Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a deadly lung disease with few therapeutic options. Apoptosis of alveolar epithelial cells, followed by abnormal tissue repair characterized by hyperplastic epithelial cell formation, is a pathogenic process that contributes to the progression of pulmonary fibrosis. However, the signaling pathways responsible for increased proliferation of epithelial cells remain poorly understood.
Objectives: To investigate the role of deoxycytidine kinase (DCK), an important enzyme for the salvage of deoxynucleotides, in the progression of pulmonary fibrosis.
Methods: DCK expression was examined in the lungs of patients with IPF and mice exposed to bleomycin. The regulation of DCK expression by hypoxia was studied in vitro and the importance of DCK in experimental pulmonary fibrosis was examined using a DCK inhibitor and alveolar epithelial cell-specific knockout mice.
Measurements and Main Results: DCK was elevated in hyperplastic alveolar epithelial cells of patients with IPF and in mice exposed to bleomycin. Increased DCK was localized to cells associated with hypoxia, and hypoxia directly induced DCK in alveolar epithelial cells in vitro. Hypoxia-induced DCK expression was abolished by silencing hypoxia-inducible factor 1α and treatment of bleomycin-exposed mice with a DCK inhibitor attenuated pulmonary fibrosis in association with decreased epithelial cell proliferation. Furthermore, DCK expression, and proliferation of epithelial cells and pulmonary fibrosis was attenuated in mice with conditional deletion of hypoxia-inducible factor 1α in the alveolar epithelium.
Conclusions: Our findings suggest that the induction of DCK after hypoxia plays a role in the progression of pulmonary fibrosis by contributing to alveolar epithelial cell proliferation.
Keywords: hyperplastic epithelial cells, airway remodeling, hypoxia-inducible factor 1α
At a Glance Commentary
Scientific Knowledge on the Subject
Idiopathic pulmonary fibrosis is a prevalent and deadly lung disease with limited treatment options. Hyperproliferating airway epithelial cells play a key role in the development of pulmonary fibrosis; however, the underlying molecular mechanisms regulating abnormal epithelial hyperplasia have not been determined.
What This Study Adds to the Field
This study demonstrates that the expression of deoxycytidine kinase is increased in pulmonary fibrosis and is up-regulated by hypoxia. In addition, deoxycytidine kinase inhibition was shown to attenuate pulmonary fibrosis by inhibiting epithelial cell proliferation and fibrotic mediator release, suggesting a novel approach to attenuate pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease characterized by excessive deposition of the extracellular matrix, persistent tissue remodeling, and recurrent injury to the alveolar epithelium (1–4). IPF has an increasing mortality rate because of its poor prognosis and lack of effective therapies (3, 5). Alveolar epithelial injury and subsequent aberrant repair processes, such as the formation of hyperplastic epithelial cells, are important in the pathogenesis of IPF (2, 4). Increased apoptosis of pulmonary epithelial cells and endothelial cells is prominent during the early stages of pulmonary fibrosis and trigger mechanisms promoting disease progression (6–8). In addition, hyperproliferation of epithelial cells is augmented in IPF to compensate for epithelial cell damage, which leads to the formation of hyperplastic epithelial cells (9). These cells are a major source of fibrotic mediators, including matrix metalloproteinase (MMP) 7, MMP1/13, and MMP19 (10, 11); cytokines, such as interleukin-1β; and growth factors (9, 12, 13). These studies suggest that release of these factors could impact differentiation and proliferation of myofibroblasts. Collectively, the imbalance between apoptosis and proliferation is important for the pathogenesis of IPF; however, the signaling pathways involved in the regulation of these pathways are not fully understood.
Deoxycytidine kinase (DCK) is the rate-limiting enzyme that catalyzes the phosphorylation of all four deoxynucleosides (deoxyadenosine, deoxycytidine [dyC], deoxyguanosine, and deoxythymidine) that are important for DNA replication (14). Elevated levels of DCK are beneficial to the build-up of deoxynucleotide (dNTP) pools necessary for the regulation of DNA repair or replication when cells are under stress. The importance of cell proliferation and apoptosis in the regulation of pulmonary fibrosis prompted us to consider DCK as a novel pathway that could influence these processes. In support of this, our recent findings demonstrate that DCK is elevated in chronic lung disease associated with alveolar airspace enlargement where it contributes to the dysregulation of apoptosis (5). Collectively, the findings of the current study demonstrate a role for DCK in pulmonary fibrosis. Some of the results of these studies have been previously reported in the form of an abstract (15).
Methods
Mice
All experiments were approved by the UTHealth Animal Welfare Committee. Bleomycin-induced fibrosis was performed as described (16). Briefly, 4- to 5-week-old male C57BL/6 mice were intraperitoneally injected with 0.035 U/g bleomycin twice a week for 4 weeks. Mice injected with phosphate-buffered saline were used as control animals. The lungs and lavage fluid were collected for analysis on Day 33. Hif1af/f(B6.129-Hif1atm3Rsjo/J), SPC-rtA [B6.Cg-Tg(SFTPC-rtTA)5Jaw/J] (17), and Tet-O-Cre [B6.Cg-Tg(tetO-cre)1Jaw/J] (18) mice were purchased from Jackson Laboratories (Bar Harbor, ME). To knockout hypoxia-inducible factor 1α (Hif-1a) specifically in the alveolar epithelium, 7- to 8-week-old triple transgenic mice (Hif1af/f Spc-rtA+ Tet-O-Cre+, Hif1af/f Spc-Cre) were induced by doxycyclin therapy over 5 days intraperitoneally and per os as described (18, 19). The first injection of bleomycin was administered 10 days after doxycycline treatment. Double transgenic (either Hif1af/f Spc-rtA− Tet-O-Cre+ or Hif1af/f Spc-rtA+ Tet-O-Cre−) sex-matched littermates also received doxycycline and were used as control animals.
Human Samples
IPF lung tissue was provided by The Methodist Hospital Cardiothoracic Transplant Center and methods for the collection and distribution of these tissues were approved by the Methodist Hospital Institutional Review Board. Lung tissues from unaffected lobes of the same patients with IPF or from patients with no IPF were used as controls (20).
Nucleoside and Nucleotide Measurement
Nucleoside levels in lavage fluid were measured using HPLC as previously described (21). To measure nucleotide levels in lung tissue, whole mouse lungs were homogenized in 60% methanol (17) and analyzed by HPLC (21).
Cell Culture and Hypoxia Exposure
Mouse MLE12 (transformed mouse alveolar epithelial type II cell line) and human A549 cells were cultured in RPMI Media 1640 (Life Technologies, Grand Island, NY) containing 10% fetal bovine serum and antibiotics. For hypoxia exposure, cells were exposed to 2% oxygen supplied with 95% N2 and 5% CO2 for various time periods. To stabilize Hif-1a expression and mimic the hypoxic condition, cells were treated with 2 mM dimethyloxaloylglycine (DMOG) or 100 μM CoCl2 for 6 or 24 hours (22). Ten micromolar 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) (LC laboratories, Boston, MA) was used to inhibit the stabilization of Hif-1a. Human Calu-3 cell lines with a stably transfected control scrambled small hairpin RNA (shRNA) or Hif-1a shRNA were also used (23).
Cell Proliferation and Caspase 3/7 Activity Assay
Cell proliferation and caspase 3/7 activity were performed as previously described (5) using the same amount of protein lysate collected from cells or lungs, and analyzed by the ApoTox-Glo Triplex Assay kit (Promega, Madison, WI).
Western Blot
Western blots were performed as previously described (5) using primary rabbit anti-DCK, Col1, or FN antibodies (Abcam, Cambridge, MA), or rabbit anti–α-tubulin antibodies (Sigma-Aldrich, St. Louis, MO), or mouse anti–Hif-1α antibodies (BD Biosciences, San Jose, CA), and then incubated with corresponding secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA). Membranes were developed with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Fair Lawn, NJ).
Hematoxylin and Eosin Staining and Immunohistochemistry
Mouse and human lungs were fixed in 10% formaldehyde at constant pressure (25 cm), dehydrated and paraffin embedded, and sections (5 μm) were collected on microscope slides. For immunostaining, sections were rehydrated, quenched with 3% hydrogen peroxide, incubated in citric buffer (VectorLabs, Burlingame, CA) for antigen retrieval, and blocked with Avidin/Biotin Blocking System (VectorLabs) and then 5% normal goat serum. Sections were then incubated with primary antibodies for DCK (1:250, rabbit polyclonal; Abcam) or Ki-67 (1:200, rabbit polyclonal; Abcam) overnight at 4°C. Slides were then incubated with appropriate secondary antibodies (1:1000; VectorLabs) and ABC Elite streptavidin reagents. Finally, slides were developed with 3,3-diaminobenzidine (Sigma-Aldrich) and counter-stained with methyl green.
To detect the hypoxia in situ, mice were injected with hypoxyprobe (60 mg/kg body weight; HPI, Burlington, MA) 90 minutes before death. Lungs were collected, sectioned, and stained with rabbit-antihypoxyprobe antibody (1:500; HPI) overnight at 4°C. Slides were then incubated with secondary antibodies and ABC reagents and developed. For TUNEL assays, sections were stained with In Situ Cell Death Detection Kit (Roche, Indianapolis, IN) as instructed. For DCK/TUNEL and DCK/Ki-67 dual staining, the sections were first stained with TUNEL or Ki-67 as described. Sections were blocked with normal goat serum, incubated with anti-DCK antibodies, proper secondary antibodies, and ABC-AP reagent (VectorLab). Slides were developed using Vector Red Substrate (VectorLab) and counter-stained with methyl green or DAPI.
To determine the percentage of proliferating cells, at least 15 microscopic fields of upper, upper-mid, lower-mid, and lower sections were randomly selected from each of Ki-67–stained lungs and were photographed with a 20-fold magnification. The number of Ki-67–positive cells were counted in a blinded manner and divided by the total number of methyl green–positive nuclei from the same photograph. Analysis of variance was used for comparisons among different treatment groups. P values less than 0.05 indicate a significant difference.
Masson Trichrome and Ashcroft Assay
Masson trichrome staining was performed using the Trichrome Stain (Masson) Kit (Sigma-Aldrich). Ashcroft scores were blindly assigned using slides stained with Masson trichrome based on a modified system of grades (24, 25).
Arterial Oxygen Saturation Measurement
Arterial oxygen saturation was measured using MouseOx oximeter (Starr Life Sciences Corp, Oakmont, PA) according to manufacturer instructions. The necks of the mice were shaved and a CollarClip Sensor (Starr Life Sciences Corp) was used to continually monitor oxygen saturation.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from MLE12 cells or frozen lung tissue using TRIzol (Life Technologies). RNA was DNase treated and reverse-transcribed using Superscript II reverse transcriptase (Life Technologies). Mouse β-actin or 18S ribosomal RNA was used as internal controls. Primer sequences used are shown in Table 1 and data were quantified using the comparative Ct method and presented as mean ratio to β-actin or 18S ribosomal RNA.
Table 1.
Primers for Real-Time Polymerase Chain Reaction
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Mouse DCK | TCTCCACGGTCTGCCCAAT | CAGAACCTCTTAGGTGGGGTG |
| Mouse Col1a1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| Mouse MCP1 | AGCATCCACGTGTTGGCTC | TGGGATCATCTTGCTGGTG |
| Mouse CCR2 | TGTGATTGACAAGCACTTAGACC | TGGAGAGATACCTTCGGAACTT |
| Mouse MMP13 | CTTCTTCTTGTTGAGCTGGACTC | CTGTGGAGGTCACTGTAGACT |
| Mouse MMP7 | CTGCCACTGTCCCAGGAAG | GGGAGAGTTTTCCAGTCATGG |
| Mouse EGFR | GCCATCTGGGCCAAAGATACC | GTCTTCGCATGAATAGGCCAAT |
| Human DCK | CCATCGAAGGGAACATCGCT | GGTAAAAGACCATCGTTCAGGT |
| Human Col1A1 | GATCTGCGTCTGCGACAAC | GGCAGTTCTTGGTCTCGTCA |
| 18S ribosomal RNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Results
DCK Expression Is Elevated in Pulmonary Fibrosis
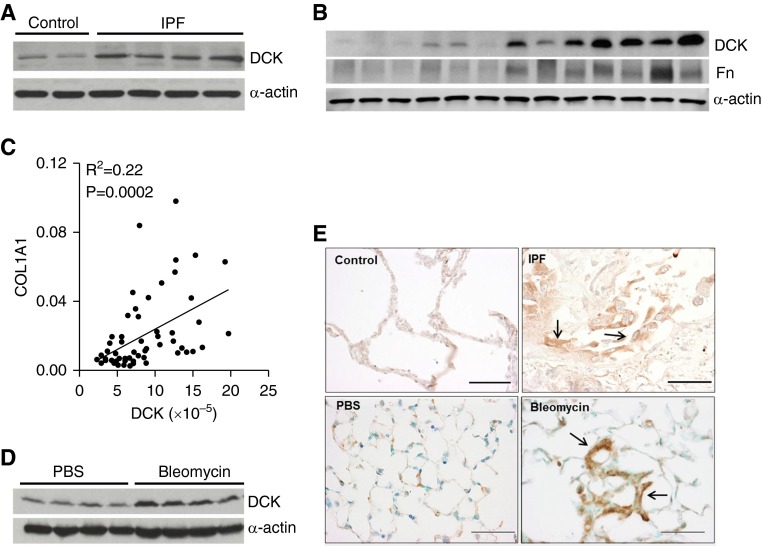
To determine whether DCK expression was elevated in pulmonary fibrosis, we examined DCK protein levels in lung tissue of control subjects and patients with IPF. DCK was consistently up-regulated in IPF lung specimens (Figure 1A; see Figure E1A in the online supplement), with its expression increasing as fibronectin (Fn) levels increased (Figure 1B). To examine the correlation between DCK levels and disease severity, we performed linear regression and Pearson correlation between DCK and Fn protein levels, as well as DCK and collagen 1α 1 (COL1A1) transcript levels from 30 patients with IPF. Results showed a strong correlation between DCK and Fn (see Figure E1B), as well as DCK and COL1A1 (Figure 1C), demonstrating that DCK is increased in association with increased fibrosis in patients with IPF.
Figure 1.
Deoxycytidine kinase (DCK) is up-regulated in airway epithelial cells in pulmonary fibrosis. (A) A representative western blot showing presence of DCK and α-actin from two control and three idiopathic pulmonary fibrosis (IPF) lung specimens. (B) A representative Western blot showing correlated expression of DCK and fibronectin (Fn) in 13 IPF lung specimens. Expression of α-actin was used as an internal control. (C) Linear regression and Pearson correlation between collagen 1 α 1 (COL1A1) and DCK transcript levels for 30 patients with IPF (R2 = 0.22, P = 0.0002). The messenger RNA levels of DCK and COL1A1 were measured by real-time polymerase chain reaction. (D) Western blot showing DCK and α-actin protein expression from whole lung lysates of mice exposed to phosphate-buffered saline (PBS) or bleomycin for 33 days. n = 4. (E) Immunohistochemistry for DCK showing cellular localization in control and IPF lung specimens (top), and the lungs from mice exposed to PBS or bleomycin for 33 days (bottom). Scale bar = 100 μm. Arrows represent hyperplastic alveolar epithelial cells.
Next, we investigated DCK expression in a mouse model of pulmonary fibrosis. Systemic exposure of mice to bleomycin is a well-characterized model of pulmonary fibrosis (26–28). Consistent with our findings in patients with IPF, DCK protein levels were increased in the lungs of mice exposed to bleomycin (Figure 1D; see Figure E1C).
Immunostaining suggested that DCK was expressed in many cells in control lung tissue at low levels, but was markedly increased in hyperplastic alveolar epithelial cells in the lungs of patients with IPF and mice exposed to bleomycin (Figure 1E, arrows). Staining was also observed in macrophages (see Figures E1D and E1E, open arrow) and in some fibroblastic cells (see Figures E1D and E1E, arrowheads). Increased DCK was colocalized with surfactant protein C in fibrotic lungs (see Figure E1F), which further confirmed that DCK is overexpressed in epithelial cells.
DCK Colocalizes with Regions of Hypoxia in Mice with Pulmonary Fibrosis
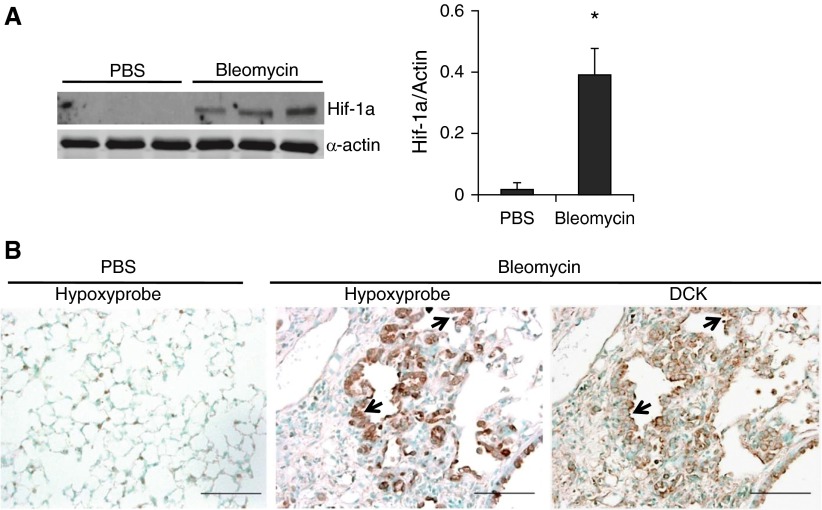
Individuals with pulmonary fibrosis exhibit hypoxemia in their lungs in association with increased levels of Hif-1a (3, 29). To determine whether hypoxia was also present in our model of pulmonary fibrosis, the expression of Hif-1a was examined by Western blot. Hif-1a was significantly increased in the lungs of bleomycin-exposed mice (Figure 2A). To determine which cells exhibit hypoxia, mice were injected with hypoxyprobe, and lungs were stained using an antihypoxyprobe antibody. Hypoxia was seen in various regions of the lung, including regions of fibrosis (data not shown); however, there was a distinct colocalization of hypoxia (Figure 2B) and increased DCK expression in regions of hyperplastic alveolar epithelial cells (Figure 2B, arrows), suggesting that hypoxia may regulate DCK expression in alveolar epithelial cells.
Figure 2.
Hypoxia is present in the lungs of mice exposed to bleomycin and colocalized with deoxycytidine kinase (DCK). (A) Western blot analysis and quantitative densitometry of the protein expressions of hypoxia-inducible factor 1α (Hif-1a) in whole lung lysates at Day 33 after phosphate-buffered saline (PBS) or bleomycin exposure. Different lanes represent samples collected from distinct mice. α-Actin was used as protein loading control. *P < 0.01 versus PBS. (B) Hypoxyprobe, to identify hypoxic cells, was injected intraperitoneally into PBS (left) or bleomycin (middle) treated mice. Lungs were collected after 90 minutes and immunohistochemistry was carried-out using antihypoxyprobe antibodies to localize the hypoxic cells (left and middle). An adjacent slide was immunostained for DCK to visualize DCK localization (right). Scale bar = 100 μm. Arrows represent hyperplastic alveolar epithelial cells.
Hypoxia Directly Regulates DCK Expression through Hif-1a Stabilization
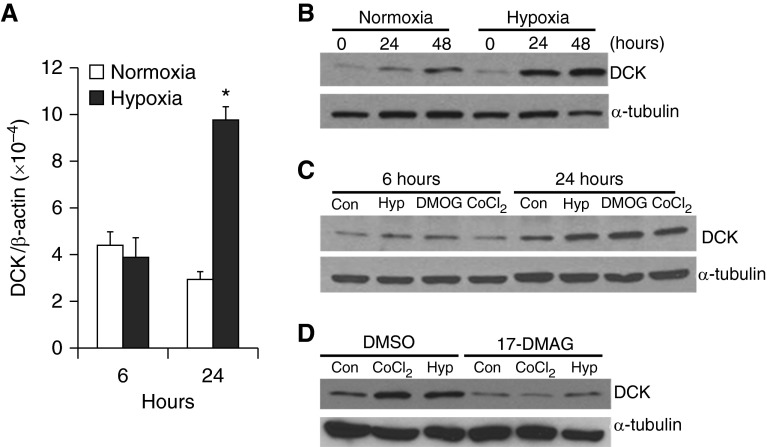
To examine whether hypoxia directly promotes DCK expression, MLE12 cells, a mouse alveolar type II cell line, were incubated in normal air or 2% oxygen for 24 or 48 hours. DCK transcript levels were significantly increased 24 hours after hypoxia exposure (Figure 3A), suggesting transcriptional activation. Under normoxia, DCK protein levels slightly increased over the culture period; however, under hypoxia, DCK protein levels were further elevated (Figure 3B; see Figure E2A). To determine whether Hif-1a mediates hypoxia-induced DCK expression, MLE12 cells were treated with DMOG or CoCl2, two chemicals capable of stabilizing Hif-1a expression under normoxic conditions (22). Both DMOG and CoCl2 markedly enhanced DCK protein expression (Figure 3C; see Figure E2B), suggesting an important role for Hif-1a in this process. To further elucidate the mechanisms involved, 17-DMAG, a heat shock protein 90 inhibitor that disrupts Hif-1a stabilization, was added before hypoxia or CoCl2 exposure. 17-DMAG significantly blocked hypoxia and CoCl2-induced DCK expression (Figure 3D; see Figure E2C). In addition, knock-down of Hif-1a by shRNA transfection inhibited hypoxia and CoCl2-induced DCK expression (see Figure E2D).
Figure 3.
Hypoxia directly regulates deoxycytidine kinase (DCK) expression via a hypoxia-inducible factor 1α (Hif-1a)–dependent pathway. (A) Real-time polymerase chain reaction was used to measure the DCK transcript levels in MLE12 (transformed mouse alveolar epithelial type II cell line) cells incubated with normal air or 2% oxygen for 6 or 24 hours (n = 3 distinct experiments, each performed twice, *P < 0.005 vs. normoxia control at corresponding time point). β-Actin was used as an internal control. (B) MLE12 cells were treated with 2% oxygen for various periods of time. Protein expression of DCK was examined using Western blot after 0, 24, and 48 hours after exposure to hypoxia. β-Tubulin protein expression levels were determined as control for protein loading. (C) MLE12 cells were incubated with normal air, 2% oxygen, 2 mM dimethyloxaloylglycine (DMOG), or 100 μM CoCl2 for 6 or 24 hours, and protein expression for DCK and α-tubulin were determined by Western blot. (D) MLE12 cells were incubated in the absence or presence of 10 μM 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) together with 2% oxygen or 100 μM CoCl2 for 24 hours; subsequently, levels of DCK and α-tubulin protein expression were determined using Western blot. n = 3 distinct experiments for B–D. DMSO = dimethyl sulfoxide.
These results are consistent with experiments in human lung epithelial cells where 24 hours after hypoxia or DMOG or CoCl2 exposure, both DCK and Hif-1a levels were up-regulated (see Figure E2E). Similarly, these cells treated with 17-DMAG showed an attenuated expression of DCK after exposure to hypoxia and CoCl2 (see Figure E2F). There are two potential Hif-1a binding sites on the mouse DCK promoter and one on the human DCK promoter (see Figure E2G), and CHIP analysis demonstrated that both hypoxia and CoCl2 enhanced the binding of Hif-1a to the DCK promoter (see Figure E2H). Collectively, these findings suggest that hypoxia regulates DCK expression via Hif-1a stabilization.
Inhibition of DCK Attenuates Pulmonary Fibrosis
Increased levels of DCK in alveolar epithelial cells suggest that this enzyme may play a role in regulating proliferation or apoptosis in these cells. dyC is an enzymatic inhibitor of DCK that binds to DCK with high affinity and thus blocks the binding of DCK to other substrates (30). To study the role of DCK in pulmonary fibrosis, bleomycin-treated mice were treated with dyC beginning on Day 20, a time point when pulmonary fibrosis was well established (see Figure E3A). The total number of bronchoalveolar lavage (BAL) cells, including macrophages, lymphocytes, and neutrophils, were increased in mice treated with bleomycin (see Figure E3B). dyC treatment did not result in a significant change in total number of BAL cells (see Figure E3B), macrophages (see Figure E3C), or lymphocytes (see Figure E3D), but the number of neutrophils was significantly decreased (see Figure E3D).
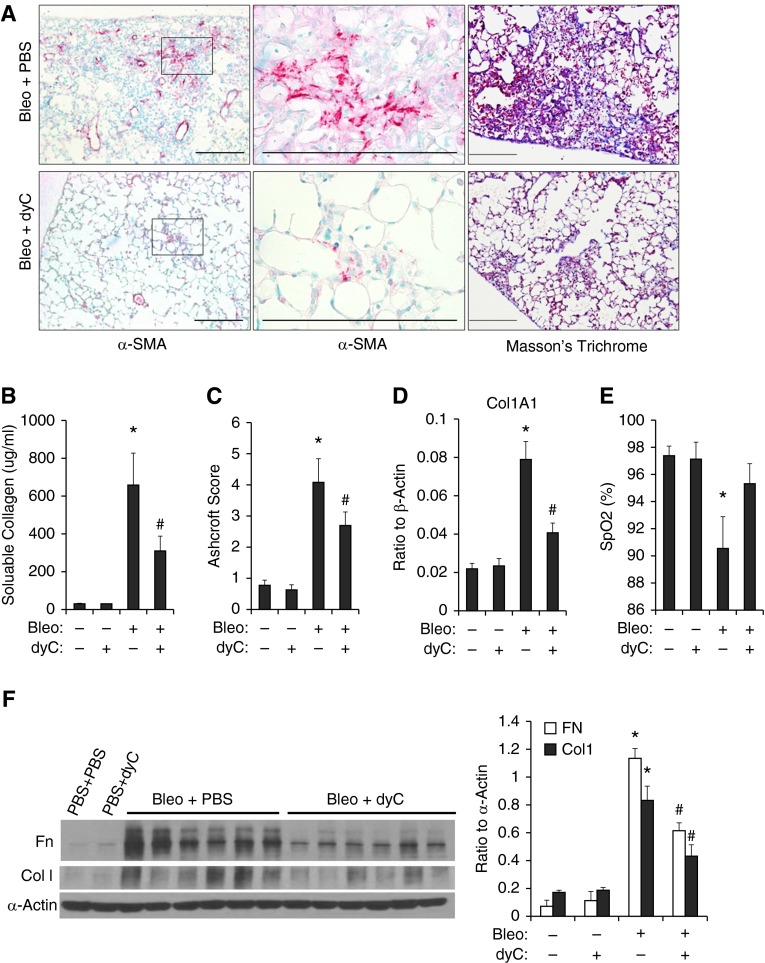
Assessment of endpoints of pulmonary fibrosis demonstrated that dyC-treated mice exhibited a reduction in α-smooth muscle actin (SMA)–positive cells (Figure 4A). Moreover, collagen deposition was decreased in Masson trichrome–stained sections (Figure 4A, right), as were soluble collagen levels (Figure 4B), Col1a1 transcripts (Figure 4D), and Fn and col1a1 protein levels (Figure 4F). Ashcroft analysis of Masson trichrome–stained sections shows an attenuated fibrosis in dyC-treated mice (Figure 4C). In addition, dyC treatment improved arterial oxygenation (Figure 4E). In summary, these studies demonstrate that DCK inhibition attenuates bleomycin-induced pulmonary fibrosis.
Figure 4.
The therapeutic effects of deoxycytidine kinase (DCK) inhibition in pulmonary fibrosis. Bleomycin (Bleo)-treated mice were intraperitoneally injected with deoxycytidine (dyC) daily starting on Day 20 through Day 33. On Day 33, mouse lungs were harvested for further analysis. (A) Lung sections were stained using α-smooth muscle actin (SMA) (left and middle) for myofibroblasts and Masson trichrome for collagen (right). Scale bar = 400 μM. (B) Total amount soluble collagen in bronchoalveolar lavage were detected by Sircol assay. (C) Ashcroft scores were blindly assigned to evaluate levels of pulmonary fibrosis. (D) Transcript levels of Col1a1 were examined by real-time polymerase chain reaction and normalized to the levels of β-actin. (E) Arterial oxygen saturation (SpO2) was measured for mice from different treatment groups. (F) The protein expression of col1a1 and fibronectin (Fn) were determined using Western blot, densitometry were analyzed and expressed as average ratio to α-actin. n = 4 for bleomycin-negative lungs, and n = 8 for bleomycin-positive lungs. *P < 0.05 versus Bleo−dyc− mice, #P < 0.05 versus Bleo+dyc− mice. PBS = phosphate-buffered saline.
Apoptosis and Proliferation Are Elevated in Pulmonary Fibrosis
DCK is the rate-limiting enzyme that catalyzes the phosphorylation of deoxynucleosides, including deoxyadenosine (dAdo) (30). DCK may play a beneficial role in the build-up of dNTP pools for DNA replication, but may also promote apoptosis if deoxyadenosine triphosphate (dATP) accumulates to a high level (31). To determine whether the elevation of DCK directly leads to dATP accumulation in our mouse model of pulmonary fibrosis, we performed HPLC analysis to measure dAdo levels in BAL fluid and dATP levels in whole lung lysates. Figure E4A shows a representative HPLC chromatogram displaying an elevation of dAdo in BAL fluid collected from a mouse exposed to bleomycin. On average, dAdo levels in BAL fluid of mice exposed to bleomycin showed a significant increase starting on Day 20, and becoming progressively elevated through Day 33 (see Figure E4B). Consistent with these findings, levels of dATP were also significantly elevated in the lungs of mice treated with bleomycin (see Figure E4C). These biochemical endpoints further verify the elevations of DCK seen in fibrotic lungs.
To determine whether the accumulation of dATP in pulmonary fibrosis promotes apoptosis or assists DNA replication and cell proliferation, we examined overall levels of apoptosis and proliferation. Increased apoptosis and proliferation were observed in the airway and alveolar epithelium in human IPF lungs (Figure 5A) and the lungs of mice exposed to bleomycin (Figure 5B). To further examine the association between activation of the DCK pathway and apoptosis or proliferation, we carried out dual-immunohistochemistry staining for DCK and TUNEL, or Ki-67. In both human and mouse lungs with fibrosis, we observed colocalization of DCK with Ki-67 predominantly in hyperplastic alveolar epithelial cells (Figures 5C and 5D). Taken together, our results suggest that overall proliferation and apoptosis are elevated in pulmonary fibrosis, and activation of DCK may promote proliferation of alveolar epithelial cells.
Figure 5.
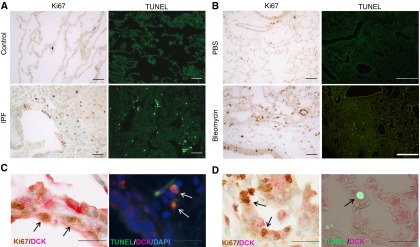
Apoptosis and proliferation is prominent in the lungs with pulmonary fibrosis. Lung sections from human (A) or mouse (B) were stained with TUNEL or Ki-67 to visualize cell apoptosis or cell proliferation, respectively. TUNEL staining was visualized under fluorescence (left) and cells positive for TUNEL appeared green in color. Ki-67 staining was visualized under light microscopy and cells positive for Ki-67 appeared brown in color. Dual immunohistochemistry was performed in human (C) or mouse lungs (D) to visualize the colocalization of deoxycytidine kinase (DCK) with TUNEL (left) or DCK and Ki-67 (right). n = 3 for control human lungs, n = 10 for idiopathic pulmonary fibrosis (IPF) lungs, and n = 5 for mouse lungs. Scale bar = 100 μM. PBS = phosphate-buffered saline. Arrows represent double positive cells.
Reduced cell proliferation and fibrotic mediator production after DCK inhibition.
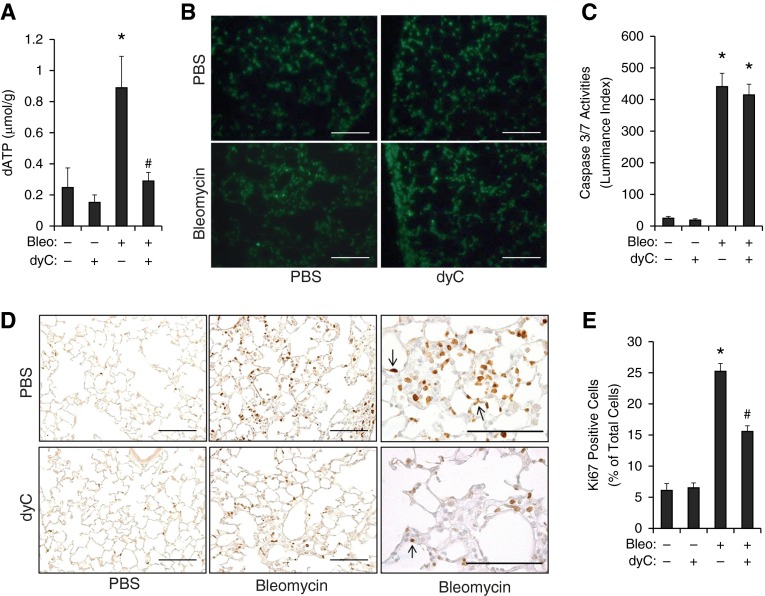
Because dATP levels in the cell is the major factor that determines whether activation of DCK leads to apoptosis or proliferation, both of which could impact fibrosis in the lung, we first determined the levels of dATP in whole lung lysates. dyC treatment significantly reduced the levels of total dATP in the lungs of both control animals and bleomycin-treated mice (Figure 6A), suggesting that inhibition of DCK directly affects dATP accumulation.
Figure 6.
Deoxycytidine kinase (DCK) inhibition suppressed deoxyadenosine triphosphate (dATP) accumulation and cell proliferation. (A) Nucleotides were extracted from the lungs of mice treated with bleomycin (Bleo) and/or deoxycytidine (dyC). HPLC was performed to measure the levels of dATP. Total dATP levels were normalized to the total protein levels. (B) TUNEL staining was visualized under fluorescence and cells positive for TUNEL appeared green in color. (C) Caspase 3/7 activities were measured using the whole lung lysates, and data were quantitated. (D) Ki-67 staining was performed to visualize proliferating cells. Scale bar = 200 μM. Arrows represent Ki-67 positive cells. (E) The number of Ki-67–positive cells were counted, and data were expressed as percentage of Ki-67–positive cells to total number of cells in the same field. n = 4 for bleomycin-negative lungs, and n = 8 for bleomycin-positive lungs. *P < 0.05 versus Bleo−dyc− mice, #P < 0.05 versus Bleo+dyc− mice. PBS = phosphate-buffered saline.
To investigate the impact of DCK inhibition on cell proliferation and apoptosis, we first examined levels of apoptosis and proliferation in 2′-chlorodeoxyadenosine (cdA)- or hypoxia-treated MLE12 cells. Consistent with our hypothesis, cdA promoted proliferation at a low dose (10 μM) (see Figure E5A) and increased apoptosis at a high dose (25 μM) (see Figure E5B). Moreover, the effect of cdA could be inhibited by dyC treatment (see Figures E5A and E5B). In vivo mouse experiments showed that the overall level of apoptosis was not affected by dyC, as shown by both TUNEL staining (Figure 6B) and caspase 3/7 activity (Figure 6C). However, cell proliferation was significantly reduced after treatment with dyC (Figures 6D and 6E). Focusing on areas with fibrosis, we observed a large number of proliferating alveolar epithelial cells in mice treated with bleomycin (Figure 6D, upper left, arrow), whereas the percentage of proliferating epithelial cells was largely decreased in mice treated with dyC (Figure 6D, bottom left, arrow, and Figure 6E), suggesting a role for DCK in promoting cell proliferation in certain cells in fibrosis.
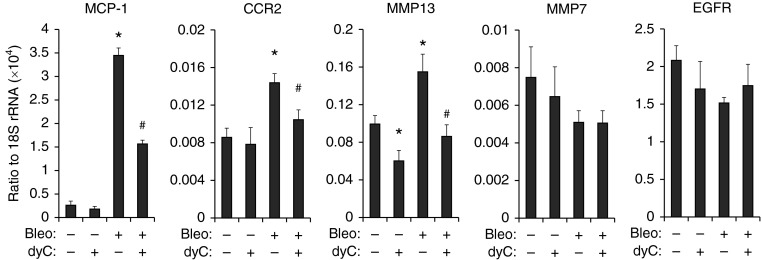
Hyperplastic epithelial cells release fibrotic mediators, including C-C chemokine receptor type 2, MMP13, MMP7, and epidermal growth factor receptor (9). To determine whether the inhibition of cell proliferation in the lungs affected the expression of these mediators, we performed real-time polymerase chain reaction to examine the transcript levels of these genes. The transcript levels of monocyte chemoattractant protein 1, C-C chemokine receptor type 2, and MMP13 were significantly increased by bleomycin and inhibited by dyC (Figure 7), whereas transcript levels of MMP7 and epidermal growth factor receptor were not affected. In addition, we performed studies in vitro on epithelial cells and demonstrated that dyC significantly attenuated cdA-induced monocyte chemoattractant protein 1 expression and hypoxia-induced MMP13 and transforming growth factor-α levels in MLE12 cells (see Figure E5C). Overall, these results suggest that inhibition of the DCK pathway attenuates pulmonary fibrosis through a mechanism that reduces cell proliferation and suppresses the release of fibrotic mediators.
Figure 7.
Mediators released by hyperplastic alveolar epithelial cells were suppressed by deoxycytidine kinase (DCK) inhibition. Real-time polymerase chain reaction was performed to examined the transcript levels of cytokines and receptors in total RNA isolated from the whole lung at Day 33, including monocyte chemoattractant protein-1 (MCP-1), C-C chemokine receptor type 2 (CCR2), matrix metalloproteinase (MMP) 13, MMP7, and epidermal growth factor receptor (EGFR). 18S ribosomal RNA (rRNA) was used as an internal control. n = 4 for bleomycin-negative lungs, and n = 8 for bleomycin-positive lungs. *P < 0.05 versus Bleo−dyc− mice, #P < 0.05 versus Bleo+dyc− mice. Bleo = bleomycin; dyC = deoxycytidine.
Conditional Knockout of Hif-1a in the Alveolar Epithelium Suppresses DCK Expression and Attenuates Pulmonary Fibrosis
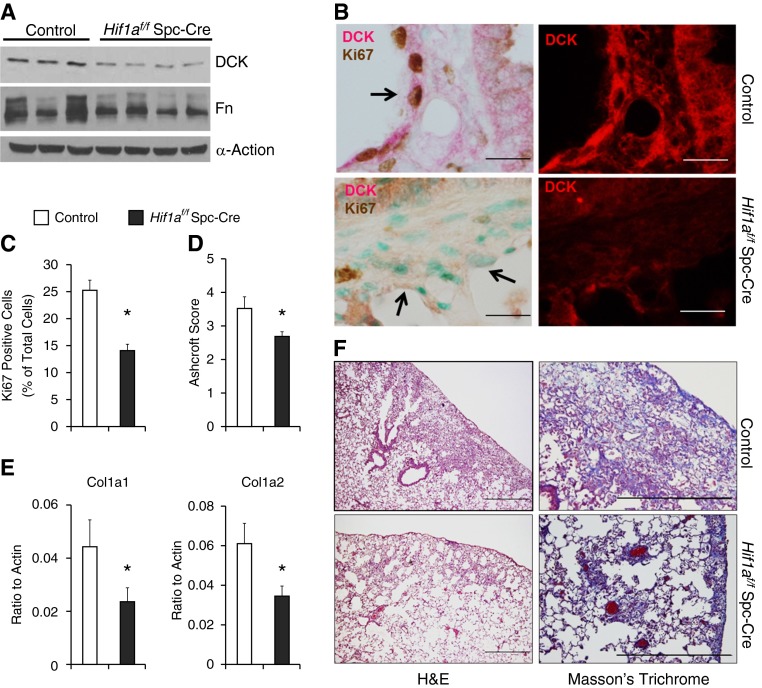
To further understand the role of hypoxia in mediating DCK expression and fibrosis in vivo, we exposed mice with conditional deletion of Hif-1a in alveolar epithelial cells (Hif1af/f Spc-Cre) to bleomycin. DCK expression was decreased in the lungs of Hif1af/f Spc-Cre mice exposed to bleomycin (Figure 8A; see Figure E6) in association with decreased proliferation of alveolar epithelial cells (Figure 8B, arrows), and the total number of proliferating cells (Figure 8C). Moreover, the degree of pulmonary fibrosis was significantly reduced in Hif1af/f Spc-Cre mice as indicated by reduced Ashcroft scores (Figure 8D), diminished collagen deposition (Figure 8F, bottom), Col1a1 and Col1a2 transcript levels (Figure 8E), and decreased Fn levels (Figure 8A). These findings demonstrate that Hif-1a in the alveolar epithelium contributes to the pathogenesis of pulmonary fibrosis by regulating DCK expression.
Figure 8.
Tissue-specific function of hypoxia-inducible factor 1α (Hif-1a) in pulmonary fibrosis. Hif1af/f Spc-Cre mice and corresponding age-, weight-, and sex-matched littermate control animals (Cre-expressing mice) were exposed to bleomycin. (A) The protein levels of deoxycytidine kinase (DCK) and fibronectin (Fn) in the lungs of mice exposed to bleomycin were determined using Western blot. α-Actin was used as an internal control. (B) Dual immunohistochemistry was performed in the lungs of mice exposed to bleomycin to visualize the colocalization of DCK with Ki-67. Scale bar = 100 μm. Arrows represent hyperplastic alveolar epithelial cells. (C) The percentage of Ki-67–positive cells were calculated. (D) Ashcroft scores were blindly assigned to evaluate levels of pulmonary fibrosis. (E) Transcript levels of Col1a1 and Col1a2 were determined using real-time polymerase chain reaction. n = 3 for control, n = 4 for Hif1af/f Spc-Cre. (F) Lung sections were stained with hematoxylin and eosin (H&E; left) or Masson trichrome (right) to visualize histology and levels of collagen deposition, respectively. Scale bar = 500 μm. n = 6 for control, n = 15 for Hif1af/f Spc-Cre. *P < 0.05 versus control.
Discussion
Injury of the alveolar epithelium and aberrant repair processes, including formation of hyperplastic alveolar epithelial cells, contributes to the progression of fibrosis (2, 9). Here we provide novel information concerning the development and regulation of hyperplastic epithelial cells in the fibrotic lung. We observed that DCK is up-regulated in hyperplastic alveolar epithelial cells in fibrotic lungs from mice and patients with IPF. Similar to what we have observed in chronic obstructive pulmonary disease (5), hypoxia directly regulated DCK expression through a Hif-1a–dependent pathway. Furthermore, inhibition of the DCK pathway attenuated pulmonary fibrosis in association with reducing epithelial cell proliferation and suppressing the expression of mediators known to be released by hyperplastic epithelial cells. In addition, selective deletion of Hif-1a in the alveolar epithelium suppressed DCK expression and dampened pulmonary fibrosis. We conclude that hypoxia-mediated up-regulation of DCK contributes to alveolar epithelial cell proliferation and the progression of pulmonary fibrosis.
A major finding of this study was the identification of DCK as a hypoxia-regulated gene in the environment of the fibrotic lung. The lungs of patients with IPF are marked with recurrent epithelial injury and remodeling, which can directly affect local blood-gas exchange and result in hypoxia (2, 32, 33). Our studies confirm previous observations that Hif-1a levels are elevated in experimental pulmonary fibrosis and in IPF (3), and demonstrate that Hif-1a–mediated up-regulation of DCK in hyperblastic epithelial cells in the lungs is necessary for the development of extensive fibrosis. These findings were further supported by our observation that DCK inhibition using dyC is associated with decreased fibrosis. dyC is an enzymatic inhibitor of DCK that competitively blocks the binding of DCK to other substrates and then feedback suppresses DCK activity by its phosphorylation product deoxycytidine triphosphate (5, 30). Interestingly, injection of dyC was initiated on Day 20, a stage when pulmonary fibrosis is well established and results demonstrated a significantly decreased lung fibrosis, suggesting a therapeutic benefit for this treatment in halting active fibrosis.
These results suggest that DCK could be a novel therapeutic target for patients with IPF. Recently, intravenous administration of dyC was found to suppress myeloma tumor growth and increase survival time in mice (34). Although the mechanisms by which dyC inhibits tumor growth is not known, they might include a mechanism similar to the one we observed here. This is supported by the notion that fibrosis and cancer share several similar features including abnormal cell proliferation, myofibroblast differentiation, and excessive extracellular matrix remodeling (35). In corroboration with our findings that DCK colocalized with hypoxia in hyperplastic epithelial cells and that hypoxia directly induced DCK expression via Hif-1a stabilization, mice with conditional deletion of Hif-1a in alveolar epithelial type II cells had decreased DCK expression and were more resistant to bleomycin-induced pulmonary fibrosis. These findings directly support a role for the hypoxia-DCK response in the progression of pulmonary fibrosis.
DCK was found to be up-regulated largely in hyperplastic alveolar epithelial cells in fibrotic lungs. These cells are highly proliferative and are found adjacent to injured epithelial cells in IPF and may play a role in the generation of new epithelial cells during repair (12). However, during the pathogenesis of IPF, persistently activated hyperplastic epithelial cells are a major source of various fibrotic mediators, including MMPs, cytokines, and growth factors (9). Activation of DCK is normally beneficial for fast nucleotide recycling and the build-up of dNTP pools for DNA repair or replication. However, in the presence of high levels of dAdo, which can result from excessive apoptosis, activation of DCK may be detrimental by facilitating dATP elevations that can enhance apoptosis (31). This was previously observed in a mouse model of airspace enlargement, where dAdo levels accumulated because of the deficiency of adenosine deaminase (5). It was shown that activation of DCK directly correlated with the formation of dATP, accumulation of dATP pools, and the enhancement of epithelial cell apoptosis (5). Conversely, in the current study, we show that increases in DCK may play more of a role in the enhancement of proliferation in fibrosis as opposed to apoptosis in emphasemic models. We show that the number of proliferative cells is increased in the lungs of mice injected with bleomycin and in patients with IPF. We also show high levels of DCK expression in hyperplastic alveolar epithelial cells in association with Ki-67 staining, indicating that DCK is associated with epithelial cell proliferation.
The differences between these models may be the degree or duration to which dAdo is allowed to accumulate, with lower levels associated with proliferation and higher levels with apoptosis. In further support of DCK regulation of epithelial proliferation in the bleomycin model, we demonstrated that treatment with a DCK inhibitor significantly decreased cell proliferation in bleomycin-treated mice without obviously affecting cell apoptosis, and largely prevented the production of fibrotic mediators known to be produced by hyperplastic alveolar epithelial cells. These findings were further supported by our in vitro data demonstrating that inhibition of the DCK pathway suppressed cell proliferation and apoptosis as well as the production of fibrotic mediators in MLE12 cells. One limitation to this study was the use of cell lines instead of primary lung epithelial cells. Expanding these studies to primary epithelial cells will be the focus of future analysis to further define the mechanisms involved (36–38). Similarly, suppressing DCK expression by selectively depleting Hif-1a in the alveolar epithelium also dramatically decreased cell proliferation. Based on these observations, we conclude that inhibition of DCK blocks epithelial cell proliferation and subsequently attenuates bleomycin-induced pulmonary fibrosis.
Targeting highly proliferative alveolar epithelial cells as a therapeutic approach for pulmonary fibrosis has recently been reported regarding dimethylarginine dimethylaminohydrolase, an enzyme that activates inducible nitric oxide synthase and promotes the generation of fibrosis mediators (39). Inhibition of dimethylarginine dimethylaminohydrolase in epithelial cells was found to prevent pulmonary fibrosis by suppressing overproliferation and increasing apoptosis of epithelial cells in bleomycin-treated mice. Similarly, induction of the expression of P21, a protein that directly inhibits proliferating cell nuclear antigen (PCNA)-dependent DNA replication, significantly suppressed bleomycin-induced pulmonary fibrosis and fibroproliferation (40). In addition to pulmonary fibrosis, inhibiting the proliferation of key cells was also observed to attenuate fibrosis in the liver (41, 42). In corroboration with these studies, we showed that suppressing the proliferation of hyperplastic alveolar epithelial cells by a natural inhibitor of DCK attenuated pulmonary fibrosis.
In conclusion, our study suggests that DCK plays an important role in the pathogenesis of pulmonary fibrosis by promoting abnormal cell proliferation. We present evidence that inhibition of the DCK pathway attenuates pulmonary fibrosis by reducing abnormal cell proliferation and the release of fibrotic mediators. Collectively, our study demonstrates a novel role for DCK in pulmonary fibrosis and provides important preclinical evidence to suggest DCK inhibition may be a potential target for the treatment of IPF. However, a major limitation of our study is the inability to directly link enhanced epithelial cell proliferation with the formation of hyperplastic epithelial cells. This is caused by our inability to effectively model the cellular process. Additional research is needed to investigate the specific role of this cell type in pulmonary fibrosis.
Footnotes
Supported by National Institutes of Health grants R01-HL070952 and P01-HL114457 (M.R.B.); an American Heart Association Fellowship (T.W.); National Heart Institute grants R01-DK097075, R01-HL0921, R01-DK083385, R01-HL098294, and P01-HL114457 (H.K.E.); and a Crohn’s and Colitis Foundation of America grant (H.K.E.).
Author Contributions: T.W., H.K.-Q., and M.R.B. developed the study concept and design. L.J.G.-M., R.R.B., B.A.B., and M.L. obtained patient consent forms, collected clinical data, and provided patient samples in a deidentified manner. C.M.E. provided expertise in hypoxia-inducible factor 1α signaling. T.W., J.M.P., E.M., F.L., N.-y.C., and H.K.-Q. performed experiments and analyzed the data. T.W. wrote the manuscript and H.K.-Q., C.M.E., J.M.P., K.A.V., H.K.E., and M.R.B. provided critical review of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201404-0744OC on October 30, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gay SE, Kazerooni EA, Toews GB, Lynch JP, III, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998;157:1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 2.Hoo ZH, Whyte MK. Idiopathic pulmonary fibrosis. Thorax. 2012;67:742–746. doi: 10.1136/thoraxjnl-2011-200515. [DOI] [PubMed] [Google Scholar]
- 3.Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D, Aidinis V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am J Respir Crit Care Med. 2007;176:1108–1119. doi: 10.1164/rccm.200705-683OC. [DOI] [PubMed] [Google Scholar]
- 4.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng T, Karmouty-Quintana H, Garcia-Morales LJ, Molina JG, Pedroza M, Bunge RR, Bruckner BA, Loebe M, Seethamraju H, Blackburn MR. Hypoxia-induced deoxycytidine kinase expression contributes to apoptosis in chronic lung disease [abstract] FASEB J. 2013;27:2013–2026. doi: 10.1096/fj.12-222067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza PM, Lindsay MA. Apoptosis as a therapeutic target for the treatment of lung disease. Curr Opin Pharmacol. 2005;5:232–237. doi: 10.1016/j.coph.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Fadeel B, Orrenius S, Zhivotovsky B. Apoptosis in human disease: a new skin for the old ceremony? Biochem Biophys Res Commun. 1999;266:699–717. doi: 10.1006/bbrc.1999.1888. [DOI] [PubMed] [Google Scholar]
- 8.Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J. 2008;32:1631–1638. doi: 10.1183/09031936.00176807. [DOI] [PubMed] [Google Scholar]
- 9.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 10.Yu G, Kovkarova-Naumovski E, Jara P, Parwani A, Kass D, Ruiz V, Lopez-Otín C, Rosas IO, Gibson KF, Cabrera S, et al. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am J Respir Crit Care Med. 2012;186:752–762. doi: 10.1164/rccm.201202-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dancer RC, Wood AM, Thickett DR. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J. 2011;38:1461–1467. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 12.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staub M, Spasokukotskaja T, Benczur M, Antoni F. DNA synthesis and nucleoside metabolism in human tonsillar lymphocyte subpopulations. Acta Otolaryngol Suppl. 1988;454:118–124. doi: 10.3109/00016488809125014. [DOI] [PubMed] [Google Scholar]
- 15.Weng T, Karmouty-Quintana H, Pedroza M, Molina JG, Blackburn MR. Hypoxia mediated up regulation of deoxycytidine kinase contributes to deoxyadenosine mediated apoptosis during pulmonary fibrosis. Presented at Keystone Symposia: Fibrosis. April 2, 2012, Big Sky, MT [Google Scholar]
- 16.Karmouty-Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD, Hemnes A, Grenz A, Eltzschig HK, Blackwell TS, et al. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J. 2012;26:2546–2557. doi: 10.1096/fj.11-200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 18.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckle T, Brodsky K, Bonney M, Packard T, Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M, Eltzschig HK. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol. 2013;11:e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One. 2010;5:e9224. doi: 10.1371/journal.pone.0009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakamiya M, Blackburn MR, Jurecic R, McArthur MJ, Geske RS, Cartwright J, Jr, Mitani K, Vaishnav S, Belmont JW, Kellems RE, et al. Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc Natl Acad Sci USA. 1995;92:3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 23.Haeberle HA, Dürrstein C, Rosenberger P, Hosakote YM, Kuhlicke J, Kempf VA, Garofalo RP, Eltzschig HK. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS One. 2008;3:e3352. doi: 10.1371/journal.pone.0003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B.Standardized quantification of pulmonary fibrosis in histological samples BioTechniques 200844507–511, 514–517 [DOI] [PubMed] [Google Scholar]
- 26.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 27.Ezzie ME, Piper MG, Montague C, Newland CA, Opalek JM, Baran C, Ali N, Brigstock D, Lawler J, Marsh CB. Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;44:556–561. doi: 10.1165/rcmb.2009-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2011;186:1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med (Berl) 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 30.Arnér ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 31.Keszler G, Spasokoukotskaja T, Csapo Z, Virga S, Staub M, Sasvari-Szekely M. Selective increase of dATP pools upon activation of deoxycytidine kinase in lymphocytes: implications in apoptosis. Nucleosides Nucleotides Nucleic Acids. 2004;23:1335–1342. doi: 10.1081/NCN-200027586. [DOI] [PubMed] [Google Scholar]
- 32.Pitsiou G, Papakosta D, Bouros D. Pulmonary hypertension in idiopathic pulmonary fibrosis: a review. Respiration. 2011;82:294–304. doi: 10.1159/000327918. [DOI] [PubMed] [Google Scholar]
- 33.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwazaki A, Imai K, Nakanishi K, Yoshioka M. Intravenously administered 2′-deoxycytidine suppresses mouse myeloma tumor growth. Biol Pharm Bull. 2012;35:251–255. doi: 10.1248/bpb.35.251. [DOI] [PubMed] [Google Scholar]
- 35.Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur Respir Rev. 2013;22:265–272. doi: 10.1183/09059180.00003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura Y, Hida W, Taguchi O, Sakurai M, Ichinose M, Inoue H, Takishima T. Respiratory muscle strength and gas exchange in neuromuscular diseases: comparison with chronic pulmonary emphysema and idiopathic pulmonary fibrosis. Tohoku J Exp Med. 1989;159:57–68. doi: 10.1620/tjem.159.57. [DOI] [PubMed] [Google Scholar]
- 37.Weitzenblum E, Ehrhart M, Rasaholinjanahary J, Hirth C. Pulmonary hemodynamics in idiopathic pulmonary fibrosis and other interstitial pulmonary diseases. Respiration. 1983;44:118–127. doi: 10.1159/000194537. [DOI] [PubMed] [Google Scholar]
- 38.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442–L452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pullamsetti SS, Savai R, Dumitrascu R, Dahal BK, Wilhelm J, Konigshoff M, Zakrzewicz D, Ghofrani HA, Weissmann N, Eickelberg O, et al. The role of dimethylarginine dimethylaminohydrolase in idiopathic pulmonary fibrosis. Sci Transl Med. 2011;3:87ra53. doi: 10.1126/scitranslmed.3001725. [DOI] [PubMed] [Google Scholar]
- 40.Inoshima I, Kuwano K, Hamada N, Yoshimi M, Maeyama T, Hagimoto N, Nakanishi Y, Hara N. Induction of CDK inhibitor p21 gene as a new therapeutic strategy against pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L727–L733. doi: 10.1152/ajplung.00209.2003. [DOI] [PubMed] [Google Scholar]
- 41.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33:2078–2090. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]