Abstract
Purpose
Activation of the c-Met and epidermal growth factor receptors (EGFR) promotes growth and survival of non-small cell lung cancer (NSCLC). Specific receptor antagonists have demonstrated efficacy in the clinic; however, tumors often become resistant to these therapies. We have investigated the ability of (-)-epigallocatechin-3-gallate (EGCG) to inhibit cell proliferation, and c-Met receptor and EGFR kinase activation in several NSCLC cell lines.
Experimental Design
NSCLC cell lines with variable sensitivity to the EGFR antagonist erlotinib were studied. Cell growth was evaluated using MTS and colony formation assays. Kinase activation was assessed via western blot analysis. Experiments were conducted with EGCG, the EGFR antagonist erlotinib and the c-Met inhibitor SU11274. The antagonists were also tested in a xenograft model using SCID mice.
Results
EGCG inhibited cell proliferation in erlotinib sensitive and resistant cell lines, including those with c-Met overexpression and acquired resistance to erlotinib. The combination of erlotinib/EGCG resulted in greater inhibition of cell proliferation and colony formation than either agent alone. EGCG also completely inhibited ligand-induced c-Met phosphorylation and partially inhibited EGFR phosphorylation. The triple combination of EGCG/erlotinib/SU11274 resulted in a greater inhibition of proliferation than EGCG with erlotinib. Finally, the combination of EGCG and erlotinib significantly slowed the growth rate of H460 xenografts.
Conclusion
EGCG is a potent inhibitor of cell proliferation, independent of EGFR inhibition, in several NSCLC cell lines, including those resistant to both EGFR kinase inhibitors and those overexpressing c-Met. Therefore, EGCG might be a useful agent to study as an adjunct to other anti-cancer agents.
Keywords: EGCG, c-Met, EGFR, erlotinib, non-small cell lung carcinoma
Introduction
Lung cancer is the most common cause of cancer death in the United States. Non-small cell lung carcinoma (NSCLC) represents 80% of lung cancers, and most patients present with stage IIIB & IV disease. Five-year survival for these patients remains poor at less than 10%. Activation of receptor tyrosine kinases such as c-Met and EGFR are known to be important in lung cancer carcinogenesis, and this has lead to the development of specific targeted therapies. Overexpression of the EGFR is found in the majority of NSCLC (1); however, in unselected patient populations, treatment success with the EGFR inhibitors erlotinib and gefitinib is poor (2, 3). Clinical studies with the use of erlotinib concurrently with cisplatin doublet chemotherapy as first-line therapy for Stage IIIB and IV NSCLC did not provide a survival advantage (4, 5). However, the BR.21 study demonstrated that erlotinib did improve survival in patients who had failed first- and second-line chemotherapy, and these results provided the basis for approval of erlotinib as second-line chemotherapy in the United States (6). It is now clear that a significant percentage of responsive tumors have activating mutations rendering the EGFR more responsive to EGF; resulting in increased signaling through pro-survival and anti-apoptotic pathways including Erk1/2, PI3K-Akt, STAT3 and STAT5 (1, 2). The activating mutations also permit greater competition by drug inhibitors for ATP in the catalytic active site (1, 2). These gain of function mutations occur more commonly in Asian patients (30 to 40%) than in Caucasians (10%) which may explain the difference in clinical responsiveness to erlotinib and gefitinib (2, 3). However, second site mutations in the EGFR render cells resistant to reversible EGFR inhibitors (1, 7). Clinically, this resistance often develops within 6 to 12 months after initiation of therapy and precludes a permanent response (7).
Activation of other receptor tyrosine kinases is important in lung cancer pathogenesis. The c-Met receptor and its downstream signaling pathways are an important example of this principle. The c-Met receptor is expressed in up to 61-75% of clinical NSCLC tissues and is often overexpressed (8, 9). Stimulation of c-Met activates Erk1/2, PI3K-Akt, STAT, and phospholipase C (10). The MET gene is amplified in some NSCLC cell lines, resulting in constitutive activation of the receptor (11). Importantly, c-Met amplification has also been detected in clinical NSCLC tissues from patients who had poor response to EGFR antagonists (12). c-Met overexpression resulted in activation of ERBB3 and PI3K/Akt thereby inducing resistance to gefitinib in vitro (12). Thus, it is increasingly apparent that inhibition of multiple signaling pathways including EGFR, c-Met, and others may be necessary to inhibit the growth of tumor cells (e.g. (13, 14)). Currently, there are no small molecule inhibitors of c-Met that have been approved for use, although a number of clinical trials have been opened.
Tea polyphenols are being investigated as possible neoadjuvant and adjuvant therapy for cancer due to their ability to inhibit multiple signaling pathways. A major component of tea polyphenols are the catechins; a family that includes (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin-3-gallate, and (-)-epigallocatechin-3-gallate (EGCG) (15). EGCG impairs cancer cell growth by a variety of mechanisms including, inhibition of receptor kinases such as EGFR, HER-2, c-Met, PDGFR, IGFR, VEGFR, and downstream kinases including Erk1/2, STAT3, PI3K, amongst others (reviewed in (16, 17)). EGCG also impairs cell signaling via effects on membrane lipids (18) and lipid rafts (Duhon et al; manuscript in preparation), and EGCG induces G0/G1 cell cycle arrest via increasing expression of p21, p18, and p53, and reduced expression of cyclins D1 and E as well as CDK2, 4, and 6 (16, 17). Cells treated with EGCG may undergo apoptosis due to effects on receptor signaling and p53, as noted above, in addition to upregulation of the pro-apoptotic protein Bax (19) and downregulation of the anti-apoptotic protein Bcl-2 (20). Finally, a clinical trial in Italy demonstrated that consumption of green tea catechins significantly delayed the appearance of prostate cancer in men with high grade prostatic intraepithelial neoplasia (HGPIN) (21). Our laboratory has also determined in a Phase II trial, that men with prostate cancer who consumed 1.3 grams of green tea catechins per day for 6 weeks demonstrated a reduction in serum levels of HGF, VEGF, and tissue levels of the phosphorylated, active forms of c-Met and Akt (McLarty et al., manuscript under review). EGCG is not without some potential risks as a recent paper reported that EGCG activity actually lowered the effectiveness of the proteasomal inhibitor Velcade (22).
Although EGCG has been tested in numerous cancer cells lines and some clinical trials, there is minimal data of its effectiveness in lung cancer. It appears that EGCG does impair growth in small cell lung cancer cells, but has a variable effect in the limited number of NSCLC cell lines tested (23, 24). The evidence that tea polyphenols exert inhibitory effects in numerous and distinct cancer cells led us to investigate the activity of these compounds on NSCLC cells in vitro and in vivo to determine if EGCG in combination with targeting strategies would be more effective than treatment with single agents. In this report, we demonstrate the effectiveness of EGCG to sensitize previously insensitive NSCLC cell lines to erlotinib in vitro and in vivo.
Materials and Methods
Cell culture
The NSCLC cell lines H2122, H358, H460, H1975 and H1993 were obtained from the American Type Culture Collection. The cells were cultured in RPMI 1640 containing 10% fetal bovine serum (complete medium). H2122, H358 and H460 cells contain a wild type EGFR gene, but all have KRAS mutations (25-28). H1975 cells contain the L858R EGFR activating mutation, but also the T790M second-site mutation conferring resistance to EGFR inhibitors (1). This cell line has wild type KRAS (26). H1993 cells demonstrate MET gene amplification and c-Met receptor overexpression (11).
(-)-Epigallocatechin-3-gallate (EGCG) and (-)-epicatechin (EC) (Sigma Chemical, St Louis, MO) were prepared as 25 mM stocks in 10 mM MES, pH 6.5 buffer. This was diluted into culture media immediately prior to the experiments. Cells were preincubated 4 hours with the polyphenols prior to the addition of growth factors. The EGFR tyrosine kinase inhibitor erlotinib was a generous gift of Genentech (San Francisco, CA). It was maintained as a 10 mM stock in DMSO for in vitro experiments. EGF (human) was obtained from Sigma (St. Louis, MO). HGF and the c-Met receptor inhibitor SU11274 were obtained from Calbiochem (San Diego, CA). SU11274 was dissolved in DMSO.
Cell growth assay
Cells were plated in 96-well plates at 2,000 cells per well in complete medium. After 24 hours, the media was replaced with RPMI, 1% FBS with or without inhibitors. Each condition in each experiment was studied in 8 replicate wells. The cells were then cultured 72 hours in the continuous presence of inhibitor. Cell viability was assessed using a tetrazolium based method (CellTiter 96 AQueous, Promega, Madison, WI). The delta between day 3 and day 0 was calculated and the delta for each condition was then divided by the control value to obtain the percent of control.
Colony Assay
Cells were plated in 24 well plates at 500 cells/well in RPMI, 10% FBS. The cells were cultured for 24 hours and the media was then replaced with RPMI, 1% FBS with or without inhibitors. For these experiments, the media was removed and replaced with fresh media with or without inhibitors every 48 hours for a total of 7 days. At the end of the experiment, the media was removed and the cells were washed with PBS. The colonies were fixed with 4% paraformaldehyde in PBS for 15 min. The wells were then washed with PBS and the colonies were stained with 0.5 % crystal violet for 10 minutes. The stain was aspirated and the wells were washed with PBS until the background was clear. The wells were then photographed. As a semi-quantitative measurement, the crystal violet was extracted from the colonies with Sorenson's solution for 10 minutes and the absorbance was measured at 570 nm (29).
Gel electrophoresis and western blotting
Cells (8 × 104) were plated in single wells of 24-well plates, grown overnight and stimulated with EGF (100 ng/ml) or HGF (33 ng/ml) for 20 min. The cells were then lysed in Laemmli buffer (125 mM Tris, 4% SDS, 0.1% bromophenol blue, 30% sucrose and 5% mercaptoethanol). Protein was resolved using 10% SDS-PAGE, transferred to PVDF, blocked with 5% milk in TBST buffer and probed with antibodies and developed using enhanced chemiluminescence (GE Healthcare, UK as previously reported (30). Phospho-EGF receptor (Tyr 1148), phospho-c-Met receptor (Tyr1234/1235), phospho-Erk1/2 (Thr 202/Tyr 204), and phospho-Akt (Ser 473) antibodies were obtained from Cell Signaling Technology (Danvers, MA).
In Vivo Experiments
All experiments were performed in accordance with guidelines set by the LSUHSC-S Institutional Animal Care and Use Committee. Six to 8 week old male SCID/bg mice were injected with 2×106 H460 cells in 100 μl PBS subcutaneously (n=15 control; n=10 each drug treatment group). Three days post-implantation, the mice were treated with 10 mg/kg erlotinib, 15 mg/kg EGCG, or both in 2% Tween-80 via gavage. Control mice received 2% Tween-80 via gavage. Mice were placed on a 5 day on, 2 days off dosing schedule. Tumors became measurable by day 10 post-implantation and were measured with digital calipers three days per week. Tumor volumes were calculated by the equation: Volume = π/6 * Length * Width2. Mice were sacrificed 22 days post-implantation and tumors were surgically removed and weighed.
Statistical Analysis
In vitro experiments were performed with 8 replicates for MTS assays and 3 replicates for western blotting experiments. The experiments were repeated 3 to 4 times. Data were expressed as means ± SEM. For comparison of 3 or more groups, we used the one-way analysis of variance. If significance was noted, the Newman-Keuls multiple comparison test was then used. For comparison of two groups, the two-tailed, paired t test was used. In vivo statistics were performed using the Mann-Whitney nonparametric two-tailed test. P values of < 0.05 were considered significant. Data analysis was performed using GraphPad Prism version 5.01 software.
Results
Effect of EGCG on growth of non-small cell lung cancer cells
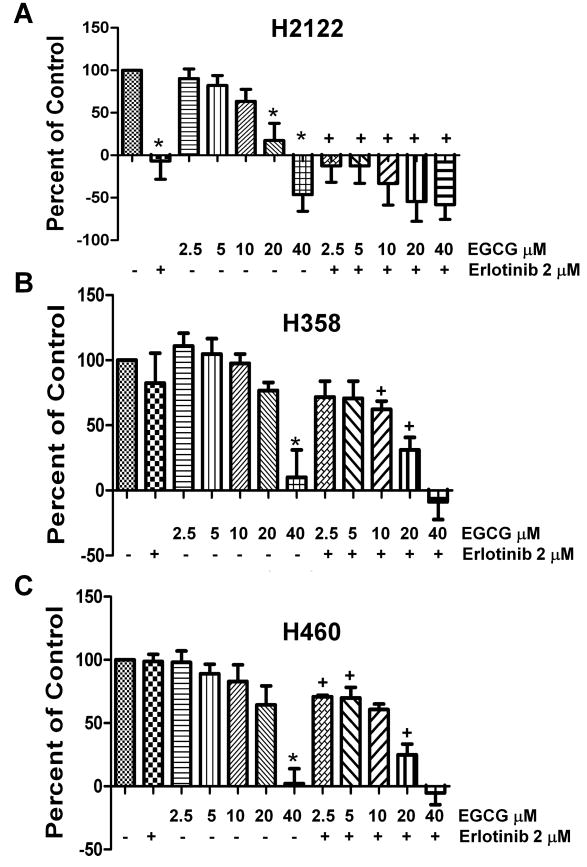
Three cell lines with variable sensitivity to erlotinib were chosen to investigate the effect of EGCG on cell growth. H2122 cells are sensitive; H358 cells have intermediate to low sensitivity, and H460 cells are resistant to erlotinib (26). An MTS proliferation assay revealed that EGCG demonstrated a dose-dependent inhibition of cell growth in all cell lines regardless of sensitivity to erlotinib (Figure 1). The erlotinib-sensitive H2122 cells were the most sensitive to EGCG with an IC50 of 13.1 μM. However, the two erlotinib-resistant cell lines were also sensitive to EGCG with IC50 values of 26.4 μM (H358) and 22.1 μM (H460).
Fig 1.
EGCG effects cell growth of NSCLC cells, and increases their sensitivity to erlotinib. Cell viability of H2122 (A), H358 (B) and H460 (C) non-small cell cancer cells in vitro in response to EGCG and erlotinib. Cells were cultured in 96-well plates in the presence of increasing concentrations of EGCG, erlotinib at 2 μM or combinations of EGCG plus erlotinib. Viability was assessed using a tetrazolium assay after allowing growth of cells for 72 h, and the change in the number of cells in treated wells were expressed as a percent relative to the changes in the number of cells in the control wells over 72 hours; normalized to 100. Negative numbers in the graph for the treated wells mean the number of cells decreased from the number of cells that were found in the control wells at the initiation of treatment (T=0).
* P < 0.05 compared with the control.
+ P < 0.05 EGCG alone vs. EGCG as same dose plus erlotinib.
To determine if EGCG could alter the sensitivity of NSCLC cells to the effects of erlotinib, increasing doses of EGCG were added to cells in combination with 2 μM erlotinib (Figure 1); a concentration of erlotinib that is a clinically achievable dose in patients (1), and that blocks the activation of EGFR (Figure 3C). The H2122 erlotinib-sensitive cells demonstrated an additive inhibitory effect of erlotinib with EGCG over a broad range; particularly at 10 and 20 μM concentrations of EGCG (Figure 1). The erlotinib-resistant cell lines H358 and H460 also demonstrated an additive effect on growth inhibition, and this occurred over a range of EGCG concentrations (Figure 1). Under these conditions, erlotinib alone had a marginal effect on proliferation of H358 cells and had no effect on H460 cells. In all cells lines, EGCG at 40 μM was sufficiently inhibitory so that there was no further additive effect in combination with erlotinib. These data suggest that exposure of cells to EGCG increases their response to erlotinib in multiple NSCLC cells.
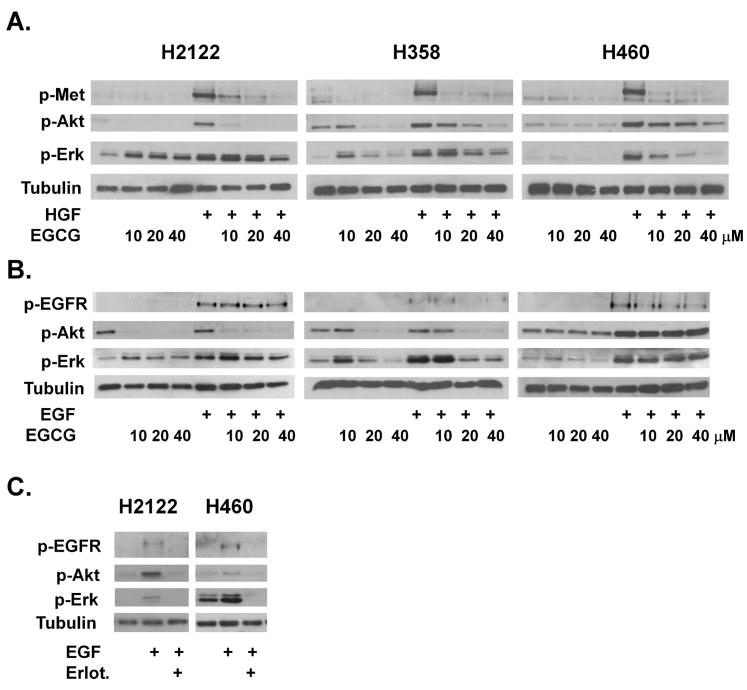
Fig 3.
EGCG effects signaling in growth factor receptor signaling in H2111, H358 and H460 NSCLC cells. Cells were stimulated with either HGF at 33 ng/ml (A) or EGF at 100 ng/ ml (B). As noted, cells were pretreated with EGCG 10 μM to 40 μM for 4 hours (A and B) or 2 mM erlotinib for 1 hour (C). Cell lysates were collected and subjected to SDS-PAGE, western blotting and analyzed with phospho-specific antibodies to the c-Met receptor (pMet), Akt (p-Akt), Erk1/2 (pErk) and the EGFR (pEGFR). Tubulin was used as a control for loading.
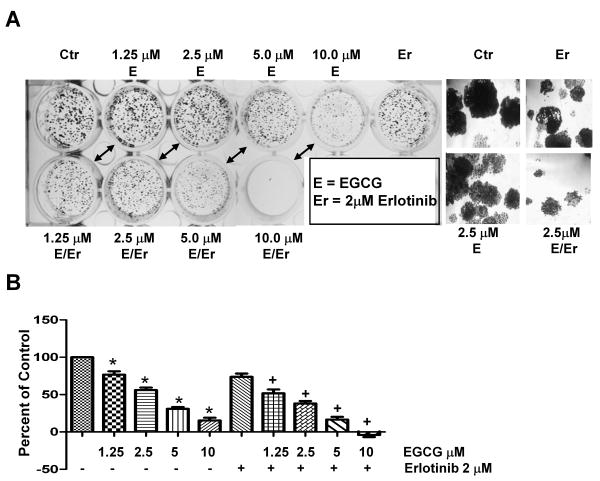
To further address the effects of EGCG combined with erlotinib, cells were dosed with EGCG every other day and examined for colony formation. For these experiments, the erlotinib-resistant cell line H460 was used. Figure 2A demonstrates that EGCG dose-dependently inhibited colony formation. This inhibition occurred with repetitive EGCG dosing of 1.25 – 10.0 μM with an IC50 of 3 μM, which was lower than the single dose IC50 inhibitory effect seen with the MTS assay (Figure 1). Combinations of EGCG with 2 μM erlotinib resulted in smaller colonies than EGCG alone (Figure 2A), suggesting that EGCG may sensitize cells to erlotinib treatment.
Fig 2.
EGCG and erlotinib are more effective than either agent alone at inhibiting growth of colonies. Cells were cultured at low density and treated with EGCG, erlotinib or combinations every 48 h for 7 days. (A) Colonies were stained with crystal violet and then photographed. In (B), the crystal violet was extracted and assayed by spectrophotometry. EGCG was used at the concentrations noted in the figure and erlotinib was used at 2 μM.
* P < 0.05 compared with FBS control.
+ P < 0.05 EGCG alone vs. EGCG as same dose plus erlotinib.
Crystal violet was extracted from the colonies and absorbance measured to provide a semi-quantitative measure of colony formation. As seen in Figure 2B, EGCG at 1.25 – 10 μM resulted in a significant inhibition of colony formation. Furthermore, the combination of erlotinib plus EGCG at 1.25, 2.5, 5.0 and 10 μM resulted in increased inhibition as compared to the corresponding dose of EGCG alone.
To investigate a potential mechanism by which EGCG inhibits cell growth, the effect of EGCG on phosphorylation of the growth factor receptor membrane tyrosine kinases c-Met and EGFR was examined. Stimulation of cells with HGF or EGF for 20 minutes induced phosphorylation of the c-Met and EGFR receptors in H2122, H358 and H460 cells (Figure 3A and 3B). The HGF-induced stimulation of c-Met was inhibited in a dose response fashion by a 4 hour pretreatment with 10-40 μM EGCG in all three cell lines (Figure 3A). These effects were specific for EGCG as the inactive analog (-)-epicatechin (EC) did not inhibit c-Met phosphorylation (data shown for H460 and H358 cells only, Figure S1). The EGF-induced stimulation of EGFR phosphorylation was inhibited up to 50% in a dose dependent fashion by 10-40 μM EGCG in H358 and H460 cells, while these concentrations had little effect on EGFR signaling in the erlotinib sensitive H2122 cells (Figure 3B). Furthermore, erlotinib prevented activation of EGFR and downstream signaling in all three cell lines (Figure 3C; H358 not shown), but only dramatically effected proliferation in H2122 cells (Figure 1).
Receptor tyrosine kinases are known to activate the downstream signaling kinases Erk1/2 and Akt, which are important in cell proliferation and survival. In a dose dependent fashion, EGCG inhibited HGF-induced phosphorylation of Akt and Erk1/2 in the H2122, H358 and H460 cells (Figure 3A). Inhibition of Akt phosphorylation was most evident in H2122 cells while inhibition of Erk1/2 was most evident in H358 and H460 cells. In contrast, the effect of EGCG on EGF signaling was cell-line dependent. In H358 and H2122 cells, increasing concentrations of EGCG reduced Akt phosphorylation. In contrast, in H460 cells, EGCG had little effect on the PI 3-Kinase pathway (Figure 3B). The MAP-Kinase pathway was inhibited by EGCG in H358 cells in a dose dependent fashion, while this pathway was relatively immune to EGCG in H460 and H2122 cells (Figure 3B).
We conclude that in H460 and H358 cells inhibition of EGFR by erlotinib is not sufficient to dramatically lower cell growth, and that EGCG reduces proliferation by a mechanism that most likely involves factors in addition to EGFR inhibition.
Effect of EGCG on growth of c-Met receptor overexpressing and EGF-receptor mutant cell lines
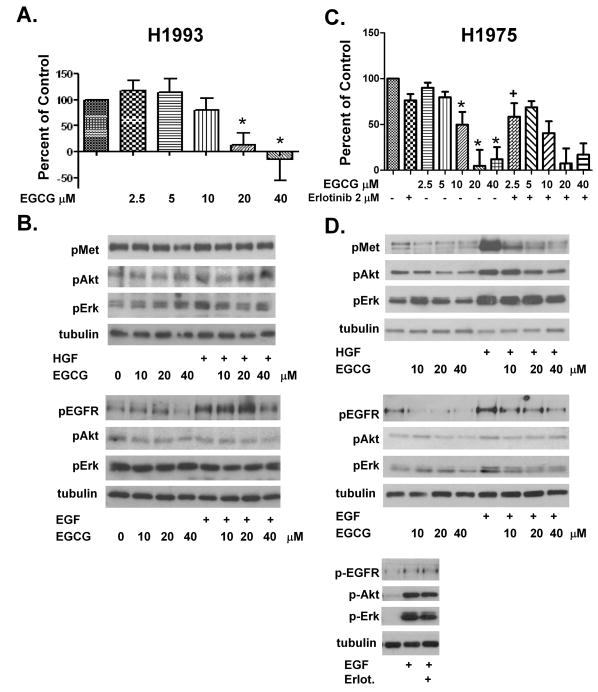
Activating mutations and overexpression of receptor tyrosine kinases such as Met and EGFR represent an important source of therapeutic resistance in NSCLC. To investigate this in our model, we used H1993 cells in which the MET gene is amplified resulting in overexpression and constitutive activation of the c-Met receptor. EGCG at 10-40 μM inhibited growth in these cells (IC50 17.9 μM) (Figure 4A). The H1993 cell line had strong basal phosphorylation of the c-Met receptor (Figure 4B) which was not further increased with HGF stimulation. Surprisingly, EGCG at concentrations between 10-40 μM did not significantly reduce c-Met phosphorylation or p-Akt and p-Erk1/2 (Figure 4B). Stimulation of H1993 cells with EGF results in phosphorylation of the EGFR, and this effect was partially blocked by pre-incubation with 40 μM EGCG, while 10 and 20 μM concentrations were not effective (Figure 4B).
Fig 4.
EGCG effects cell proliferation and kinase activity in the H1993 c-Met overexpressing cell line and the H1975 T790M EGFR TKI resistant cell line. In (A) (H1993) & (C) (H1975), cells were cultured in the presence of EGCG 2.5, 5.0, 10, 20 and 40 μM, or erlotinib 2 μM or combinations as noted. Viability was assessed after 72 h using a tetrazolium based assay and the data were expressed as described in the legend to Figure 1. In (B), H1993 cells were pretreated with EGCG 10-40 μM and then left unstimulated or EGF or HGF was added. In (D), H1975 cells were pretreated with EGCG 10-40 μM and left unstimulated or stimulated with HGF at 33 ng/ml or EGF at 100 ng/ml as noted. Cells were also treated with 2 μM erlotinib prior to exposure to EGF. In (B), and (D), cell lysates were subjected to SDS-PAGE and western blotting with phospho-specific antibodies to the c-Met receptor (p-Met), Akt (p-Akt), Erk1/2 (p-Erk) and the EGFR (pEGFR). Tubulin was used as a control for loading.
* P < 0.05 compared with FBS control.
+ P < 0.05 EGCG alone vs. EGCG as same dose plus erlotinib.
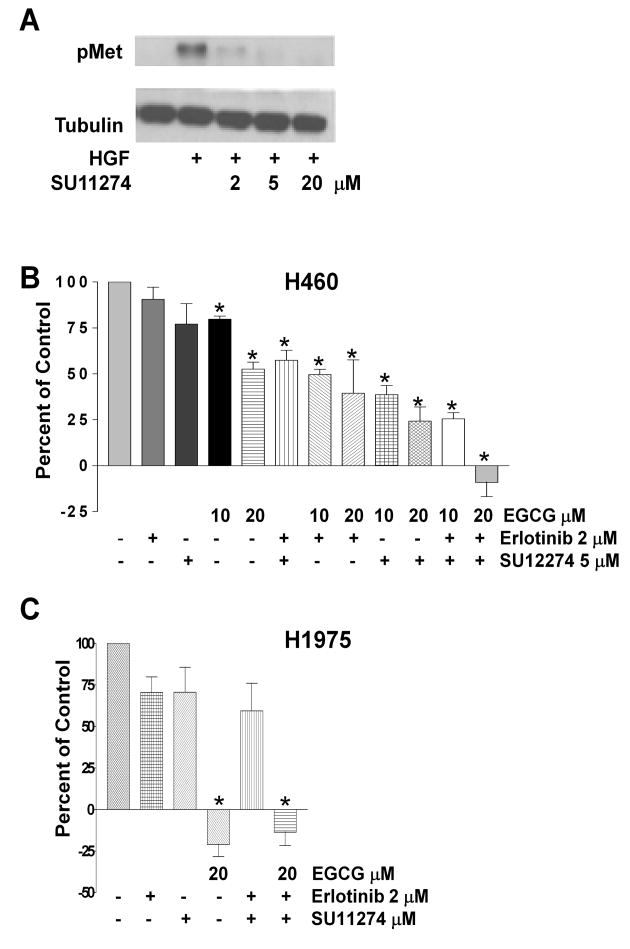
We also examined the effect of EGCG on the H1975 cell line which contains the EGFR second-site mutation T790M resulting in constitutive activation of EGFR and resistance to reversible receptor tyrosine kinase inhibitors (31, 32). Unlike the other NSCLC cells examined, we found that 10 and 20 μM EGCG profoundly inhibited cell growth in the H1975 cells (IC50 10.2 μM) (Figure 4C). These cells demonstrated basal phosphorylation of the c-Met receptor that was further induced by stimulation with HGF (Figure 4D). Pre-incubation with 10-40 μM EGCG decreased the level of c-Met phosphorylation in unstimulated and HGF-stimulated cells (Figure 4D) as well as the HGF-induced phosphorylation of p-Akt and p-Erk1/2. EGFR is phosphorylated under basal, unstimulated conditions in H1975 cells and phosphorylation increased in the presence of EGF (Figure 4D). Preincubation with 10-40 μM EGCG decreased EGFR phosphorylation by greater than 75% (at 20 μM) while downstream Akt and Erk signaling in the absence or presence of EGF was only slightly attenuated (Figure 4D). Erlotinib had no effect on EGFR phosphorylation (Figure 4D)
Effect of EGCG in combination with the c-Met inhibitor SU11274 and Erlotinib
It is increasingly recognized that therapeutic resistance can be due to activation of multiple kinase pathways (12-14, 33). These studies have demonstrated that inhibition of multiple pathways including the c-Met signaling pathway is necessary to induce cell death in numerous cancer cell lines. Therefore, we examined the effect of the c-Met kinase inhibitor SU11274 on NSCLC cells. Western blot analysis revealed that the concentrations of SU11274 used in these experiments prevented HGF-induced phosphorylation of c-Met (Figure 5A).
Fig 5.
Combinations of EGCG, a c-Met inhibitor and erlotinib synergistically block proliferation of H460 cells. In (A), H460 cells were pretreated with SU11274 at varying doses as indicated prior to stimulation with HGF 33 ng/ml. Lysates were subjected to PAGE and Western blotting with a phosphospecific antibody to the c-Met receptor. Tubulin was uses as a control for loading. In (B), H460 cells were cultured in the presence of EGCG at 10 or 20 μM, erlotinib at 2μM, or SU11274 at 5 μM alone or in combination. Viability was assessed after 72 h using a tetrazolium based assay and the data were expressed as described in the legend to Figure 1. In (C), H1975 cells were cultured in the presence of erlotinib at 2 μM, SU11274 at 5 μM, EGCG at 20 μM or combinations as noted. Viability was again assessed as described in the legend to Figure 1.
* P < 0.05 compared with FBS control.
In the erlotinib resistant cell-line H460, SU11274 was no more effective at inhibiting proliferation than erlotinib, while 10 and especially 20 μM EGCG slowed proliferation (Figure 5B). In fact, 20 μM EGCG alone was as effective as the combination of erlotinib and SU11274. However, combinations of EGCG plus erlotinib or SU11274 were more effective than EGCG alone (Figure 5B). Finally, a triple combination, EGCG plus erlotinib and SU11274, was most effective at inhibiting proliferation (Figure 5B) and cell numbers actually decreased when 20 μM EGCG was part of the combination.
In the erlotinib resistant cell-line H1975 (containing the second site mutation T790M), EGCG at 20 μM completely prevented proliferation while erlotinib and SU11274 alone or in combination were not effective (Figure 5C). Unlike what was observed with the H460 cells, the triple combination (EGCG, erlotinib and SU1124) was not more effective than EGCG.
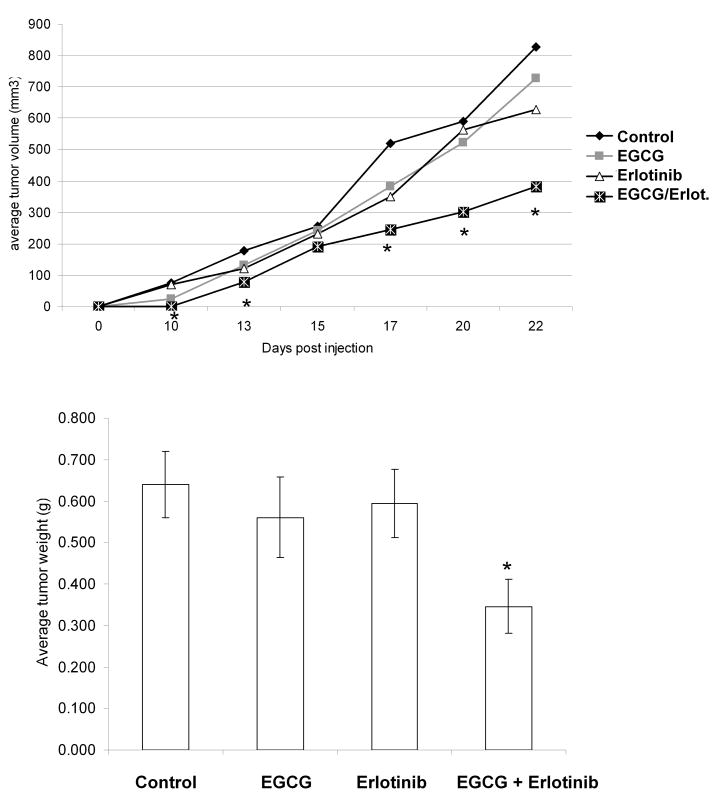
To determine if combinations of erlotinib and EGCG had in vivo efficacy, H460 cells (erlotinib resistant cells) were injected subcutaneously into the hind flank of ∼6-8 week old male SCID mice. Three days after tumor cell implantation, mice were treated with 10 mg/kg erlotinib, 15 mg/kg EGCG, or both in 2% Tween-80 via daily oral gavage. Beginning ten days post-injection, tumor volumes were measured as indicated in Figure 6. Administration of EGCG and erlotinib alone did not have any statistically significant effect on tumor volume versus control (p=0.56 and 0.16, respectively) (Figure 6A) or tumor weight (p=0.53 and 0.70, respectively) (Figure 6B). However, the combination of EGCG and erlotinib significantly reduced both tumor volume (p=0.03) and weight (p=0.011) versus control, confirming the combination results seen in vitro. Thus, EGCG appears to re-sensitize previously erlotinib-resistant cell lines to inhibit tumor growth in vivo.
Fig 6.
Combinations of EGCG and erlotinib are effective a slowing the growth of H460 tumors. Two million H460 cells were injected subcutaneously in male SCID mice. After 3 days, the mice were gavaged with EGCG (15/mg/kg), erlotinib (10/mg/kg), both EGCG and erlotinib or with 2% Tween-80 control as indicated. In (A), tumor volumes were measured. Data is expressed as cubic mm volume. In (B), animals were sacrificed at day 22 and tumors were dissected and weighed.
* P < 0.05 EGCG + erlotinib vs. control.
Discussion
Our results demonstrate that the tea polyphenol EGCG inhibited cell growth in a number of erlotinib sensitive and resistant NSCLC cell lines. Moreover, these effects were seen in the resistant H1975 cell line containing the EGFR second site T7980M activating mutation, and EGCG inhibited growth in cell lines containing K-Ras mutations. Several reports have recently demonstrated that MET gene amplification is an important mechanism of resistance to EGFR tyrosine kinase inhibitors in patients previously treated with erlotinib or gefitinib (34). We found that EGCG was also equally effective in inhibiting cell growth and c-Met signaling in H1993 cells with the MET gene amplification.
EGFR activating mutations in NSCLCs represent an example of oncogene addiction (1), and tumors harboring these mutations are quite sensitive to erlotinib. Indeed, recent clinical studies have suggested that the use of erlotinib could expand from its current second line use to primary therapy in patients with known EGFR activating mutations (2). Despite the frequent expression of EGFR, as shown by immunohistochemistry, in unselected clinical series of NSCLCs, most patients do not contain activating mutations; therefore, many but not all patients are poorly responsive to EGFR-targeted therapy (3, 12). Furthermore, even in cancers with activating mutations, resistance to reversible EGFR TKI frequently develops after several months of therapy. This has lead to the development of multiple irreversible EGFR TKI's. Although resistance to these irreversible inhibitors seems to occur less frequently (35), resistance to these inhibitors has been described (31). The mechanisms accounting for chronic or acquired resistance to erlotinib remain largely unknown, but it has been proposed that other signaling pathways may be activated, bypassing the need for EGFR signaling.
An example of this is the c-Met receptor, another strongly overexpressed receptor in NSCLCs (8). Signaling through the c-Met pathway is important in cancer cell proliferation, survival, motility and invasion (36). Some NSCLCs demonstrate MET gene amplification, implicating yet another pathway of oncogene addiction in lung cancer (11), and consistent with this, inhibition of c-Met in these cells results in growth inhibition and cell death (8, 11). Recent studies have linked EGFR TKI resistance to c-Met signaling. Predictably, combinations of the c-Met inhibitor SU11274 with erlotinib, or the irreversible inhibitor CL-387,785 resulted in growth inhibition of the erlotinib-resistant cell line H1975 (34, 37). Also, the multikinase-inhibitor XL880, which blocks both EGFR and c-Met, inhibited growth of H1975 cells. The effectiveness of multikinase inhibitors in lung cancer and other cell lines is encouraging as it may delay the development of resistance as seen using single agent targeted therapies.
Based on the studies reported here, we propose EGCG may also have important preclinical ramifications as a multi-modality inhibitor in lung cancer. A large number of reports have concluded that EGCG inhibits a variety of growth factor receptors and signaling proteins. We found EGCG was an effective inhibitor of HGF-induced c-Met phosphorylation in H2122, H358, and H460 cells as well as in the EGFR TKI-resistant H1975 cell line. EGCG is also a potent inhibitor of c-Met in other tumor models including breast and prostate ((30); Duhon et al.; manuscript in preparation). Surprisingly, EGCG had little effect on c-Met-phosphorylation in H1993 cells where the receptor is constitutively activated and HGF does not further induce activity. Perhaps, EGCG is most effective at blocking activation of c-Met and not attenuating the already activated receptor.
In contrast to the effect on c-Met activity, EGCG had a variable effect on EGF induced phosphorylation of EGFR in the NSCLC cells we studied. EGFR activity in H1975 and H358 cells were reduced by EGCG, while activity in H460, H1975 and particularly H2122 cells was less affected.
EGCG also inhibited growth of all the cell lines we tested. Surprisingly, H1975 cells, which have the second site mutation T790M rendering the EGFR receptor completely insensitive to erlotinib, were the most sensitive to EGCG as regards to cell growth of all the cell lines examined. The reason for this is not presently known (see below for more discussion), although EGCG did inhibit activation of the EGFR in these cells. Regardless, this result suggests that many patients that have acquired resistance to erlotinib may be good candidates for EGCG therapy. This is especially germane since no FDA approved inhibitor is available to treat patients who have acquired this mutation.
To examine the effect of inhibitor combinations, we combined EGCG with erlotinib and found that there was greater inhibition of cell growth than with EGCG alone in the H2122, H358, and H460 cells. This was also noted in the H460 cell colony assay in which EGCG as low as 1.25 μM resulted in significant decreases in cell growth when combined with erlotinib compared to EGCG alone. EGCG is more effective at lower concentrations at inhibiting colony growth versus the MTS assay most likely because we dosed cells every other day with EGCG and cells were seeded at lower densities. This combinatorial effect was further confirmed using a xenograft mouse model in which EGCG and erlotinib were tested alone and in combination. EGCG in combination with erlotinib resulted in a significant decrease in tumor volume and weight versus the control group and the groups treated with single agents.
Tang and coworkers found that erlotinib combined with the c-Met inhibitor SU11274 inhibited cell growth in vitro and in mouse H1975 xenografts (37). In contrast, our in vitro studies with H1975 cells did not show inhibition with the dual combination of SU11274 and erlotinib, under conditions where c-Met activation was blocked by more than 90%. Furthermore, combinations of SU11274 or erlotinib plus EGCG did not inhibit cell growth more than EGCG alone. The reason for this difference in results remains unclear, although it is possible that the residual c-Met activity in the presence of SU11274 was sufficient to maintain growth under our culture conditions. Strikingly though, EGCG by itself was much more effective than combined erlotinib and SU11274, and the H1975 cell line was more sensitive to EGCG than any of the other cell lines we tested.
In contrast, in H460 cells, the dual combination of SU11274 with erlotinib resulted in significant inhibition of cell growth, and the addition of 10 μM EGCG, a concentration having a small effect on cell growth by itself, induced further growth inhibition. Zhang and coworkers have also used the combination of EGCG and erlotinib in a model of head and neck squamous cell carcinoma (38). The combined treatment decreased cell growth greater than either agent alone both in vitro and in mouse xenografts (38). However, their laboratory did not investigate the role of c-Met signaling in the head and neck model system.
By what mechanisms does EGCG act to lower proliferation of a number of different NSCLC cells? Our data suggest for some cell lines, it is most likely independent of effects on EGFR activation. First, concentrations of EGCG that profoundly effect proliferation of H2122 cells and H1993 cells had little effect on EGFR activity and downstream signaling. Second, erlotinib does inhibit EGFR activity in H460 cells, but it did not effect proliferation. A combination of erlotinib and EGCG had an additive effect on H460 cell proliferation in vitro and in vivo. This suggests that erlotinib resistant cells may regain sensitivity to agents like erlotinib, if other signaling pathways that bypass the need for EGFR activity are inhibited by agents such as EGCG. In contrast, inhibition of EGFR signaling by EGCG may play a role in reducing proliferation of H358 and H1975 cells.
There was also an additive effect on H460 cell growth using c-Met inhibitors combined with erlotinib, consistent with multiple growth factor receptors playing a role in proliferation. Our data also suggest that EGCG works independent of EGFR activity and at least partially independent of c-Met inactivation, since a combination of EGCG with erlotinib and SU12774 was more effective than the combination of the two specific RTK inhibitors.
It has been demonstrated in numerous publications that EGCG has multiple targets including RTKs, matrix metalloproteinases, and other signaling enzymes to name a few. A variety of mechanisms have been proposed to account for the pleiotropic anti-cancer activities of tea polyphenols, including 1) inhibiting the production of reactive oxygen species necessary for receptor signaling, 2) directly inhibiting receptor activation by competition with the receptor ligand; 3) inhibiting signaling proteins like Akt directly and 4) altering fluidity of membranes. Lipid rafts represent ordered domains within cell membranes, and several receptor and non-receptor kinases including EGFR co-localize within the rafts (39, 40). We hypothesize that EGCG-induced alterations in lipid rafts could be an important mechanism by which these compounds exert their effectiveness in NSCLC, since recent studies have demonstrated that EGCG may interact with these lipid rafts. For instance, Weinstein and colleagues have recently demonstrated that EGCG can “reduce the content” of the rafts within the membrane of colon cancer cells, resulting in a decrease in activated (phosphorylated) EGFR (18). In another model using head and neck cancer cells, EGCG was found to induce EGFR internalization, but in this case the receptor underwent degradation (38). Our laboratory has recently shown that the active c-Met receptor also co-localizes to lipid rafts in prostate cancer cells, and EGCG may prevent c-Met activation by disrupting rafts (Duhon et al. manuscript submitted). Thus, we propose that the ability of EGCG to affect raft function may represent part of the mechanism by which this catechin acts to inhibit multiple growth factor receptors, as growth factor receptors are dependent upon lipid rafts for complete activity. Also, the EGFR harboring the T790M mutation may be more functionally dependent on lipid rafts than the wild-type receptor, while for H1993 cells, where c-Met is already highly active, lipid rafts may no longer be critical for activity. This could explain why EGCG is more effective at lower concentrations at blocking EGFR in H1975 cells and less effective against c-Met in H1993 cells. Future experiments will test this possibility.
In summary, our study demonstrates that EGCG alone or in combination with targeted agents to c-Met and EGFR may be a viable addition to approaches to control lung cancer progression. Clinical studies using erlotinib in combination with chemotherapy for patients with NSCLC have not demonstrated a benefit from the addition of the EGFR TKI (4, 5). However, the promising results of EGCG in combination with additional kinase inhibitors suggest that this approach may be clinically effective and therefore warrant further investigation. We predict that the percentage of patients with lung cancer that respond to EGCG therapy could be greater than observed for using erlotinib alone. Consequently, we have opened a Phase I/II in which NSCLC patients are being treated with a combination of erlotinib and polyphenon E (Polyphenon Pharma), which contains EGCG and the three other green tea catechins.
Supplementary Material
Supplemental Figure 1: EGCG but not EC blocks HGF-induced activation of c-Met. H358 and H460 cells were pretreated for 4 hours with 10 μM EGCG or EC prior to the addition of HGF for 20 minutes. Western blot analysis was performed and blots were probed for p-Met, p-Erk p-Akt and tubulin.
Acknowledgments
The authors would like to thank members of the Cardelli and Williams laboratory for careful reading of the manuscript. We would also like to thank Genentech for the kind gift of Erlotinib.
Grant Support: Feist-Weiller Cancer Center.
Footnotes
Conflict of interest statement: The authors express no conflicts of interest.
Statement of Translational Relevance: Long-term survival of patients with lung cancer remains poor. The growth of non-small cell lung cancer (NSCLC) can be driven by activation of growth factor receptors such as EGFR and c-Met. Unfortunately, inhibition of EGFR activates alternative pathways, permitting cancer cell growth, suggesting that development of alternative therapies that can inhibit multiple pathways should be a priority.
The tea polyphenol, (-)-epigallocatechin-3-gallate (EGCG), inhibits multiple signal transduction pathways in cancer cells. EGCG inhibited proliferation in a variety of NSCLC cell lines, many of which were resistant to erlotinib. Greater inhibition occurred when EGCG was combined with the EGFR tyrosine kinase inhibitor erlotinib and the c-Met inhibitor SU11274. The potential clinical relevance of this finding was supported by increased inhibition of tumor growth of erlotinib resistant cell-lines, using combined EGCG and erlotinib. These results suggest that EGCG and other polyphenols could prove efficacious alone or in combination with other targeted agents in the treatment of NSCLC.
References
- 1.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 2.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 3.Uramoto H, Mitsudomi T. Which biomarker predicts benefit from EGFR-TKI treatment for patients with lung cancer? Br J Cancer. 2007;96:857–63. doi: 10.1038/sj.bjc.6603665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 5.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 8.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–88. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 9.Cheng TL, Chang MY, Huang SY, et al. Overexpression of circulating c-met messenger RNA is significantly correlated with nodal stage and early recurrence in non-small cell lung cancer. Chest. 2005;128:1453–60. doi: 10.1378/chest.128.3.1453. [DOI] [PubMed] [Google Scholar]
- 10.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081–8. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- 12.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 13.Huang PH, Mukasa A, Bonavia R, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–72. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–91S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 16.Hou Z, Lambert JD, Chin KV, Yang CS. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555:3–19. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 17.Manson MM. Inhibition of survival signalling by dietary polyphenols and indole-3-carbinol. Eur J Cancer. 2005;41:1842–53. doi: 10.1016/j.ejca.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Adachi S, Nagao T, Ingolfsson HI, et al. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 19.Adhami VM, Malik A, Zaman N, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 20.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–21. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 21.Bettuzzi S, Brausi M, Rizzi F, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 22.Golden EB, Lam PY, Kardosh A, et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009 doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 23.Sadava D, Whitlock E, Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun. 2007;360:233–7. doi: 10.1016/j.bbrc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Suganuma M, Kurusu M, Suzuki K, Tasaki E, Fujiki H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int J Cancer. 2006;119:33–40. doi: 10.1002/ijc.21809. [DOI] [PubMed] [Google Scholar]
- 25.Balko JM, Potti A, Saunders C, et al. Gene expression patterns that predict sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer cell lines and human lung tumors. BMC Genomics. 2006;7:289. doi: 10.1186/1471-2164-7-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yauch RL, Januario T, Eberhard DA, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–98. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 27.Helfrich BA, Raben D, Varella-Garcia M, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–25. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 28.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HT, Adam RM, Bride SH, et al. Cyclic stretch activates p38 SAPK2-, ErbB2-, and AT1-dependent signaling in bladder smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1155–67. doi: 10.1152/ajpcell.2000.279.4.C1155. [DOI] [PubMed] [Google Scholar]
- 30.Bigelow RL, Cardelli JA. The green tea catechins, (-)-Epigallocatechin-3-gallate (EGCG) and (-)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25:1922–30. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, Boggon TJ, Kobayashi S, et al. Resistance to an irreversible epidermal growth factor receptor (EGFR) inhibitor in EGFR-mutant lung cancer reveals novel treatment strategies. Cancer Res. 2007;67:10417–27. doi: 10.1158/0008-5472.CAN-07-1248. [DOI] [PubMed] [Google Scholar]
- 32.de La Motte Rouge T, Galluzzi L, Olaussen KA, et al. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007;67:6253–62. doi: 10.1158/0008-5472.CAN-07-0538. [DOI] [PubMed] [Google Scholar]
- 33.Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–7. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–70. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006;12:3657–60. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 37.Tang Z, Du R, Jiang S, et al. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. Br J Cancer. 2008;99:911–22. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Zhang H, Tighiouart M, et al. Synergistic inhibition of head and neck tumor growth by green tea (-)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–14. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–67. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–26. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: EGCG but not EC blocks HGF-induced activation of c-Met. H358 and H460 cells were pretreated for 4 hours with 10 μM EGCG or EC prior to the addition of HGF for 20 minutes. Western blot analysis was performed and blots were probed for p-Met, p-Erk p-Akt and tubulin.