Abstract
Rationale: Phosphorylation of myristoylated alanine-rich C kinase substrate (phospho-MARCKS) at the phosphorylation site domain (PSD) is crucial for mucus granule secretion and cell motility, but little is known concerning its function in lung cancer.
Objectives: We aimed to determine if MARCKS PSD activity can serve as a therapeutic target and to elucidate the molecular basis of this potential.
Methods: The clinical relevance of phospho-MARCKS was first confirmed. Next, we used genetic approaches to verify the functionality and molecular mechanism of phospho-MARCKS. Finally, cancer cells were pharmacologically inhibited for MARCKS activity and subjected to functional bioassays.
Measurements and Main Results: We demonstrated that higher phospho-MARCKS levels were correlated with shorter overall survival of lung cancer patients. Using shRNA silencing and ectopic expression of wild-type and PSD-mutated (S159/163A) MARCKS, we showed that elevated phospho-MARCKS promoted cancer growth and erlotinib resistance. Further studies demonstrated an interaction of phosphoinositide 3-kinase with MARCKS, but not with phospho-MARCKS. Interestingly, phospho-MARCKS acted in parallel with increased phosphatidylinositol (3,4,5)-triphosphate pools and AKT activation in cells. Through treatment with a 25-mer peptide targeting the MARCKS PSD motif (MPS peptide), we were able to suppress tumor growth and metastasis in vivo, and reduced levels of phospho-MARCKS, phosphatidylinositol (3,4,5)-triphosphate, and AKT activity. This peptide also enhanced the sensitivity of lung cancer cells to erlotinib treatment, especially those with sustained activation of phosphoinositide 3-kinase/AKT signaling.
Conclusions: These results suggest a key role for MARCKS PSD in cancer disease and provide a unique strategy for inhibiting the activity of MARCKS PSD as a treatment for lung cancer.
Keywords: MARCKS phosphorylation, PI3K/AKT, PIP3, lung cancer, erlotinib
At a Glance Commentary
Scientific Knowledge on the Subject
Myristoylated alanine-rich C kinase substrate (MARCKS), particularly phosphorylated MARCKS (phospho-MARCKS), has emerged as a potential therapeutic target for asthma and other respiratory diseases characterized by mucus hypersecretion and inflammation. However, the understanding of phospho-MARCKS function and its contribution to lung cancer remains unclear.
What This Study Adds to the Field
This study points to the up-regulation of phospho-MARCKS as a predictor and a potential target for lung cancer therapy. Phosphorylation of MARCKS at the phosphorylation site domain releases MARCKS from the membrane and allows phosphatidylinositol (4,5)-bisphosphate pools to become available for phosphoinositide 3-kinase to convert to phosphatidylinositol (3,4,5)-triphosphate, a major driver of cancer progression and drug resistance. Targeting MARCKS phosphorylation site domain with the MPS peptide suppresses cell growth and malignancy of cancers characterized by elevated phosphatidylinositol (3,4,5)-triphosphate levels. Based on our findings, patients with advanced-stage lung cancer may benefit from combined treatment with MPS peptide and epidermal growth factor receptor tyrosine-kinase inhibitors.
Cancer metastasis and drug resistance are the main reasons for the poor survival of patients with lung cancer. Standard treatment approaches are often associated with unsatisfying outcomes (1, 2) and alternatives to conventional treatment are in dire need. New molecular-targeted therapies, such as targeting the epidermal growth factor receptor (EGFR), have played a central role recently in lung cancer treatment. Two EGFR tyrosine-kinase inhibitors (TKIs), gefitinib and erlotinib, are currently used as first-line treatment options for patients with the EGFR mutation (3, 4). Unfortunately, variable rates of responsiveness to this targeted therapy and the ability of lung cancer to develop resistance to these inhibitors has brought about new challenges in clinical practice (5, 6). The development of novel biomarkers and effective therapeutic interventions are greatly needed.
Many cancers have activated phosphoinositide 3-kinase (PI3K)/AKT pathway, which is involved in promoting cell proliferation, survival, motility, and metabolism (7, 8). Of note, PI3K signaling is frequently activated by receptor tyrosine kinases, such as EGFR, and eventually an overly activated PI3K/AKT signaling can confer drug resistance (9, 10). Recent research has indicated inhibition of PI3K/AKT signaling as a possible therapeutic target, but it remains unknown which cancer types will benefit most from such an intervention (11, 12). An important function of PI3K is to synthesize phosphatidylinositol (3,4,5)-triphosphate (PIP3), resulting in AKT activation and the subsequent regulation of various biologic processes. Once the regulatory subunit (p85) of PI3K binds to phosphotyrosine residues on receptor tyrosine kinases and/or adaptors, the catalytic subunit (p110) is free to catalyze the phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2) to PIP3. However, it remains unclear how PIP2 is accessible to PI3K (13, 14).
Myristoylated alanine-rich C kinase substrate (MARCKS) is a PIP2-associated protein through its phosphorylation site domain (PSD), also known as the basic effector domain. Phosphorylation by protein kinase C (PKC) within MARCKS PSD (Ser159, Ser163, and Ser170) enhances phosphorylated MARCKS (phospho-MARCKS) detachment from membrane and suppresses PIP2 sequestering effect (15, 16). In the lung, MARCKS, predominantly phospho-MARCKS, is crucial for controlling mucin secretion and inflammation (17–21), but only a few studies have revealed its relevance with lung cancer (22, 23). Recently, our laboratory discovered that the use of a MANS peptide, targeting MARCKS N-terminal myristoylation site, was able to reduce lung cancer metastasis. However, the treatment had no effect on tumor growth in vivo (22). Because the understanding of the contribution of MARCKS activity to lung cancer is incomplete, there is a need to test if other parts of MARCKS, especially the PSD motif, can be targeted for lung cancer treatment. In this study, we test this potential and elucidate the molecular basis of this potential.
Methods
Details are provided in the Methods section of the online supplement.
Results
High Phospho-MARCKS Is Correlated with Poor Survival of Lung Cancer Patients and EGFR-TKI–based Therapy
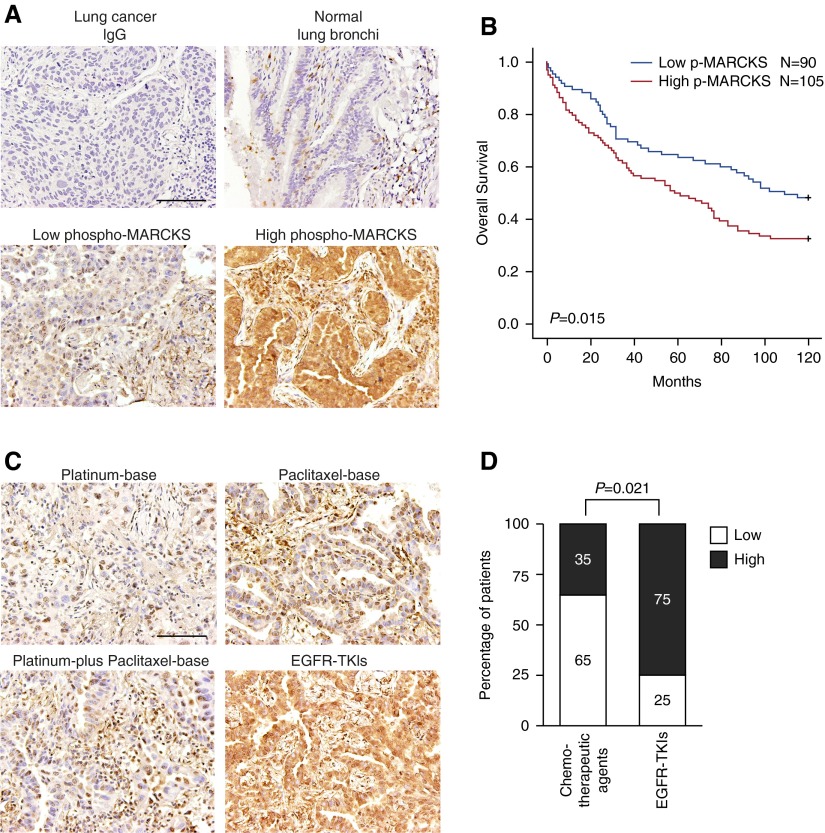
We previously reported an association between elevated phospho-MARCKS (pSer159/163) and lung cancer malignancy (22, 23). To expand on this previous finding, we retrospectively evaluated the relationship between MARCKS activity and overall survival of 195 lung cancer patients by immunohistochemical (IHC) staining. Consistent with our previous reports, phospho-MARCKS was very low in normal lung bronchi and staining was not detected when normal IgG was used (Figure 1A). Patient samples were further grouped into low and high phospho-MARCKS categories and their clinical characteristics are summarized in Table E1 in the online supplement. Importantly, the patients with high phospho-MARCKS levels had a significantly shorter overall survival as compared with the low phospho-MARCKS group (P = 0.015; log rank test) (Figure 1B). Additionally, to examine whether MARCKS becomes activated in response to chemotherapy, we assessed phospho-MARCKS levels in primary lung tumors from 52 patients who were concurrently receiving therapeutic agents (see Table E2). Strong phospho-MARCKS staining was observed in tumors from lung cancer patients undergoing EGFR-TKI–based therapy (Figure 1C) and was positively correlated (P = 0.021; Fisher exact test) (Figure 1D), as compared with chemotherapy other than EGFR-TKI.
Figure 1.
Clinical relevance of phosphorylation of myristoylated alanine-rich C kinase substrate (phospho-MARCKS) in overall survival of lung cancer patients and epidermal growth factor receptor (EGFR)- tyrosine-kinase inhibitor (TKI)–based therapy. (A) The levels of phospho-MARCKS were examined by immunohistochemical staining in lung cancer specimens from 195 patients who underwent surgical resections. Representative images by using IgG as a negative control or using anti-pSer 159/163 MARCKS monoclonal antibody in normal lung tissue and lung cancer specimens with low and high levels of phospho-MARCKS. Scale bar: 100 μm. (B) These patients were further grouped by high and low phospho-MARCKS levels and their overall survival analyzed by Kaplan-Meier plot and two-sided log-rank tests (n = 195). (C and D) Fifty-two lung cancer patients who have metastatic disease and received first-line chemotherapeutic agents or EGFR-TKI–based therapy (without prior chemotherapy) were included in our retrospective analysis. (C) Representative images of immunohistochemical staining (phospho-MARCKS) in lung cancer specimens from patients treated with different therapeutic agents. (D) These tumor specimens were stratified by low and high levels of phospho-MARCKS. Percentage of 52 patients with low and high MARCKS phosphorylation according to chemotherapeutic agents versus EGFR inhibitors (EGFR-TKIs). P values were obtained from Fisher's exact test.
Elevated Phospho-MARCKS Levels Support Lung Cancer Growth
Because a hallmark of cancer is an increase of growth rate, we investigated whether phospho-MARCKS (Ser159 and Ser163) has a role in growth and tumorigenesis. V5-tagged wild-type and PSD-mutated (S159/163A) MARCKS constructs were used to transfect into low MARCKS-expressing CL1-0 cells (see Figure E1A) (22). We observed an approximate threefold increase of colony-forming ability in cells with ectopic expression of V5-tagged wild-type MARCKS, as compared with the mock controls and cells transfected with PSD-mutated MARCKS construct (Figure 2A). In addition, silencing MARCKS expressions through the use of three independent MARCKS-specific short hairpin RNAs (MARCKS-shRNA-a, MARCKS-shRNA-b, and MARCKS-shRNA-c) in high MARCKS-expressing A549 cells (see Figure E1A) (22) had resulted in reducing cancer growth in a MARCKS expression-dependent manner (see Figure E1B). Interestingly, MARCKS-shRNA-c was especially potent in reducing both MARCKS and phospho-MARCKS at the protein levels. These reductions were correlated with the suppression of clonogenic capacity of A549 cells by this shRNA (Figure 2B). These effects were also seen in various cancer cell lines with knockdown of MARCKS expression (see Figure E1C).
Figure 2.
Myristoylated alanine-rich C kinase substrate (MARCKS) activation in promoting lung cancer growth. (A) Effects of ectopic V5-tagged wild-type or phosphorylation site domain–mutated (S159/163A) MARCKS expression on the growth (left) and MARCKS phosphorylation (right) in CL1-0 cells. (B) Effects of MARCKS shRNA silencing on cell growth (left) and MARCKS phosphorylation (right) in A549 cells. Anchorage-dependent colony formation (upper left) was carried for cell growth assay and colonies were counted in a blind fashion and quantified (lower left). *P < 0.05 versus mock (A; n = 4) or control shRNA (B; n = 4). Western blot analyses were performed to determine phospho-MARCKS and total MARCKS levels in these cells (right). (C) A549 cells treated with control or MARCKS-specific shRNA were xenografted subcutaneously to nude mice as described in the Methods section of the online supplement. Top, Representative sizes of tumors. Bottom, Tumor weights presented as the mean ± SE (n = 8). *P < 0.05 versus control shRNA. (D) Paraffin histology sections were subjected to immunohistochemical staining with various antibodies as indicated. A representative image was shown and positive nuclear staining of PCNA was quantified (mean ± SD). *P < 0.01 versus control shRNA. Scale bar: 20 μm. (E) Implication of phospho-MARCKS levels in erlotinib resistance. V5-tagged wild-type or phosphorylation site domain–mutated MARCKS-expressing CL1-0 cells (left) and MARCKS-knockdown A549 cells (right) were subjected to various doses of erlotinib for treatment. After 72 hours, cell viability was determinate by MTS assays. Data shown as mean ± SD. *P < 0.05 versus mock (left; n = 3) or control shRNA (right; n = 4).
To confirm that phospho-MARCKS promotes cell proliferation in vivo, a subcutaneous xenograft experiment was performed. As shown in Figure 2C, the average weight of tumors derived from MARCKS shRNA-silenced A549 cells was significantly smaller than that of unaltered control cells. Moreover, IHC staining revealed also the down-regulation of proliferating cell nuclear antigen, a proliferation marker, in MARCKS-silenced cells (Figure 2D). Because cell proliferation supports the development of drug resistance in cancer cells, we evaluated whether phospho-MARCKS could alter the sensitivity of lung cancer cells to EGFR-TKIs. MARCKS-overexpression and -knockdown cell lines, CL1-0 and A549, respectively, were exposed to increasing concentrations of erlotinib for 72 hours. Data from MTS cell viability assays showed that erlotinib-mediated cytotoxicity was significantly enhanced in the cells with low phospho-MARCKS levels (Figure 2E). Taken together, these results reveal a novel function of phospho-MARCKS in supporting tumor proliferation and erlotinib resistance.
Activated MARCKS Modulates PIP3 Pools and Contributes to AKT Activation
AKT activation is recognized as a key player in cell survival and may account for erlotinib resistance (24–26). To elucidate whether phospho-MARCKS can regulate AKT activation, we performed a shRNA silencing approach followed by reexpression of wild-type or PSD-mutated MARCKS to determine if knockdown activities are rescued by wild-type or PSD-mutated MARCKS expression. As shown in Figure E2A, reexpression of the wild-type MARCKS could restore clonogenic abilities of silenced cells. Restorations in AKT phosphorylation both at Ser473 and Thr308 and its downstream GSK3-β activity were also seen in these silenced cells with overexpression of V5-tagged wild-type MARCKS, but not in cells transfected with PSD-mutated MARCKS (Figure 3A).
Figure 3.

Myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation site domain (PSD) activity directly regulates phosphatidylinositol (3,4,5)-triphosphate (PIP3) pools and AKT signaling. (A) Western blot analysis of AKT signaling activation in the MARCKS-knockdown A549 cells with reexpression of wild-type and mutant V5-tagged MARCKS. (B) PIP3 levels in these genetically modified A549 cells. *P < 0.05 versus control shRNA (n = 4; mean ± SD). (C) Determination of the interaction between MARCKS/phospho-MARCKS and phosphoinositide 3-kinase (PI3K) by using coimmunoprecipitation and immunoblotting with indicated antibodies. (D) Proposed hypothetical model to account for the contribution of MARCKS in the enhancement of PIP3 levels via a two-step process: at membrane level, phosphatidylinositol (4,5)-bisphosphate (PIP2) lipids in inner membrane domains are concentrated by MARCKS, which is also interacted with PI3K; and after phosphorylation by protein kinase C (PKC), phospho-MARCKS is detached from membrane and PI3K interaction, and the newly release PIP2 serves as a substrate for PI3K to produce PIP3 that leads to AKT activation.
Because AKT activation occurs as a consequence of PIP3 generated on the plasma membrane (7), we examined PIP3 levels in these genetically modified cells. As shown in Figure 3B, a suppressive effect on PIP3 levels occurred in MARCKS-silenced A549 cells, whereas ectopic expression of V5-tagged wild-type MARCKS reversed this suppression and increased PIP3 pools. PI3K is known to catalyze the synthesis of PIP3 from PIP2. Unphosphorylated MARCKS has been reported to bind a significant fraction of PIP2 in the cell membrane (16). Therefore, we then asked whether there could be an interaction between MARCKS and PI3K. Sequences analysis of the MARCKS protein revealed that MARCKS potentially binds to the SH3 domain of p85, a regulatory subunit of PI3K (see Figure E2B). A coimmunoprecipitation assay further confirmed an association between MARCKS and PI3K in various lung cancer cell lines (Figure 3C). However, this interaction did not occur between phospho-MARCKS and PI3K, implying that MARCKS is disassociated from PI3K after its PSD motif is phosphorylated. These results led us to propose a model for the contribution of MARCKS PSD in regulation of PIP3 levels and AKT activation (Figure 3D).
MPS Peptide Treatment Has a Cancer-Specific Growth-Inhibitory Effect through Targeting MARCK PSD
According to the sequence of MARCKS PSD, we designed a 25–amino-acid peptide, termed the MPS peptide, to inhibit the functions of MARCKS PSD. As expected, treatment with MPS peptide reduced MARCKS phosphorylation in TKI-resistant cancer cells (Figure 4A); concurrently, a decrease of PIP3 pools in whole cell lysates of MPS-treated cells was observed (Figure 4B). Next, we confirmed the importance of serine residues present in the MPS peptide for its binding to the cell membrane by using peptides with substitutions at these serine residue sites (see Figures E3A and E3B). As shown in Figure 4C, phosphorylation levels of MARCKS and AKT were repressed in MPS-treated cells but not in aspartates-substituted MPS (Mut) peptide-treated cells. Furthermore, exposure to MPS peptide was demonstrated to dose-dependently depress ERK1/2 MAP kinase activity (see Figure E3C), which has been reported to be modulated by PI3K signaling (27).
Figure 4.
MPS peptide has a cancer-inhibitory effect. (A) Western blot analysis of phosphorylation of myristoylated alanine-rich C kinase substrate (phospho-MARCKS) levels in MPS peptide-treated cells. (B) Levels of phosphatidylinositol (3,4,5)-triphosphate (PIP3) in epidermal growth factor receptor tyrosine-kinase inhibitor–resistant non–small cell lung cancer cells after MPS peptide treatment. *P < 0.05 compared with untreated cells. (C) Cells were treated with 100 μM MPS or Mut peptide and lysates were immunoblotted with the indicated antibodies. (D) Cell viability analysis of six cancer cell lines and human bronchial epithelial cells (HBE1) upon MPS treatment. Cells were incubated with various concentrations of MPS peptide for 72 hours and then subjected to MTS assays. 50 μM polylysine and Mut (aspartates-substituted mutant) peptide served as peptide controls. n = 4, *P < 0.05 compared with untreated cells (Con). (E) Cells were treated with the indicated concentrations of MPS peptide and colonies were counted after 10 days of treatment using crystal violet staining. *P < 0.05 versus untreated cells (0 μM). (F) Cells were exposed to either MPS or Mut (aspartates-substituted mutant) peptide for 48 hours. The percentages of apoptotic cells were quantified by flow cytometry (top), and presented as mean ± SD of three experiments (bottom). *P < 0.05 versus untreated cells (Con). (G) Western blot analysis of cleaved caspase-3 and PARP in MPS-treated cells. Mut peptide (aspartates-substituted mutant; 100 μM) used as a control peptide.
Based on the molecular results, we examined whether the MPS peptide could serve as a cancer growth inhibitor. Six cancer cell lines and one normal epithelial cell line were treated with various doses of MPS peptide for 72 hours. Impaired cell viability was found in MPS-treated cancer cells but not in normal ones (Figure 4D). Similarly, we did not observe any cytotoxicity in normal human bronchial epithelial cells after treatment with various concentrations of MPS peptide (see Figure E3D). Of note, we found an obvious antiproliferative effect of the MPS peptide on various TKI-resistant cancer cells, including H1975, HCT116, H1650, and CL1–5 cells, all of which have either a PI3K CA (constitutively activated) mutation or loss of PTEN function (see Table E3). Consistent with the previous observations, the clonogenic abilities of drug-resistant cancer cells were repressed by MPS peptide treatment and this inhibition seemed to be concentration-dependent (Figure 4E; see Figure E3E).
Because we noticed some MPS-treated cells displayed typical apoptotic morphology, we assessed the sub-G1 fraction via flow cytometry. We found a significant increase in the sub-G1 fraction of MPS-treated groups as compared with untreated or Mut-peptide–treated cells to a level of over 30% higher (Figure 4F). Additionally, Western blots revealed an MPS dose-dependent occurrence for both cleaved caspase-3 and PARP in these treated cells (Figure 4G). Our results suggest a strong cancer-specific suppressive activity of MPS peptide.
MPS Peptide Inhibits Lung Cancer Progression In Vivo
To determine the anticancer effect of MPS peptide in vivo, H1975 cells were injected subcutaneously into nude mice. Mice were randomly grouped and then received phosphate-buffered saline, Mut peptide, or MPS peptide for seven injections (21 d of treatment). As shown in Figures 5A and 5B, the MPS-treated group showed significantly reduced tumor growths, as compared with either the phosphate-buffered saline–treated or Mut-treated group. The average tumor weights were significantly decreased, from 0.69 g in the phosphate-buffered saline–treated group to 0.28 g in the MPS-treated group (Figure 5B, right). Specifically, IHC staining showed that both phospho-MARCKS and phospho-AKT levels were reduced in MPS-treated xenograft tumor sections (Figure 5C), in agreement with in vitro observations (Figure 4C).
Figure 5.
Suppressive effects of MPS peptide on cancer growth and metastasis in vivo. (A–C) Nude mice bearing subcutaneous tumors were treated for 21 days with intraperitoneal injections of phosphate-buffered saline (PBS), Mut, or MPS peptide at the dosage of 28 mg/kg/3 days. (A) Tumor volumes were measured and data were presented as mean ± SD (n = 5). *P < 0.05 as compared with PBS group. (B) A representative photograph of primary tumors (left) and tumor weights (right) (n = 5, mean ± SE). *P < 0.05 as compared with PBS-treated group. (C) Immunohistochemical staining of phosphorylation of myristoylated alanine-rich C kinase substrate (phospho-MARCKS) (Ser159/163) and phospho-AKT (Thr308) in xenograft tumor sections as described in B. (D) Nude mice bearing subcutaneous tumors formed from PC9 cells were treated for 21 days with PBS, RNS, MANS, or MPS peptide. Top, A representative photograph of primary tumors from subcutaneous tissue of nude mice. Bottom, Tumor weights are average values from six mice per group. Data were shown as mean ± SE. *P < 0.01 as compared with PBS-treated group. (E) Inhibition of lung cancer metastasis by MPS peptide. Dissociated PC9 cells were orthotopically injected into the left lobe of the mouse lung. After mice were injected intraperitoneally with PBS, RNS, or MPS peptide (14 mg/kg/3 d) for a total of 10 injections, the organs were examined. Left, Gross photographs of various organs removed from the mice. The arrows indicate metastatic tumor nodules in each organ and the arrowhead indicates the primary lung tumor at the injected lobe. Right, Quantification of the average pulmonary metastasis nodules from mice with injected cancer cells and treated with RNS or MPS peptide as described (*P < 0.05 vs. PBS).
We recently have shown that the MANS peptide, a 24–amino-acid peptide corresponding to the myristoylated N-terminus of MARCKS, had an effect in reducing lung cancer metastasis but not in tumorigenesis (22). To further characterize the effect of these two peptides on tumor growth, we performed the treatment on subcutaneous xenograft tumors with these peptides. As expected, MPS peptide was very effective in the inhibition of tumor growth, whereas MANS and the control scramble RNS peptides were not (Figure 5D). Moreover, MPS treatment also showed a marked suppression of cell migration (see Figure E4). To determine whether the MPS peptide inhibits metastatic activities in vivo, we orthotopically inoculated highly metastatic PC9 cancer cells into the left lung of mice and examined the metastatic nodules of the right lung of mice that received various treatments for 28 days. Similar to MANS peptide (22), MPS was very effective in suppressing lung cancer metastasis and the size of the tumor nodule in the inoculated lung lobe (Figure 5E). These data suggest that targeting MARCKS PSD with MPS peptide may be more potent than MANS in the control of lung cancer progression.
MPS Peptide Acts Synergistically with EGFR Inhibitor Erlotinib in Lung Cancer Treatment
Based on the previously mentioned findings, we presumed that cotreatment with MPS peptide may enhance drug sensitivity of EGFR-TKI–resistant cells. The erlotinib-resistant cells were cotreated with various doses of erlotinib and 50 μM MPS peptide for 48 hours. MTS viability assays have shown that the combined erlotinib (0.5–5 μM) and 50-μM MPS peptide treatment resulted in a significant decrease of erlotinib IC50 (half maximal inhibitory concentration) (see Figure E5A). Likewise, we noticed an increase of floating or dead cells in lung cancer cells receiving erlotinib and MPS peptide cotreatment, compared with cells receiving erlotinib alone (Figure 6A). In particular, combination indices indicated a synergistic interaction between erlotinib and MPS peptide treatment in erlotinib-resistant cells (see Figure E5B; Figure 6B).
Figure 6.
MPS peptide and erlotinib administered in combination decrease lung cancer growth. (A) H1975 cells were individually treated with 5 μM erlotinib, 50 μM MPS peptide, or combinations of 5 μM erlotinib and 50 μM MPS peptide. After 48 hours, cell morphology (left) was photographed and cell viability (right) was determined by trypan blue exclusion assay (n = 4). Cell viability was calculated by the number of viable cells per the number of total cells × 100. *P < 0.05. (B) Combination indices of epidermal growth factor receptor (EGFR) inhibitor erlotinib with MPS peptide in EGFR tyrosine-kinase inhibitor–resistant non–small cell lung cancer cells. Cells were treated with combinations of erlotinib plus MPS peptide at various concentrations and combination indices (CI) were determined by using CalcuSyn software. The experimental values of the fixed dose ratios of erlotinib/MPS combinations showed a synergistic effect (CI < 1) at median effective dose (ED50). (C and D) Growth curves and tumor weights of xenograft tumors generated by subcutaneous injection of H1975 cells into the nude mice were shown. Once tumor volume reached an average of 60–80 mm3 at the injected site, mice were randomly grouped for intraperitoneal injection, once for every 3 days with vehicle, Mut peptide (28 mg/kg), MPS peptide (28 mg/kg), or erlotinib (14 mg/kg) alone or together with MPS peptide (28 mg/kg). Tumor measurements were made every 3 days and data were presented as mean ± SD (C, n = 5). *P < 0.05 as compared with erlotinib alone. After 21 days of treatment, the subcutaneous tumors were removed and weighed. Data were expressed as the mean ± SE (D, n = 7). (E) Immunohistochemical staining of PCNA and activated caspase-3 in xenograft tumors as described in D. A representative image was shown and positive staining was quantified as mean ± SD (n = 7). Scale bar: 50 μm.
To further investigate the synergistic effect of MPS peptide with erlotinib in vivo, we subcutaneously injected EGFR-TKI–resistant H1975 cells into nude mice and divided them randomly into several treatment groups. There was a significant amount of growth inhibition seen in the cotreatment of erlotinib and MPS peptide as compared with erlotinib alone after 12 days of treatment (Figure 6C). As indicated by Figure 6D, the combination of erlotinib and MPS peptide treatment led to the greatest reduction in tumor size and its weight. We also found an obvious reduction of proliferating cell nuclear antigen expression and increase of activated caspase-3 in these xenograft tumors with the combination treatment (Figure 6E). These results suggest the potential of using MPS peptide alongside with erlotinib for the cotreatment of lung cancer.
Discussion
MARCKS phosphorylation is thought to be involved in cell motility and exocytic vesicle release through actin cytoskeletal remodeling. Emerging evidence has suggested that phospho-MARCKS can specifically regulate cancer migration and metastasis (22, 28–31). Here, we show that elevated phospho-MARCKS (pSer159/163) is a predictor for poor outcomes and also correlated with EGFR-TKI therapy in lung cancer patients. Mechanistically, our results provide the first demonstration of phospho-MARCKS in promoting PIP3 pools and AKT activity as well as cancer growth. These findings have shown additional functions of phospho-MARCKS in cancer and extend its role beyond cell motility. Additionally, we have identified a peptide derived from the MARCKS PSD, the MPS peptide, as a potential therapeutic agent to block phospho-MARCKS–associated functions in lung cancer.
In clinical practice, most lung cancer patients with EGFR-activating mutations are treated with EGFR-TKIs. Unfortunately, most patients ultimately develop drug resistance and relapse (1, 5, 6). Discovery of an alternative targeted therapy in addition to EGFR signaling inhibition would be extremely helpful for the treatment of lung cancers with EGFR-TKI resistance. In this work, we hypothesized that MARCKS signaling may potentially be an alternative pathway and act as a critical regulator for the crosstalk of signaling between EGFR and PI3K/AKT pathways. Initially, our clinical data indicated an association of increased phospho-MARCKS with shortened patient survival and EGFR-TKI therapy. Experimentally, we have shown that cells with elevated phospho-MARCKS levels are more resistant to erlotinib treatment. These results suggest the importance of phospho-MARCKS status as a good candidate for predicting the response of lung cancer to erlotinib treatment. Both in vitro and in vivo evidence concerning combined treatment of erlotinib with MPS peptide clearly supports the notion further that an inhibition of MARCKS phosphorylation may be able to reduce the occurrence of drug resistance.
PI3K is well known to drive tumor progression through an activation of AKT, triggering a cascade of responses, including cell survival, proliferation, invasiveness, and motility (7, 24). Although many ATP-binding site inhibitors have been generated for targeting various kinases (PI3K, AKT, and mTOR) (11), it is important to consider alternative modes for pathway interruption. Reducing the availability of PIP2 to PI3K may represent a powerful approach to alter PI3K/AKT signaling, because PIP3 synthesis from PIP2 is a universal upstream step in PI3K signaling. Our studies provide evidence that is indicative of the fact that the PIP3 required for AKT activation is exclusively supplied after MARCKS phosphorylation. The coimmunoprecipitation assays revealed a unique interaction between MARCKS and PI3K, depending on the phosphorylation status of MARCKS. Moreover, shRNA knockdown of MARCKS and MPS-mediated inhibition of MARCKS phosphorylation showed both a reduction in PIP3 pools and AKT phosphorylation. Particularly, MPS peptide is specific against MARCKS PSD and has been reported to not only directly attract PIP2 pools (32, 33) but also to be responsible for the down-regulation of MARCKS phosphorylation (34, 35).
Based on these observations, we speculated that phosphorylation within MARCKS PSD may contribute to the availability of PIP2 to PI3K. MARCKS unphosphorylated PSD motif can trap PIP2 and facilitate accumulation of PIP2 levels at the plasma membrane (32, 36). Because of the binding of PI3K with unphosphorylated MARCKS, PI3K may be able to immediately catalyze PIP2 to PIP3 after MARCKS PSD motif is phosphorylated, which releases PIP2 pools (Figure 3D). On the other hand, higher levels of phospho-MARCKS may be an indicator that cellular PIP3 levels are increased, leading to the promotion of AKT-dependent and -independent activations, especially in cancer cells with PTEN loss or PI3K dysregulation. Overall, inhibition of MARCKS PSD activity, leading to a decrease in PIP2 recruitment and availability of PIP2 to PI3K, may lead to a promising strategy for cancer drug development.
MARCKS PSD has been shown to be crucial for the multifunctions of MARCKS, mediating its membrane binding and release, calcium-calmodulin binding, actin binding, and phosphorylation (37). Many studies used MPS peptide (also termed ED peptide) to elucidate the role of MARCKS PSD (32, 33, 35, 38–40). Yet, the pharmacologic function of MPS peptide has been investigated in only a few papers (34, 41, 42). Our data indicate that MPS specifically inhibits the growth of a broad spectrum of cancer cells, whereas cytotoxicity did not occur in MPS-treated human normal cells, suggesting a cancer-specific suppressive activity.
Several possibilities may explain the mechanisms of how the MPS peptide can inhibit cancer progression and improve AKT-driven erlotinib resistance. First, the MPS peptide may trap PIP2 pools and compete with membrane-associated MARCKS for PIP2 binding because the MPS peptide has been previously confirmed to be inserted into the plasma membrane and directly interact with PIP2 (32, 33, 39). Reduction in MARCKS-mediated PIP2 accumulation may impair the availability of PIP2 to PI3K leading to a decrease in PIP3 synthesis and subsequently resulting in down-regulation of AKT activation. Second, it is well documented that both PKC and calmodulin can associate with MARCKS PSD and result in the detachment of MARCKS from membrane, leading to PIP2 release (37). There is a theoretical possibility that MPS peptide may interfere with the PKC-MARCKS and/or calmodulin-MARCKS interaction in addition to blocking the crosstalk of the previously mentioned signaling pathways, all of which are crucial pathways for cancer progression (43, 44). Lastly, MARCKS is recognized to be a cytoplasmic component of motility signaling complex, so we considered that MPS-mediated inhibition of cell migration may partly result from a decrease of phospho-MARCKS.
Previously, we had used a peptide against the N-terminus of MARCKS (the MANS peptide) to reduce lung cancer metastasis (22). Compared with MANS peptide, MPS peptide can comprehensively suppress cancer growth and malignancy, whereas the inhibitory effect of MANS peptide was only on cancer metastasis. This result suggests that targeting MARCKS PSD may be more useful than targeting the myristoylation domain. This notion is consistent with a previous study showing that myristoylation of MARCKS is not required for many of the in vivo functions of MARCKS through the use of a nonmyristoylatable MARCKS in MARCKS-null mice (45). MANS peptide could only repress some but not all of MARCKS signaling; in contrast, MPS peptide diminishes both PIP3 pools and phospho-MARCKS levels through specifically suppressing MARCKS PSD activity. Therefore, MPS peptide can trigger a variety of cellular responses, including the inhibition of cell growth, induction of apoptosis, restoration of cellular sensitivity to erlotinib, and a suppression of cell motility. These effects are preferentially induced in PTEN-deficient H1650 or PIK3CA mutant H1975 cells, suggesting that targeting PSD may be an effective therapy against human tumors characterized by elevated PIP3 levels.
PIP3 signaling has been documented to activate several proteins, such as PDK1 and P-Rex, which are AKT-independent and have been implicated in promoting cancer progression (46, 47). Thus, it is possible that the suppressive effects of MPS peptide on cancer growth may also be through an inhibition of these AKT-independent signalings. Additional work is needed to clarify the importance between these AKT-dependent and -independent activations in cells after MPS peptide treatment. In addition, we have to point out the superiority of inhibiting MARCKS PSD activity by MPS peptide for cancer treatment over the use of PKC inhibitors because MARCKS PSD activity is targeted by PKC and Rho kinase (22). However, for many years, the use of PKC inhibitors for cancer treatment had disappointing results in clinical trials. This is because of a large number of PKC isoforms and their diverse signaling effects (48) and various signaling crosstalks, which make the specificity of the inhibitor treatment difficult. To date, targeting the PKC pathways has been considered to narrow the inhibition to downstream mediators of PKCs. Phospho-MARCKS is known to be a convergent downstream among multiple PKC isoforms (22); thus, given our results, targeting the MARCKS PSD motif by MPS peptide, instead of PKC inhibitors, is an intriguing strategy for cancer therapy. Importantly, the in vivo data represent a unique step toward the potential application of a peptide-based therapy in cancer treatment and also provide a proof-of-concept basis for identifying additional small molecules that inhibit the activity of MARCKS PSD.
Acknowledgments
Acknowledgment
The authors thank Dr. Christopher M. Barker (Department of Pathology, University of California Davis) for statistic advice.
Footnotes
Supported by National Institutes of Health grants HL077902, HL096373, HL36982, and T32 HL007013 and California Tobacco-Related Disease Research Program grant 23FT-0104.
Author Contributions: Conception and design, C.-H.C. and R.W. Development of methodology, C.-H.C., P.T., and R.W. Acquisition of data (i.e., functional bioassays and molecular analyses), C.-H.C., M.A., and R.W. Analysis and interpretation of data (i.e., statistical and computational analysis), C.-H.C., S.S., and C.-L.C. Writing, review, and/or, revision of the manuscript, C.-H.C., S.S., K.B.A., and R.W. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases), C.-H.C. and R.W. Study supervision, R.W.
Originally Published in Press as DOI: 10.1164/rccm.201408-1505OC on October 15, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Crinò L, Weder W, van Meerbeeck J, Felip E ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 4.Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, McShane LM, Milton DT, Strawn JR, Wakelee HA, Giaccone G. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 5.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. doi: 10.1513/pats.200809-110LC. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Shigematsu H, Nomura M, Lockwood WW, Sato M, Okumura N, Soh J, Suzuki M, Wistuba II, Fong KM, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008;68:6913–6921. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T, Seeger JM, Weiss J, Fischer F, Frommolt P, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck E, Eyzaguirre A, Brown E, Petti F, McCormack S, Haley JD, Iwata KK, Gibson NW, Griffin G. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 11.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer. 2013;14:322–332. doi: 10.1016/j.cllc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter CL, Auger KR, Chanudhuri M, Yoakim M, Schaffhausen B, Shoelson S, Cantley LC. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268:9478–9483. [PubMed] [Google Scholar]
- 15.Gambhir A, Hangyás-Mihályné G, Zaitseva I, Cafiso DS, Wang J, Murray D, Pentyala SN, Smith SO, McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–2207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–40990. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- 19.Green TD, Crews AL, Park J, Fang S, Adler KB. Regulation of mucin secretion and inflammation in asthma: a role for MARCKS protein? Biochim Biophys Acta. 2011;1810:1110–1113. doi: 10.1016/j.bbagen.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol. 2010;42:586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein. Am J Pathol. 2007;171:1822–1830. doi: 10.2353/ajpath.2007.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CH, Thai P, Yoneda K, Adler KB, Yang PC, Wu R. A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis. Oncogene. 2014;33:3696–3706. doi: 10.1038/onc.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CH, Chiu CL, Adler KB, Wu R. A novel predictor of cancer malignancy: up-regulation of myristoylated alanine-rich C kinase substrate phosphorylation in lung cancer. Am J Respir Crit Care Med. 2014;189:1002–1004. doi: 10.1164/rccm.201401-0053LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 25.Sangodkar J, Dhawan NS, Melville H, Singh VJ, Yuan E, Rana H, Izadmehr S, Farrington C, Mazhar S, Katz S, et al. Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J Clin Invest. 2012;122:2637–2651. doi: 10.1172/JCI62058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamasaki F, Johansen MJ, Zhang D, Krishnamurthy S, Felix E, Bartholomeusz C, Aguilar RJ, Kurisu K, Mills GB, Hortobagyi GN, et al. Acquired resistance to erlotinib in A-431 epidermoid cancer cells requires down-regulation of MMAC1/PTEN and up-regulation of phosphorylated Akt. Cancer Res. 2007;67:5779–5788. doi: 10.1158/0008-5472.CAN-06-3020. [DOI] [PubMed] [Google Scholar]
- 27.Chetram MA, Hinton CV. PTEN regulation of ERK1/2 signaling in cancer. J Recept Signal Transduct Res. 2012;32:190–195. doi: 10.3109/10799893.2012.695798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Techasen A, Loilome W, Namwat N, Takahashi E, Sugihara E, Puapairoj A, Miwa M, Saya H, Yongvanit P. Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci. 2010;101:658–665. doi: 10.1111/j.1349-7006.2009.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Rotenberg SA. PhosphoMARCKS drives motility of mouse melanoma cells. Cell Signal. 2010;22:1097–1103. doi: 10.1016/j.cellsig.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama Y, Ito T, Hanson V, Schwartz GK, Aderem AA, Holland JF, Tamaya T, Ohnuma T. PMA-induced reduction in invasiveness is associated with hyperphosphorylation of MARCKS and talin in invasive bladder cancer cells. Int J Cancer. 1998;75:774–779. doi: 10.1002/(sici)1097-0215(19980302)75:5<774::aid-ijc18>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A, Rebecchi M, Scarlata SF, Runnels LW, Prestwich GD, et al. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem. 1996;271:26187–26193. doi: 10.1074/jbc.271.42.26187. [DOI] [PubMed] [Google Scholar]
- 33.Ellena JF, Burnitz MC, Cafiso DS. Location of the myristoylated alanine-rich C-kinase substrate (MARCKS) effector domain in negatively charged phospholipid bicelles. Biophys J. 2003;85:2442–2448. doi: 10.1016/s0006-3495(03)74667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elzagallaai A, Rosé SD, Trifaró JM. Platelet secretion induced by phorbol esters stimulation is mediated through phosphorylation of MARCKS: a MARCKS-derived peptide blocks MARCKS phosphorylation and serotonin release without affecting pleckstrin phosphorylation. Blood. 2000;95:894–902. [PubMed] [Google Scholar]
- 35.Graff JM, Rajan RR, Randall RR, Nairn AC, Blackshear PJ. Protein kinase C substrate and inhibitor characteristics of peptides derived from the myristoylated alanine-rich C kinase substrate (MARCKS) protein phosphorylation site domain. J Biol Chem. 1991;266:14390–14398. [PubMed] [Google Scholar]
- 36.Kalwa H, Michel T. The MARCKS protein plays a critical role in phosphatidylinositol 4,5-bisphosphate metabolism and directed cell movement in vascular endothelial cells. J Biol Chem. 2011;286:2320–2330. doi: 10.1074/jbc.M110.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbuzova A, Schmitz AA, Vergères G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theis T, Mishra B, von der Ohe M, Loers G, Prondzynski M, Pless O, Blackshear PJ, Schachner M, Kleene R. Functional role of the interaction between polysialic acid and myristoylated alanine-rich C kinase substrate at the plasma membrane. J Biol Chem. 2013;288:6726–6742. doi: 10.1074/jbc.M112.444034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton LA, Yang H, Saludes JP, Fiorini Z, Beninson L, Chapman ER, Fleshner M, Xue D, Yin H. MARCKS-ED peptide as a curvature and lipid sensor. ACS Chem Biol. 2013;8:218–225. doi: 10.1021/cb300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinrichsen RD, Blackshear PJ. Regulation of peptide-calmodulin complexes by protein kinase C in vivo. Proc Natl Acad Sci USA. 1993;90:1585–1589. doi: 10.1073/pnas.90.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gay EA, Klein RC, Melton MA, Blackshear PJ, Yakel JL. Inhibition of native and recombinant nicotinic acetylcholine receptors by the myristoylated alanine-rich C kinase substrate peptide. J Pharmacol Exp Ther. 2008;327:884–890. doi: 10.1124/jpet.108.144758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timofeeva OA, Eddins D, Yakel JL, Blackshear PJ, Levin ED. Hippocampal infusions of MARCKS peptides impair memory of rats on the radial-arm maze. Brain Res. 2010;1308:147–152. doi: 10.1016/j.brainres.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teicher BA. Protein kinase C as a therapeutic target. Clin Cancer Res. 2006;12:5336–5345. doi: 10.1158/1078-0432.CCR-06-0945. [DOI] [PubMed] [Google Scholar]
- 44.Hait WN, Lazo JS. Calmodulin: a potential target for cancer chemotherapeutic agents. J Clin Oncol. 1986;4:994–1012. doi: 10.1200/JCO.1986.4.6.994. [DOI] [PubMed] [Google Scholar]
- 45.Swierczynski SL, Siddhanti SR, Tuttle JS, Blackshear PJ. Nonmyristoylated MARCKS complements some but not all of the developmental defects associated with MARCKS deficiency in mice. Dev Biol. 1996;179:135–147. doi: 10.1006/dbio.1996.0246. [DOI] [PubMed] [Google Scholar]
- 46.Tan J, Li Z, Lee PL, Guan P, Aau MY, Lee ST, Feng M, Lim CZ, Lee EY, Wee ZN, et al. PDK1 signaling toward PLK1-MYC activation confers oncogenic transformation, tumor-initiating cell activation, and resistance to mTOR-targeted therapy. Cancer Discov. 2013;3:1156–1171. doi: 10.1158/2159-8290.CD-12-0595. [DOI] [PubMed] [Google Scholar]
- 47.Pandiella A, Montero JC. Molecular pathways: P-Rex in cancer. Clin Cancer Res. 2013;19:4564–4569. doi: 10.1158/1078-0432.CCR-12-1662. [DOI] [PubMed] [Google Scholar]
- 48.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]