN-γ KO mice. (A) Histograms showing NKG2A+ (the right peak) in the gated iNKT cells in the liver. (B) Percentage of NKG2A+ iNKT cells in the total iNKT cells in the thymus, BM, liver and spleen of wild type mice. (C) Percentage of NKG2A+ iNKT cells in the total iNKT cells in the PBL, BM, liver and spleen of IFN-γ KO mice. Experiments were repeated twice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD for groups containing 6–10 mice. *p<0.05, ** p<0.01, ***p<0.001.
Activated iNKT cells produce IFN-γ, which, in turn, induces the expression of MHC molecules. These MHC molecules are the ligands of Ly49 receptors and NKG2A. NKG2A is an inhibitory receptor (Brooks et al., 1997). Therefore, enhanced expression of MHC molecules will activate NKG2A to induce an inhibitory signal that will inhibit further activation of iNKT cells (Ota et al., 2005). We examined if the increase in NKG2A+ iNKT cells is dependent on the IFN-γ signaling pathway. We found that chronic alcohol consumption also increases the percentage of NKG2A+ iNKT cells in IFN-γ KO mice (Fig. 5C), indicating that the increase in these cells is independent of the IFN-γ signaling pathway.
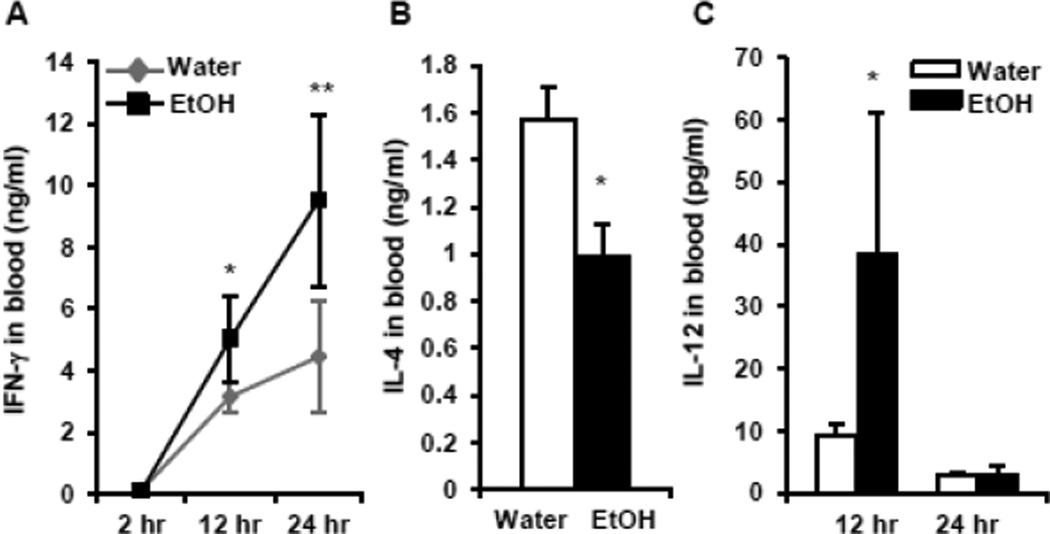
Chronic alcohol consumption increases IFN-γ and IL-12 levels in the blood after iNKT cell activation by αGalCer in vivo
One of the important features of iNKT cells is the production of multiple cytokines upon the activation. These cytokines further activate DC, NK, and T cells and regulate the downstream immune response. The most important cytokines produced by activated iNKT cells are the Th1 cytokine, IFN-γ, and the Th2 cytokine, IL-4. We sought to determine how alcohol consumption affects the IFN-γ and IL-4 concentration in the blood after in vivo activation of iNKT cells by αGalCer and found that chronic alcohol consumption increases IFN-γ concentration in the blood 12 and 24 hr after αGalCer injection (Fig. 6A). IL-4 could only be detected 2–4 hr after αGalCer injection, and alcohol consumption decreases the concentration of IL-4 as measured at 2 hr after αGalCer injection (Fig. 6B).
Fig. 6.
Concentration of IFN-γ, IL-4 and IL-12 in the plasma of water-drinking and alcohol-consuming mice. (A) Dynamic changes of IFN-γ at 2 hr, 12 hr, 24 hr after αGalCer injection. (B) Concentration of IL-4 in the blood 2 hr after αGalCer injection. (C) Dynamic changes of IL-12 in the blood at 12 and 24 hr after αGalCer injection. Experiments were repeated twice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD for groups containing 5–7 mice. *p<0.05, **p<0.01.
The IFN-γ produced by activated iNKT cells will further activate DC to produce IL-12, which can activate NK, NKT, and T cells. Chronic alcohol consumption significantly increases the concentration of IL-12 in the blood of mice 12 hr after αGalCer injection (Fig. 6C). At 24 hr the concentration of IL-12 is very low, and there are no differences attributable to alcohol consumption (Fig. 6C). IL-12 in the blood is undetectable at 2 hr after αGalCer injection (data not shown).
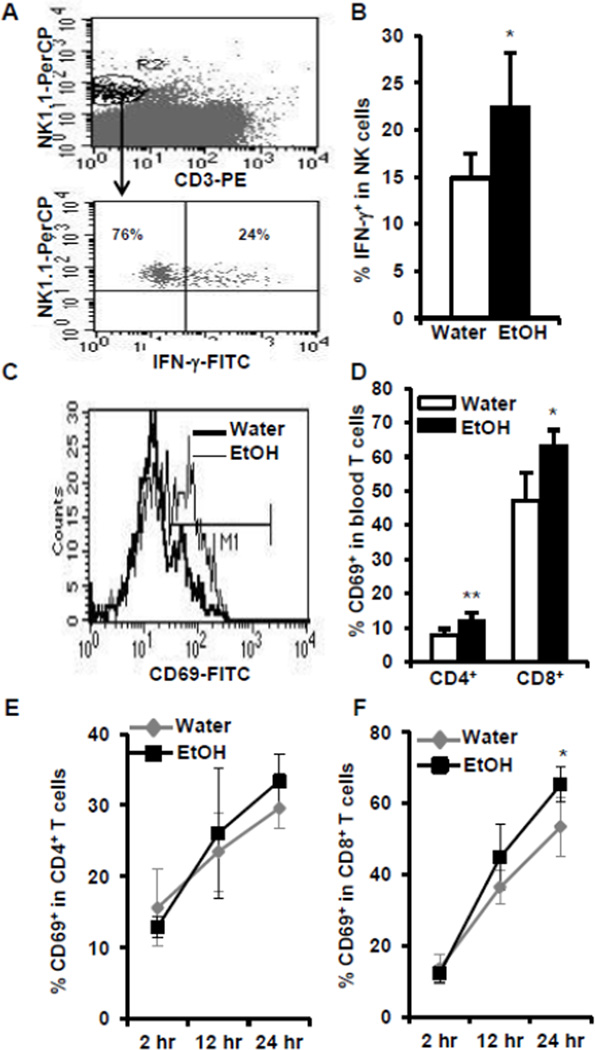
Chronic alcohol consumption enhances the activation of NK and T cells after in vivo αGalCer stimulation
Activation of iNKT cells will induce these cells to produce cytokines. These cytokines further regulate NK cell, T cell, and antigen presenting cell function. The IFN-γ produced by iNKT cells will activate NK and T cells. Chronic alcohol consumption increases αGalCer-induced production of IFN-γ, which should lead to the enhanced activation of NK and T cells in the alcohol-consuming mice. The activation of NK and T cells will up-regulate the expression of the cell activation marker, CD69, and induce these cells to produce IFN-γ. To examine these functions, we determined IFN-γ in NK and T cells after in vivo αGalCer activation of iNKT cells. Indeed, 12 hr after αGalCer injection, the percentage of IFN-γ-producing NK cells is significantly higher in the alcohol-consuming group compared to their water-drinking counterparts (Fig. 7A, 7B). The percentage of CD69+ CD4+ T cells and CD69+ CD8+ T cells increases significantly as a function of chronic alcohol consumption in the blood (Fig. 7C, 7D) 24 hr after αGalCer injection. In the spleen there is a significant increase in the percentage of CD69+ CD8+ T cells at 24 hr (Fig. 7F) but no significant differential effect in CD69+CD4+ T cells at any time points relative to alcohol (Fig. 7E). These results suggest that alcohol consumption enhances NK and T cell activation as a result of activating iNKT cells in vivo.
Fig. 7.
Chronic alcohol consumption increases INF-γ-producing NK cells and enhances CD69 expression in T cells after αGalCer injection. (A) Upper panel showing CD3−NK1.1+ NK cells (gated region R2) in splenocytes, lower panel showing IFN-γ-producing cells (IFN-γ+, upper right quadrant) in the gated splenic NK cells. Numbers are the indicated cells in the total NK cells. (B) Percentage of IFN-γ+ NK cells in the total NK cells in spleen. (C) CD69+ cells (M1) in the gated CD8+ T cells in the blood. (D) Percentage of CD69+ cells in CD4+ T cells and CD8+ T cells in the blood 24 hr after αGalCer injection. (E–F) Dynamic changes of CD69+ cells in CD4+ (E) and CD8+ (F) T cells at 2 hr, 12 hr and 24 hr after αGalCer injection. Experiments were repeated twice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD for groups containing 7–10 mice. *p<0.05, **p<0.01.
Chronic alcohol consumption up-regulates the expression of the co-stimulatory molecules, CD80 and CD86, in blood DC and B cells
iNKT cell activation induces DC maturation as well as the antigen presenting functions of DC and B cells. One of the features of antigen presenting cell activation is the up-regulation of the co-stimulatory molecules, CD80 and CD86. The expression of CD80 and CD86 on splenic and blood B cells and DC reached their peak 12 hr after αGalCer injection. The percentage of CD80+ B cells in the spleen and blood reached their peak at 12 hr after αGalcer injection, and rapidly decreases thereafter. There is no difference between the two groups of mice, except that the percentage of CD80+ B cells in the blood of alcohol-consuming mice is slightly higher than in the blood of water-drinking mice 24 hr after αGalCer injection (data not shown). At 2 hr after αGalCer injection, the percentage of CD80+ DC in the spleen is significantly higher in the alcohol-consuming mice than in the water-drinking mice. Twelve and 24 hr after αGalCer injection, all of the splenic DC were CD80+, and there are no differences between the two groups of mice. Two hr after αGalCer injection, there is no difference in the percentage of CD80+ DC in the blood between the two groups of mice (Fig. 8C). However, after 12 and 24 hr the percentage of CD80+ DC in the blood of alcohol-consuming mice is much higher compared to water-drinking mice (Fig. 8B, 8C). Twelve hr after αGalCer injection, all of the splenic DC as well as the splenic and blood B cells express CD86. There are no differences between the two groups of mice with regards to their expression of CD86 in terms of the mean fluorescence intensity (MFI) of these co-stimulatory molecules on these cells (data not shown). The percentage of CD86+ DC in the blood reached a peak 12 hr after αGalCer injection, and the percentage of CD86+ DC in the blood of alcohol-consuming mice is higher than in the water-drinking mice (Fig. 8D). However, after 12 hr the percentage of CD86+ DC in the blood of water-drinking mice starts to decrease; whereas, the percentage of these cells in the blood of alcohol-consuming mice only slightly decreases at 24 hr (Fig. 8D). Thus, at the 24 hr time point the percentage of CD86+ DC in the blood of alcohol-consuming mice is significantly higher than in the blood of water-drinking mice (Fig. 8D). In summary, chronic alcohol consumption increases CD80 and CD86 expression on blood DC and B cells after αGalCer activation in vivo.
Fig. 8.
Effects of chronic alcohol consumption on the expression of CD80 and CD86 in blood DC after αGalCer injection. (A) Dot plot showing the gated CD11chi cells (gated region R3) in PBL. Number is the percentage of the gated CD11chi cells in the PBL. (B) Histograms showing the CD80+ cells (M1) in the gated DC in PBL 24 hr after αGalCer injection. (C) Dynamic changes of CD80+ cells on DC in PBL at 2 hr, 12 hr, and 24 hr αGalCer after injection. (D) Dynamic changes of CD86+ DC in PBL at 2 hr, 12 hr, and 24 hr after αGalCer injection. Experiments were repeated twice. Water=water-drinking mice. EtOH=alcohol-consuming mice. Values are mean ± SD for groups containing 7–10 mice. *p<0.05, **p<0.01.
DISCUSSION
In this study, we demonstrate that chronic alcohol consumption increases the percentage and number of iNKT cells in the thymus and liver, but does not alter the distribution of these cells in the spleen, or blood. Chronic alcohol consumption increases the proportion of NK1.1+ mature iNKT cells in all of these organs, but only enhances NK1.1−CD44lo stage I iNKT cell proliferation in the thymus. Alcohol consumption does not alter iNKT cell proliferation in the spleen or liver. Our findings support the following concepts: a) Chronic alcohol consumption increases iNKT cells by enhancing stage I iNKT cell proliferation in the thymus; b) The distribution of the increased iNKT cells is organ specific and these cells are selectively retained in the thymus, and/or emigrate into the liver; and c) Chronic alcohol consumption enhances iNKT cell maturation and induces an IFN-γ-dominant Th1 cytokine profile.
The increase of iNKT cells in the thymus results from the enhanced proliferation of stage I cells and prolonged retention of stage III mature cells. iNKT cells develop in the thymus (Benlagha et al., 2002) and their expansion takes place in the stage I iNKT cells (Benlagha et al., 2002; Benlagha et al., 2005). The stage II iNKT cells migrate to the periphery, or are retained in the thymus for further maturation to stage III mature iNKT cells, which is featured by the acquisition of NK1.1 (Benlagha et al., 2002). These NK1.1+ iNKT cells do not divide in the steady state (Benlagha et al., 2002). However, with TCR or cytokine stimulation, NK1.1+ iNKT cells proliferate vigorously (Crowe et al., 2003). Since chronic alcohol consumption neither alters the proliferation of iNKT cells in the spleen and liver, nor alters the proliferation of NK1.1+ iNKT cells in the thymus, it is apparent that the increase of iNKT cells in the thymus and liver results from the enhanced thymic generation and proliferation of Stage I cells. Because the percentage and number of iNKT cells in the spleen and blood are normal in the alcohol-consuming mice compared to the water-drinking mice, the release of iNKT cells from the thymus and subsequent emigration to the spleen is likely not compromised. The increase of NK1.1+ iNKT cells in the thymus could be a result of the increased retention of iNKT cells in the thymus. CXCR3 is the key chemokine receptor that governs the retention of iNKT cells in the thymus (Drennan et al., 2009). The fact that chronic alcohol consumption significantly increases CXCR3+ iNKT cells in the thymus, but not in the spleen or liver (Fig. 4C, 4D), strongly supports the concept that chronic alcohol consumption increases NK1.1+ iNKT cells in the thymus by increasing their retention.
Chronic alcohol consumption enhances the proliferation of CD44loNK1.1− Stage I iNKT cells (Fig. 3). The portion of these cells should be increased in the alcohol-consuming mice, however, we still found that the percentage of these cells was significantly lower in these mice compared to the water-drinking control mice (Fig. 2). This is because Stage I and Stage II iNKT cells are small populations compared to the Stage III iNKT cells in the thymus (Fig. 2). A slight increase in the percentage of Stage III iNKT cells will cause a significant decrease in the percentage of Stage I and Stage II iNKT cells in total thymic iNKT cells as we showed in Fig. 2, although the total number of Stage I and Stage II iNKT cells does not decrease in the alcohol-consuming mice. Therefore, alcohol consumption 1) enhances Stage I iNKT cell proliferation, 2) accelerates iNKT cell maturation, and 3) increases mature iNKT cell retention in the thymus. The final outcome is the increase in the percentage of mature iNKT cells and the decrease in the percentage of Stage I and Stage II iNKT cells in the thymus.
The increase of iNKT cells in the liver could result from the increased retention or liver-specific migration of these cells from the thymus. LFA-1 plays an important role in iNKT cell migration to the liver (Emoto et al., 1999); however, alcohol consumption does not significantly alter the expression of LFA on iNKT cells (data not shown). CXCR6 is a key chemokine receptor that regulates iNKT cell activation and homeostasis in the liver (Germanov et al., 2008), but it is not required for the retention of iNKT cells in the liver (Germanov et al., 2008). Chronic alcohol consumption does not alter the expression of CXCR6 in liver iNKT cells (Fig. 4E, 4F). The mechanism of how chronic alcohol consumption increases liver iNKT cells remains to be elucidated.
The enhanced proliferation of stage I iNKT cells in the thymus could be induced by the activation of iNKT cells through TCR engagement. Indeed, we found that there are more CD69+ iNKT cells in the thymus of alcohol-consuming mice than in the thymus of their water-drinking counterparts (Fig. 4A. 4B), suggesting that the iNKT cells are activated in the alcohol-consuming mice. The result that chronic alcohol consumption does not alter the expression of CD69 on iNKT cells in the spleen and liver is consistent with the observation that the proliferation of iNKT cells in the spleen and liver of alcohol-consuming mice is not changed. These results further support the notion that the increase of iNKT cells in the liver of alcohol-consuming mice results from organ-specific migration or retention, but does not result from activation-induced proliferation.
Some endogenous lipids in the thymus can act as ligands for iNKT cell receptors, and activate iNKT cells. It was recently reported that peroxisome-derived plasmalogen lysophosphatidylethanolamine (p-LPE) in thymocytes is a potent agonist of the iNKT cell receptor (Facciotti et al., 2012). p-LPE plays an important role in the regulation of iNKT cell development, maturation, and activation. It is well-documented that alcohol consumption modulates the metabolism of lipids (Sozio and Crabb, 2008; Clugston et al., 2011). Thus, it is possible that chronic alcohol consumption activates iNKT cells and induces iNKT cell proliferation through increasing specific lipids such as p-LPE in the thymus.
iNKT cells leave the thymus and migrate to the peripheral organs during stage II (NK1.1− CD44hi). It can take several days, or even weeks, in the periphery before these cells mature into NK1.1+CD44hi iNKT cells (Stage III). The acquisition of NK1.1 is an important marker of iNKT cell maturation. The acquisition of NK1.1 is CD1d-dependent (McNab et al., 2005), indicating that iNKT cell maturation in the periphery needs the engagement of TCR and CD1d. However, the acquisition of NK1.1 and maturation of iNKT cells in the peripheral organs does not require cell division (Gadue and Stein, 2002). It is known that a lipid is required to stimulate iNKT cell maturation in the periphery; however, it is not known whether there is any organ specificity for these lipids (McNab et al., 2005). Chronic alcohol consumption decreases the proportion of NK1.1− iNKT cells in the thymus, spleen, BM and liver, suggesting that alcohol enhances iNKT cell maturation not only in the thymus, but also in the periphery. Alternatively, chronic alcohol consumption also could enhance iNKT cell maturation in the thymus resulting in the release of more mature iNKT cells to the periphery.
NKG2A is an inhibitory NK cell receptor, which is expressed by some subsets of NK cells, NKT cells, and activated T cells (Mingari et al., 1998; Sivakumar et al., 1999). The ligand of this receptor is an MHC molecule – Qa-1b in mice, and HLA-E in humans (Lee et al., 1998; Vance et al., 1998). Activated iNKT cells produce IFN-γ, which up-regulates the expression of Qa-1b in mice (Ota et al., 2005). The interaction between up-regulated Qa-1b and NKG2A/CD94 inhibits further NKT cell activation. NKG2A plays important protective roles in Con-A, and αGalCer-induced liver injury by inhibiting NKT cell activation (Kawamura et al., 2009). The increase in NKG2A+ iNKT cells in the alcohol-consuming mice could be induced by iNKT cell activation. Since activated iNKT cells produce IFN-γ, we examined whether or not the increase in NKG2A+ iNKT cells was induced by IFN-γ. The results from IFN-γ KO mice indicate that the increase of NKG2A+ iNKT cells induced by alcohol consumption is independent from IFN-γ. NKG2A can be induced by IL-12 (Saez-Borderias et al., 2009) and activation of iNKT cells further triggers DC to produce IL-12. Indeed, in vivo activation of iNKT cells with αGalCer significantly increases the level of IL-12 in the blood of alcohol-consuming mice compared to water-drinking mice (Fig. 6C). Therefore, the increase in NKG2A+ iNKT cells in the alcohol-consuming mice could be associated with the activation of iNKT cells. This activation-induced NKG2A expression could prevent further iNKT cell activation. The increase in NKG2A+ iNKT cells in the alcohol-consuming mice also could be associated with the increase in the NK1.1+ mature iNKT cells.
We found that chronic alcohol consumption increases IL-12 and IFN-γ production, and decreases IL-4 production after in vivo αGalCer stimulation (Fig. 6), suggesting that chronic alcohol consumption induces a Th1cytokine-dominant cytokine profile. Upon activation, iNKT cells produce Th1, Th2, and Th17 cytokines, (Coquet et al., 2008; Watarai et al., 2012). The production of each type of cytokine is determined by the specific iNKT subsets and their activation status. NK1.1−CD44lo iNKT cells primarily produce IL-4 and no IFN-γ. NK1.1−CD44hi iNKT cells primarily produce IL-4 and lower amounts of IFN-γ. NK1.1+CD44hi iNKT cells primarily produce IFN-γ and a lower amount of IL-4 cytokines (Benlagha et al., 2002). However, strong stimulation of iNKT cells induces anergy, and anergic iNKT cells are characterized by their IL-4 dominant Th2 cytokine profile (Parekh et al., 2005). We found that chronic alcohol consumption increases NK1.1+ iNKT cells and that these cells exhibit an IFN-γ-dominant cytokine profile (Fig. 6). In addition, alcohol facilitates CD1d loading and increases IFN-γ production in iNKT cells (Buschard et al., 2011). This could partially contribute to the increase in IFN-γ production in alcohol-consuming mice relative to water-drinking controls.
It is well-defined that the balance between Th1 and Th2 cytokines produced by iNKT cells will differentially regulate the cytokine production of DC, and further shape the downstream immune response. A Th1 dominant cytokine profile produced by iNKT cells will induce DC to produce IL-12, which will further activate NK, NKT, and T cells to produce IFN-γ (Fujii et al., 2003). A Th2 dominant cytokine profile produced by iNKT cells will induce DC to produce IL-10, which will inhibit NK and CD8+ T cell activation (Kojo et al., 2005). We found that in vivo injection of αGalCer increases IFN-γ and IL-12 production in the blood (Fig. 6A, 6C), increases the IFN-γ producing NK cells in the alcohol-consuming mice (Fig. 7A, 7B), and increases the activation of CD8+ T cells in the blood and spleen (Fig. 7C, 7D, 7F). The in vivo activation of iNKT cells also enhances the expression of the costimulatory molecules, CD80 and CD86, on B cells and DC in alcohol-consuming mice (Fig. 8). The up-regulation of these costimulatory molecules is known to enhance CD8+ T cell activation, and boost the Th1 immune response. Taken together, chronic alcohol consumption increases IFN-γ-producing mature iNKT cells, and the activation of these iNKT cells induces a strong Th1 immune response.
A Large body of research indicates that iNKT cells play important roles in hepatitis and liver injury in other models (Ishikawa et al., 2011; Kumar, 2013; Wang et al., 2013). Lieber and co-workers (Lieber and DeCarli, 1970; Lieber et al., 1989) demonstrated early on that a high fat (35–40% of total calories) diet consumed with alcohol administered in a liquid diet was essential to induce liver injury. In an intragastric alcohol infusion model where mice were fed with high fat diet, chronic alcohol consumption increases iNKT cells in the liver and that activation of iNKT cells with αGalcer induces severe liver injury (Minagawa et al., 2004). In the present study mice were fed a normal diet containing 4.5% fat and given alcohol in the drinking water. While chronic alcohol consumption in this model significantly increases iNKT cells in the liver, no liver injury is observed. Histological examination of the livers from mice administered alcohol using this model showed a lack of inflammation and fibrosis (Blank et al., 1991). In human alcoholics without liver disease, a significant increase in NKT cells (CD56+ T cells) was observed in the PBL; whereas the NKT cells in the PBL of people who had alcoholic liver disease, although increased somewhat, was not statistically different from healthy people (Laso et al., 2010).
Currently the biological relevance of the increase of iNKT cells in the thymus and liver in alcohol consuming mice is not known. It is known that liver is an iNKT cell rich organ. It is possible that alcohol consumption alters the metabolism of lipid, which in turn increases the lipids that serve as iNKT cell antigens in the thymus to enhance iNKT cell proliferation and activation. The increased iNKT cells are stored in the thymus and liver in steady state and would be available for participation in immune responses when they are needed. In a mouse model of melanoma, we found that chronic alcohol consumption significantly increases circulating iNKT cells in the PBL after two weeks of tumor injection (Zhang et al., 2012). The biological significance of the increased iNKT cells in the alcohol-consuming, melanoma-bearing mice also is under investigation in our laboratory.
In summary, chronic alcohol consumption induces iNKT cell maturation and activation, and enhances proliferation of the thymic NK1.1−CD44lo stage I iNKT cells. Alcohol consumption selectively increases iNKT cells in the thymus and liver. The increase of iNKT cells in the thymus likely results from increased thymic retention, which could be associated with the enhanced CXCR3 signaling pathway. Chronic alcohol consumption induces iNKT cells to produce an IFN-γ dominant cytokine profile, which favors a Th-1 immune response.
Highlights.
Chronic alcohol consumption:
-
➢
Increases iNKT cells in the thymus and liver;
-
➢
Enhances thymic Stage I iNKT cell proliferation;
-
➢
Enhances iNKT cell maturation in thymus and periphery;
-
➢
Induces Th1 immune response upon iNKT cell in vivo activation.
Ackanwledgements
This project was supported by National Institutes of Health grant K05AA017149 and by funds provided for medical biological research by the State of Washington Initiative Measure No. 171. Mouse CD1d/PBS57-tetramer was provided by NIH tetramer facility (Atlanta, GA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins SP, McNab FW, Jones CM, Smyth MJ, Godfrey DI. Long-term retention of mature NK1.1+ NKT cells in the thymus. J. Immunol. 2006;176:4059–4065. doi: 10.4049/jimmunol.176.7.4059. [DOI] [PubMed] [Google Scholar]
- Blank SE, Duncan DA, Meadows GG. Suppression of natural killer cell activity by ethanol consumption and food restriction. Alcohol. Clin. Exp. Res. 1991;15:16–22. doi: 10.1111/j.1530-0277.1991.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Blom KG, Qazi MR, Matos JB, Nelson BD, DePierre JW, Abedi-Valugerdi M. Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained. Clin Exp Immunol. 2009;155:320–329. doi: 10.1111/j.1365-2249.2008.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AG, Posch PE, Scorzelli CJ, Borrego F, Coligan JE. NKG2A complexed with CD94 defines a novel inhibitory natural killer cell receptor. J. Exp. Med. 1997;185:795–800. doi: 10.1084/jem.185.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschard K, Hansen AK, Jensen K, Lindenbergh-Kortleve DJ, de Ruiter LF, Krohn TC, Hufeldt MR, Vogensen FK, Aasted B, Osterbye T, Roep BO, de Haar C, Nieuwenhuis EE. Alcohol facilitates CD1d loading, subsequent activation of NKT cells, and reduces the incidence of diabetes in NOD mice. PLoS One. 2011;6:e17931. doi: 10.1371/journal.pone.0017931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, Yagita H, Kang CY. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J. Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- Clugston RD, Jiang H, Lee MX, Piantedosi R, Yuen JJ, Ramakrishnan R, Lewis MJ, Gottesman ME, Huang LS, Goldberg IJ, Berk PD, Blaner WS. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J Lipid Res. 2011;52:2021–2031. doi: 10.1194/jlr.M017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, Godfrey DI. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J. Immunol. 2003;171:4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- Drennan MB, Franki AS, Dewint P, Van Beneden K, Seeuws S, van de Pavert SA, Reilly EC, Verbruggen G, Lane TE, Mebius RE, Deforce D, Elewaut D. Cutting edge: the chemokine receptor CXCR3 retains invariant NK T cells in the thymus. J. Immunol. 2009;183:2213–2216. doi: 10.4049/jimmunol.0901213. [DOI] [PubMed] [Google Scholar]
- Emoto M, Mittrucker HW, Schmits R, Mak TW, Kaufmann SH. Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J. Immunol. 1999;162:5094–5098. [PubMed] [Google Scholar]
- Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13:474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J. Immunol. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- Germanov E, Veinotte L, Cullen R, Chamberlain E, Butcher EC, Johnston B. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J. Immunol. 2008;181:81–91. doi: 10.4049/jimmunol.181.1.81. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- Horie Y, Yamagishi Y, Kajihara M, Kato S, Ishii H. National survey of hepatocellular carcinoma in heavy drinkers in Japan. Alcohol. Clin. Exp. Res. 2003;27:32S–36S. doi: 10.1097/01.ALC.0000078605.33391.20. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, Arai K, Takeda K, Watanabe S. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol. 2011;54:1195–1204. doi: 10.1016/j.jhep.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology. 2011;53:219–229. doi: 10.1002/hep.23983. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Takeda K, Kaneda H, Matsumoto H, Hayakawa Y, Raulet DH, Ikarashi Y, Kronenberg M, Yagita H, Kinoshita K, Abo T, Okumura K, Smyth MJ. NKG2A inhibits invariant NKT cell activation in hepatic injury. J. Immunol. 2009;182:250–258. doi: 10.4049/jimmunol.182.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kojo S, Seino K, Harada M, Watarai H, Wakao H, Uchida T, Nakayama T, Taniguchi M. Induction of regulatory properties in dendritic cells by Valpha14 NKT cells. J. Immunol. 2005;175:3648–3655. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- Kumar V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laso FJ, Almeida J, Torres E, Vaquero JM, Marcos M, Orfao A. Chronic alcohol consumption is associated with an increased cytotoxic profile of circulating lymphocytes that may be related with the development of liver injury. Alcohol. Clin. Exp. Res. 2010;34:876–785. doi: 10.1111/j.1530-0277.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Quantitative relationship between amount of dietary fat and severity of alcoholic fatty liver. Am J Clin Nutr. 1970;23:474–478. doi: 10.1093/ajcn/23.4.474. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. J. Immunol. 2005;175:3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol. Clin. Exp. Res. 2011;35:787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa M, Deng Q, Liu ZX, Tsukamoto H, Dennert G. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-alpha during alcohol consumption. Gastroenterology. 2004;126:1387–1399. doi: 10.1053/j.gastro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, Moretta A, Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci U S A. 1998;95:1172–1177. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Jarman DW, Rehm J, Greenfield TK, Rey G, Kerr WC, Miller P, Shield KD, Ye Y, Naimi TS. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health. 2013;103:641–648. doi: 10.2105/AJPH.2012.301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defense and society. Nat. Rev. Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Ota T, Takeda K, Akiba H, Hayakawa Y, Ogasawara K, Ikarashi Y, Miyake S, Wakasugi H, Yamamura T, Kronenberg M, Raulet DH, Kinoshita K, Yagita H, Smyth MJ, Okumura K. IFN-gamma-mediated negative feedback regulation of NKT-cell function by CD94/NKG2. Blood. 2005;106:184–192. doi: 10.1182/blood-2004-11-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J. Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J. Exp. Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubsky S, Speak AO, Salio M, Jennemann R, Bonrouhi M, Zafarulla R, Singh Y, Dyson J, Luckow B, Lehuen A, Malle E, Muthing J, Platt FM, Cerundolo V, Grone HJ. Globosides but not isoglobosides can impact the development of invariant NKT cells and their interaction with dendritic cells. J. Immunol. 2012;189:3007–3017. doi: 10.4049/jimmunol.1201483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Borderias A, Romo N, Magri G, Guma M, Angulo A, Lopez-Botet M. IL-12-dependent inducible expression of the CD94/NKG2A inhibitory receptor regulates CD94/NKG2C+ NK cell function. J. Immunol. 2009;182:829–836. doi: 10.4049/jimmunol.182.2.829. [DOI] [PubMed] [Google Scholar]
- Sivakumar PV, Gunturi A, Salcedo M, Schatzle JD, Lai WC, Kurepa Z, Pitcher L, Seaman MS, Lemonnier FA, Bennett M, Forman J, Kumar V. Cutting edge: expression of functional CD94/NKG2A inhibitory receptors on fetal NK1.1+Ly-49-cells: a possible mechanism of tolerance during NK cell development. J. Immunol. 1999;162:6976–6980. [PubMed] [Google Scholar]
- Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10–E16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Tsutsumi M. National survey of alcoholic liver disease in Japan (1968–91) J Gastroenterol Hepatol. 1995;10:509–516. doi: 10.1111/j.1440-1746.1995.tb01339.x. [DOI] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J. Exp. Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology. 2013;58:1474–1485. doi: 10.1002/hep.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TJ, Li X, Giuntoli RL, 2nd, Lopez PH, Heuser C, Schnaar RL, Tsuji M, Kurts C, Oelke M, Schneck JP. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012;72:3744–3752. doi: 10.1158/0008-5472.CAN-11-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Rao P, Carreno LJ, Kim S, Lawrenczyk A, Porcelli SA, Cresswell P, Yuan W. Human CD1d knock-in mouse model demonstrates potent antitumor potential of human CD1d-restricted invariant natural killer T cells. Proc Natl Acad Sci U S A. 2013;110:2963–2968. doi: 10.1073/pnas.1300200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Meadows GG. Chronic alcohol consumption perturbs the balance between thymus-derived and bone marrow-derived natural killer cells in the spleen. J. Leukoc. Biol. 2008;83:41–47. doi: 10.1189/jlb.0707472. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhu Z, Meadows GG. Chronic alcohol consumption impairs distribution and compromises circulation of B cells in B16BL6 melanoma-bearing mice. J. Immunol. 2012;189:1340–1348. doi: 10.4049/jimmunol.1200442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]