Abstract
Rationale: The rate of progression of most interstitial lung diseases (ILD) is unpredictable. Fibrocytes are circulating bone marrow–derived cells that have been implicated in the pathogenesis of lung fibrosis. Hermansky-Pudlak syndrome (HPS), a genetic cause of ILD in early adulthood, allows for study of biomarkers of ILD in a homogeneous population at near-certain risk of developing fibrotic lung disease.
Objectives: To test the hypothesis that, in subjects with HPS, the number or phenotype of circulating fibrocytes predicts progression and outcome of ILD.
Methods: We measured circulating fibrocyte counts and chemokine levels in a cohort of subjects with HPS and healthy control subjects and correlated the results to disease outcome.
Measurements and Main Results: In a cross-sectional analysis, peripheral blood fibrocyte concentrations were markedly elevated in a subset of subjects with HPS who had ILD but not subjects without lung disease or normal control subjects. The blood concentration of fibrocytes expressing the chemokine receptor CXCR4 correlated significantly with the plasma concentration of the CXCR4 ligand, CXCL12. In a longitudinal study, we found marked episodic elevations in circulating fibrocyte counts over a median follow-up period of 614 days. Elevations in both maximal values and final values of peripheral blood CXCR4+ fibrocyte concentration were strongly associated with death from ILD.
Conclusions: CXCR4+ fibrocyte concentration may be useful as a biomarker for outcome of ILD in subjects with HPS.
Keywords: interstitial lung diseases, stem cells, chemokines, CXCR4 receptors
At a Glance Commentary
Scientific Knowledge on the Subject
A key limitation in the care of patients with interstitial lung diseases (ILDs) is that progression of these diseases is unpredictable. Identification of biomarkers that identify individuals at risk for progression is important because these individuals may benefit from directed therapy or can be targeted for enrollment into clinical trials of new interventions. Fibrocytes are a population of circulating progenitor cells that are found in higher concentration in the blood of patients with ILD.
What This Study Adds to the Field
We studied the usefulness of fibrocytes as biomarkers of disease activity in a relatively homogeneous population of patients with ILD caused by the genetic illness Hermansky-Pudlak syndrome. We report that subjects experienced intermittent but marked elevations in circulating fibrocyte counts, and fibrocyte concentrations exceeding specific thresholds were predictive of poor outcome. These data suggest that fibrocytes may be useful as a biomarker of prognosis in ILDs.
Diffuse parenchymal lung diseases are a heterogeneous group of illnesses characterized by multifocal inflammation and fibrosis of the lung (1, 2). In a subset of these diseases, exemplified by idiopathic pulmonary fibrosis (IPF), progressive scarring and consequent loss of gas exchange surface results in premature death from respiratory failure (3). Critically, the rate of decline in lung function is highly variable and currently unpredictable among patients with fibrotic interstitial lung diseases (ILD), and lung damage is only detectable when severe enough to cause symptoms, impair physiology (as detected on pulmonary function testing [PFT]), or derange anatomy (as seen on imaging). Identification of biomarkers that predict the future course in these diseases would constitute a critical advance in the field by providing prognostic information and identifying patients most likely to benefit from therapeutic interventions.
Fibrocytes are circulating bone marrow–derived progenitor cells that home to injured tissues, differentiate into myofibroblasts, and contribute to fibrosis (4, 5). In the peripheral blood, fibrocytes can be identified as cells coexpressing the leukocyte marker CD45 and collagen. In animal models of lung fibrosis, fibrocytes expand in the blood before their accumulation in the lungs and have been causally implicated in lung scarring (6–8). Fibrocytes are markedly elevated in the blood and lungs of patients with ILD compared with healthy control subjects (9, 10), and the proportion of circulating fibrocytes correlated with IPF exacerbations and mortality in a cross-sectional study (11), suggesting that these cells may be useful as biomarkers. Systematic studies of longitudinal fibrocyte counts in subjects with ILD have not, however, been reported to date.
Hermansky-Pudlak syndrome (HPS) is a group of autosomal recessive disorders causing oculocutaneous albinism. Three subtypes, HPS-1, HPS-2, and HPS-4, invariably result in ILD that typically becomes clinically manifest in early adulthood (12). The ILD in HPS has the imaging and histologic patterns of usual interstitial pneumonia and is the cause of death in nearly all patients (13, 14). HPS is rare in the general population, but, due to a founder effect, HPS-1 is the most common cause of albinism in Puerto Rico, allowing affected individuals to be diagnosed before the onset of lung disease (15) and providing the unique opportunity to serially study a population at different stages of developing fibrotic ILD from the same etiology. We therefore tested the hypothesis that, in subjects with HPS, the number or phenotype of circulating fibrocytes predicts progression and outcome of ILD. Some of these data were previously published as an abstract (16).
Methods
Subjects and Sample Collection
Sixty-five subjects with HPS and 12 healthy control subjects were enrolled under institutionally approved protocols and provided written informed consent. The diagnosis of HPS was based on both the absence of platelet-dense granules on whole-mount electron microscopy and the presence of HPS1 or HPS4 mutations. Among subjects with HPS, 12 were treated with pirfenidone during the study period as part of a previously published trial (17). Control individuals were healthy volunteers older than 18 years of age without acute or chronic medical illnesses who were nonsmokers. During study visits, PFTs were obtained and venous blood samples were collected in heparinized tubes, anonymized, and shipped on wet ice. The group performing sample processing were blinded to the identity of subjects until after the measurements were completed.
Sample Processing
Samples were processed the day after blood draw without ex vivo manipulations such as cell enrichment, freezing, or culture. Plasma was separated by centrifugation and stored at −80°C, and fibrocytes were identified by flow cytometry as described (6, 10, 11) and as detailed in the online data supplement. Plasma proteins were quantified using multiplex assays (Milliplex MAG, Millipore, Billerica, MA) on a Bioplex 200 instrument (BioRad, Hercules, CA) using Bioplex Manager software (version 6.1), using manufacturer’s instructions.
Statistical Analysis
Data were analyzed using Prism (version 6.0c for Mac, GraphPad Software, La Jolla, CA) and R (version 3.1.0; open source, www.R-project.org). Euler diagrams were generated with EulerAPE (version 3.0.0; open source, http://www.eulerdiagrams.org/eulerAPE/). Descriptive data were expressed as median and interquartile range (IQR). In cross-sectional comparisons, medians were compared using the Kruskal-Wallis test with Dunn multiple comparison test to adjust for multiple candidate populations. For subjects with serial data, the first value was used for cross-sectional analyses. The distributions of fibrocyte populations between groups were compared using the sample quantile test. Changes in fibrocyte populations and PFTs over time were compared by one-way analysis of variance with repeated measurements, and correlations between variables were quantified using nonparametric Spearman rank correlation coefficient. For prospective survival outcome stratification, an optimal threshold value of fibrocytes was determined by maximizing overall prediction performance with Youden J index from a receiver operating characteristics analysis. Association between fibrocyte count exceeding a threshold value and death was tested using Fisher exact test. Survival data were expressed using Kaplan-Meier curves and compared with log- rank test. Hazard ratio (HR) was computed using Mantel-Haenszel test. Results were considered statistically significant if two-sided P values were less than 0.05.
Results
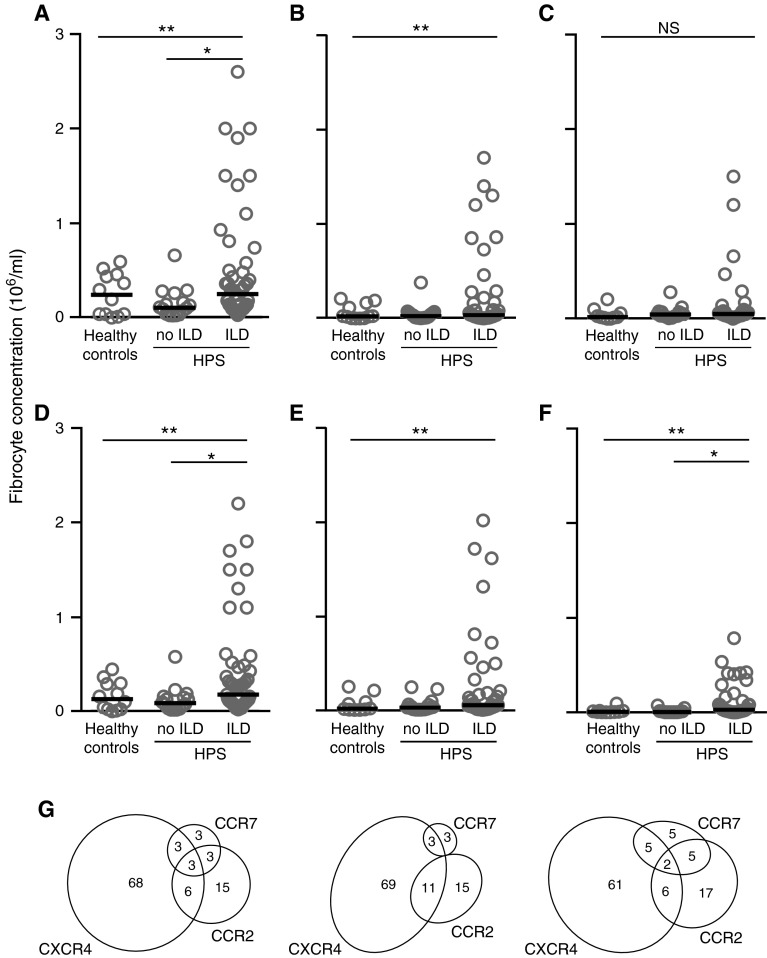
We enrolled 66 subjects with HPS and 12 healthy control subjects (Table 1). All subjects were nonsmokers and did not have other comorbidities. One subject with HPS carried an HPS4 mutation, and all others carried mutations in the HPS1 gene. In a cross-sectional comparison, the median concentration of circulating fibrocytes was higher in subjects with HPS and ILD compared with subjects with HPS without clinical ILD (Figure 1A). This difference was driven by a subset of subjects with HPS with ILD with very high absolute concentrations of circulating fibrocytes. We compared this skewing of distribution of blood fibrocyte concentrations between groups and found that the top quintile of total fibrocytes was significantly higher in subjects with HPS with ILD compared with other groups, whereas the distribution did not differ significantly between subjects with HPS without ILD and healthy control subjects. The concentration of activated subset of fibrocytes, defined by coexpression of the myofibroblast marker α-SMA, was close to undetectable in healthy subjects and in subjects with HPS without ILD but was again markedly elevated in a subset of subjects with HPS and ILD (Figure 1B). In contrast, the subset of circulating fibrocytes that expressed CD34 was elevated in only four subjects with ILD, and the concentration of this subset did not differ between groups (Figure 1C), indicating that CD34 is not an optimal marker for assessing fibrocyte concentrations in this population. The concentration of fibrocytes and fibrocyte subsets had no detectable relationship to age, sex, or treatment with pirfenidone (data not shown). In addition, circulating fibrocyte concentration did not correlate significantly with peripheral leukocyte counts (r = 0.22; P = 0.08).
Table 1.
Summary of Demographic Data and Lung Function Tests of Study Subjects at Enrollment
| Group | N | Male Sex [n (%)] | Age (yr) [Median (IQR)] | % Predicted FVC [Median (IQR)] | % Predicted DlCO [Median (IQR)] |
|---|---|---|---|---|---|
| Healthy control subjects | 12 | 5 (42) | 41 (30–48) | ||
| Subjects with HPS | 66 | ||||
| Without clinical ILD | 17 | 6 (38) | 41 (30–47) | 90 (84–94) | 87 (82–98) |
| With clinical ILD | 49 | 19 (39) | 40 (33–48) | 71 (60–80) | 66 (53–77) |
| Longitudinal study | 42 | 14 (40) | 40 (33–46) | 72 (63–81) | 72 (54–80) |
Definition of abbreviations: DlCO = diffusion capacity for carbon monoxide; HPS = Hermansky-Pudlak syndrome; ILD = interstitial lung disease; IQR = interquartile range.
Figure 1.
Cross-sectional comparison of peripheral blood fibrocytes in subjects with Hermansky-Pudlak syndrome (HPS) and healthy control subjects. Concentration of cells was determined by flow cytometry. For subjects with serial data, the first value is shown. (A–F) Circles indicate individual subjects and horizontal bars represent medians. (A) Total fibrocytes (CD45+Col1+). (B) Activated fibrocytes (CD45+Col1+α-SMA+). (C) CD34+ fibrocytes (CD45+Col1+CD34+). (D) CXCR4+ fibrocytes (CD45+Col1+CXCR4+). (E) CCR2+ fibrocytes (CD45+Col1+CCR2+). (F) CCR7+ fibrocytes (CD45+Col1+CCR7+). (G) Distribution of median fibrocyte concentrations expressing various chemokine receptors. Left, Healthy controls; middle, subjects with HPS without ILD; right, subjects with HPS with ILD. The numeric values represent percentage of total fibrocytes in that group. *P < 0.05 by Dunn multiple comparison test; **P < 0.05 by Kruskal Wallis test. α-SMA = α-smooth muscle actin; Col1 = collagen-1; ILD = interstitial lung disease; NS = no significant difference.
Because the chemokine receptors CXCR4, CCR2, and CCR7 have been previously implicated in fibrocyte trafficking (7, 8, 18), we compared the concentration of subsets of fibrocytes expressing these receptors and the plasma concentration of their ligands. Consistent with prior publications, CXCR4 was expressed by most fibrocytes, and CCR2 and CCR7 were expressed by smaller subsets (Figures 1D–1G). In addition, the distribution of CXCR4+ fibrocytes was the most skewed of all assessed subsets. The plasma concentration of CXCL12 (ligand of CXCR4) and CCL2, CCL8, and CCL13 (ligands of CCR2) were elevated in subjects with HPS with ILD as compared with subjects without ILD, whereas the concentration of the CCR2 ligand CCL7 and the CCR7 ligands CCL19 and CCL21 did not differ significantly between groups (Figure 2). Because these ligands can act on their cognate receptors on many cells other than fibrocytes, we examined the correlations between the concentration of each ligand and the concentrations of fibrocytes expressing its receptor in individual subjects. Among the ligands examined, only the concentration of CXCL12 was found to correlate significantly with that of CXCR4+ fibrocytes (Figure 3), whereas the concentration of CCR2 and CCR7 ligands had no detectable relationship to that of fibrocytes expressing these receptors (r = −0.05 to 0.17; P = 0.23–0.90). This association is consistent with the notion that interaction of CXCL12 with fibrocyte CXCR4 may be relevant to the trafficking of these cells in subjects with HPS.
Figure 2.
Cross-sectional comparison of plasma chemokine ligands in subjects with Hermansky-Pudlak syndrome. (A) CXCL12; (B) CCL2; (C) CCL7; (D) CCL8; (E) CCL13; (F) CCL19; (G) CCL21. Circles indicate individual subjects and horizontal bars represent medians. *P < 0.05 by Mann–Whitney test. ILD = interstitial lung disease; NS = no significant difference.
Figure 3.
Correlation between plasma CXCL12 level and concentration of CXCR4+ fibrocytes in cross-sectional study of subjects with Hermansky-Pudlak syndrome. Circles indicate individual subjects. P and r values determined by Spearman rank correlation.
We obtained serial blood samples and PFTs from 42 of the subjects with HPS, 37 of whom had clinical evidence of ILD at the beginning of the study, on a median of four visits over 614 days (IQR, 3–6 visits over 266–742 d). There was no significant change in median FVC or gas transfer of the cohort over time (Figure 4), although some individual subjects experienced deterioration in lung function over time (see Figure E2 in the online supplement). Surprisingly, however, we found episodes of marked elevation in the circulating fibrocyte counts among individual subjects over time (median intraindividual variation of 12-fold minimum value; IQR, 3- to 34-fold change; Figure 5A). As a group, both the total and activated blood fibrocyte concentrations increased over time with marked interindividual variation (Figure 5B); a change was most notably reflected in the CXCR4+ fibrocytes (Figure 5C). Among individual subjects, there was no significant relationship between total or activated fibrocyte counts, or changes in these values, and decline in FVC on the subsequent visit. There was a weak but statistically significant correlation between an increase in activated fibrocyte concentration on a given visit and decline in gas transfer at the following visit (r = −0.2, P = 0.017).
Figure 4.
Change in pulmonary function tests over time in longitudinal study of subjects with Hermansky-Pudlak syndrome. Data are represented as median and interquartile range. No change was found over time in either variable by one-way analysis of variance with repeat measurements. DlCO = diffusion capacity for carbon monoxide.
Figure 5.
Change in peripheral blood fibrocytes over time in longitudinal study of subjects with Hermansky-Pudlak syndrome. (A) Each line represents data from a single subject. (B, C) Data are represented as median and interquartile range. All curves showed significant change over time by one-way analysis of variance with repeat measurements.
Survival data were collected for a median period of 6.1 years (IQR, 4.1–6.8 yr). Among the 57 subjects in whom survival data were available, 11 died of progressive lung disease during this period, 7 of whom had had serial blood sampling. Deaths occurred a median of 347 days after the last blood draw (IQR, 159–843 d). We sought to identify a threshold fibrocyte concentration that would identify risk of death before the next visit. In a receiver operating characteristics analysis, we found that the last measured CXCR4+ fibrocyte count greater than 3.35 × 105 cells/ml had 92% negative predictive value and 33% positive predictive value for death and was significantly associated with death (P = 0.03; HR, 3.8; Figure 6A) but was unrelated to age, sex, or treatment with pirfenidone. Given the correlation between the concentrations of plasma CXCL12 and CXCR4+ fibrocytes, we also performed a similar analysis to determine whether plasma CXCL12 elevations were associated with mortality. Although serial plasma CXCL12 levels showed substantial variability over time, no threshold concentration of CXCL12 was associated with death (Figure E3). Finally, we found that a highest ever CXCR4+ fibrocyte count greater than 1.0 × 106 cells/ml had 91% negative predictive value and 37% positive predictive value for death and was again significantly associated with death (P = 0.01; HR, 5.1; Figure 6B).
Figure 6.
Fibrocyte counts as predictors of mortality in subjects with Hermansky-Pudlak syndrome. (A) Kaplan-Meier survival analysis of subjects, separated by last measured circulating CXCR4+ fibrocyte concentration threshold value of 3.35 × 105 cells/ml. Of the 57 subjects with available survival data, 33 had values below and 24 above the threshold. (B) Kaplan-Meier survival analysis of subjects, separated by highest measured circulating CXCR4+ fibrocyte concentration above or below threshold value of 1.0 × 106 cells/ml. Of the 57 subjects, 40 had values below and 17 above the threshold. *Statistically significant difference by log-rank test.
Discussion
A key limitation in the care of patients with ILDs is the unpredictable nature of the clinical course. For many years, the natural history of progressive fibrotic ILDs was believed to be one of steady and persistent decline in lung function, with some patients having a more accelerated deterioration than others (1, 19). Observations in the control groups of early trials of IPF, however, showed that the typical natural history is one of prolonged periods of relative stability, punctuated by abrupt and step-wise deteriorations (20, 21). These exacerbations, which have since been described in other fibrotic ILDs (22), vary in severity from asymptomatic to severe enough to precipitate respiratory failure and are the most common cause of death in these illnesses (20). The inciting cause of ILD exacerbations, and deterioration of lung fibrosis in general, is usually unknown and is hypothesized to involve episodes of epithelial injury from diverse etiologies.
Circulating fibrocytes have previously been shown to home to diverse tissues in response to injury and differentiate into fibroblasts and myofibroblasts, thus contributing to both physiologic and pathologic scarring. Fibrocytes express the extracellular matrix proteins vimentin, fibronectin, collagens -I and -III, together with the common leukocyte antigen, CD45RO. In addition, a progressively smaller subset of fibrocytes in the bone marrow, blood, and target tissues express the progenitor marker CD34. In contrast, under profibrotic conditions such as exposure to transforming growth factor-β, fibrocytes gain the expression of the myofibroblast marker, α-SMA, consistent with an activated phenotype (4–6). Prior work has shown that, in animal models of lung fibrosis, traffic of fibrocytes to the lungs is dependent on chemokine signals and that the interruption of this traffic attenuates the degree of fibrosis (6–8, 23). The circulating pool of fibrocytes composes less than 0.5% of nucleated cells in peripheral blood of healthy subjects, but this pool is greatly expanded in both the blood and lungs in patients with fibrotic ILD (9, 10), and this expansion was an independent predictor of death in a cross-sectional study of patients with IPF (11), raising the possibility that these cells may be useful as biomarkers in fibrotic lung disease. To our knowledge, however, fibrocytes have not been investigated longitudinally as biomarkers of ILD to date.
We chose to study the usefulness of fibrocytes as biomarkers of disease activity in the HPS because studying individuals within a genetically homogeneous cohort has the potential to reduce the variability inherent to studying groups of heterogeneous subjects with ILD. In addition, the ability to identify subjects with asymptomatic subclinical ILD provides a unique window to study the early events in the development of ILD. In this context, HPS is an autosomal recessive disease manifested by oculocutaneous albinism caused by one of nine human mutations that affect the formation of specialized intracellular vesicles known as lysosome-related organelles (12, 24). Lysosome-related organelles are present in specific cell types, including melanocytes, platelets, and type-II alveolar epithelial cells (25). The most common subtype of this disease, HPS type 1 (HPS-1), invariably results in ILD in early adulthood; this phenotype also occurs in the far less common HPS-2 and HPS-4 but not in the other HPS genotypes (26–28). The lung disease in HPS has the chest computed tomography scan and histology of usual interstitial pneumonia and is the cause of death in nearly all patients (13, 14, 29). Thus, studying subjects with HPS provides a unique opportunity to evaluate a homogeneous group of subjects with a broad range of ILD severity.
Fibrocytes are known to express several chemokine receptors and to display chemotaxis in response to the ligands of these receptors. CXCR4 is the most commonly expressed chemokine receptor on peripheral blood fibrocytes in both humans and mice and is involved in fibrocyte traffic in mouse models of lung fibrosis and in vitro chemotaxis with human cells (6, 8, 23, 30). Smaller and overlapping subsets of fibrocytes express CCR2 and CCR7, and the ligands for these receptors have also been implicated in fibrocyte traffic in several animal models of fibrotic diseases (7, 18, 31, 32). In this context, our data revealed elevations in plasma levels of ligands of CXCR4 and CCR2, but not CCR7, in subjects with HPS with ILD. The significant correlation between plasma CXCL12 level and the concentration of circulating CXCR4-expressing fibrocytes support the hypothesis that, similar to animal models of lung fibrosis (6, 8), this ligand–receptor pair is mechanistically involved in the traffic of fibrocytes in this patient population. On the other hand, we interpret the lack of such a correlation between CCR2-expressing fibrocytes and CCR2 ligands as evidence of redundancy of these ligands for fibrocyte traffic and potentially their involvement in mobilization of other cells, such as monocytes.
Another finding in this study was that fibrocyte counts were predictive of death but had only a weak relationship to worsening in diffusing capacity of carbon monoxide (DlCO) and no detectable relationship to decline in FVC. In this context, decline in FVC and DlCO have been shown to predict early mortality in IPF and other ILDs, both alone (33–35) and as part of clinical scoring systems (36, 37). Because all deaths in our study were due to lung disease, it is possible that our study was underpowered to detect a relationship between fibrocytes and decline in PFTs where one existed. We note, however, that a dissociation between fibrocyte levels and PFTs in providing prognostic information was also found in a cross- sectional study in patients with IPF (11). Taken together, we speculate that fibrocytes and PFTs may be measures of independent mechanisms that influence outcome, with decline in PFTs representing the cumulative effect of past episodes of deterioration and fibrocytes predicting future events.
In summary, we report that, in a relatively homogeneous population of patients with progressive ILD, individual subjects experience episodic elevations of the concentration of CXCR4+ circulating fibrocytes, and these episodes correlated with subsequent death from progressive lung disease. Based on these data, we propose a general model wherein episodic events of lung injury, some of which may be clinically silent, result in CXCR4-dependent recruitment of fibrocytes from the bone marrow to the bloodstream and then to the lungs, where they contribute to fibrosis. Future work should determine whether fibrocytes are also useful as biomarkers of deterioration in more common types of ILD and whether the combination of fibrocytes with other biomarkers, such as markers of epithelial injury, is more effective in identifying at-risk patients. If these results are reproduced in other forms of ILD, we envision measuring fibrocytes in patients at each clinic visit, thus identifying individuals at high risk of poor outcomes who can then be targeted for clinical interventions or enrollment in trials.
Footnotes
Funded by National Institutes of Health grants HL098526 and HL098329 (both to B.M.).
Author Contributions: Conception, hypothesis delineation, and study design: W.A.G., R.M.S., and B.M.; data acquisition, analysis and interpretation: A.T., B.R.G., T.C.M., R.F., J.K.L., Y.K., M.D.B., and B.M.; writing first the draft of manuscript: A.T. and B.M.; revision of manuscript: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201407-1287OC on October 27, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeley EC, Mehrad B, Strieter RM. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2010;42:535–542. doi: 10.1016/j.biocel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrad B, Strieter RM. Fibrocytes and the pathogenesis of diffuse parenchymal lung disease. Fibrogenesis Tissue Repair. 2012;5:S22. doi: 10.1186/1755-1536-5-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson-Sjöland A, de Alba CG, Nihlberg K, Becerril C, Ramírez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 11.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 12.Seward SL, Jr, Gahl WA. Hermansky-Pudlak syndrome: health care throughout life. Pediatrics. 2013;132:153–160. doi: 10.1542/peds.2012-4003. [DOI] [PubMed] [Google Scholar]
- 13.Brantly M, Avila NA, Shotelersuk V, Lucero C, Huizing M, Gahl WA. Pulmonary function and high-resolution CT findings in patients with an inherited form of pulmonary fibrosis, Hermansky-Pudlak syndrome, due to mutations in HPS-1. Chest. 2000;117:129–136. doi: 10.1378/chest.117.1.129. [DOI] [PubMed] [Google Scholar]
- 14.Nakatani Y, Nakamura N, Sano J, Inayama Y, Kawano N, Yamanaka S, Miyagi Y, Nagashima Y, Ohbayashi C, Mizushima M, et al. Interstitial pneumonia in Hermansky-Pudlak syndrome: significance of florid foamy swelling/degeneration (giant lamellar body degeneration) of type-2 pneumocytes. Virchows Arch. 2000;437:304–313. doi: 10.1007/s004280000241. [DOI] [PubMed] [Google Scholar]
- 15.Witkop CJ, Almadovar C, Piñeiro B, Nuñez Babcock M. Hermansky-Pudlak syndrome (HPS). An epidemiologic study. Ophthalmic Paediatr Genet. 1990;11:245–250. doi: 10.3109/13816819009020986. [DOI] [PubMed] [Google Scholar]
- 16.Trimble A, Burdick MD, Fischer R, Gahl WA, Gochuico BR, Mehrad B. Elevation in peripheral fibrocyte counts correlates with development of interstitial lung disease in patients with Hermansky-Pudlak syndrome. Presented at the American College of Chest Physicians Meeting. October 26–31, 2013, Chicago, IL [Google Scholar]
- 17.O’Brien K, Troendle J, Gochuico BR, Markello TC, Salas J, Cardona H, Yao J, Bernardini I, Hess R, Gahl WA. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011;103:128–134. doi: 10.1016/j.ymgme.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panos RJ, Mortenson RL, Niccoli SA, King TE., Jr Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med. 1990;88:396–404. doi: 10.1016/0002-9343(90)90495-y. [DOI] [PubMed] [Google Scholar]
- 20.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, Lasky JA, Loyd JE, Noth I, Olman MA, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyzy R, Huang S, Myers J, Flaherty K, Martinez F. Acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2007;132:1652–1658. doi: 10.1378/chest.07-0299. [DOI] [PubMed] [Google Scholar]
- 22.Park IN, Kim DS, Shim TS, Lim CM, Lee SD, Koh Y, Kim WS, Kim WD, Jang SJ, Colby TV. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 23.Song JS, Kang CM, Kang HH, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp Mol Med. 2010;42:465–472. doi: 10.3858/emm.2010.42.6.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gahl WA, Huizing M.Hermansky-Pudlak syndromePagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews. Seattle: University of Washington [created 2000 Jul 24; revised 2013 Feb 28]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1287/ [Google Scholar]
- 25.Shotelersuk V, Gahl WA. Hermansky-Pudlak syndrome: models for intracellular vesicle formation. Mol Genet Metab. 1998;65:85–96. doi: 10.1006/mgme.1998.2729. [DOI] [PubMed] [Google Scholar]
- 26.Gahl WA, Brantly M, Kaiser-Kupfer MI, Iwata F, Hazelwood S, Shotelersuk V, Duffy LF, Kuehl EM, Troendle J, Bernardini I. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome) N Engl J Med. 1998;338:1258–1264. doi: 10.1056/NEJM199804303381803. [DOI] [PubMed] [Google Scholar]
- 27.Anderson PD, Huizing M, Claassen DA, White J, Gahl WA. Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum Genet. 2003;113:10–17. doi: 10.1007/s00439-003-0933-5. [DOI] [PubMed] [Google Scholar]
- 28.Gochuico BR, Huizing M, Golas GA, Scher CD, Tsokos M, Denver SD, Frei-Jones MJ, Gahl WA. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol Med. 2012;18:56–64. doi: 10.2119/molmed.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avila NA, Brantly M, Premkumar A, Huizing M, Dwyer A, Gahl WA. Hermansky-Pudlak syndrome: radiography and CT of the chest compared with pulmonary function tests and genetic studies. AJR Am J Roentgenol. 2002;179:887–892. doi: 10.2214/ajr.179.4.1790887. [DOI] [PubMed] [Google Scholar]
- 30.Keeley EC, Mehrad B, Janardhanan R, Salerno M, Hunter JR, Burdick MM, Field JJ, Strieter RM, Kramer CM. Elevated circulating fibrocyte levels in patients with hypertensive heart disease. J Hypertens. 2012;30:1856–1861. doi: 10.1097/HJH.0b013e32835639bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reich B, Schmidbauer K, Rodriguez Gomez M, Johannes Hermann F, Göbel N, Brühl H, Ketelsen I, Talke Y, Mack M. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013;84:78–89. doi: 10.1038/ki.2013.84. [DOI] [PubMed] [Google Scholar]
- 33.Peelen L, Wells AU, Prijs M, Blumenthal JP, van Steenwijk RP, Jonkers RE, Peek N, Bresser P. Fibrotic idiopathic interstitial pneumonias: mortality is linked to a decline in gas transfer. Respirology. 2010;15:1233–1243. doi: 10.1111/j.1440-1843.2010.01862.x. [DOI] [PubMed] [Google Scholar]
- 34.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, King TE, Jr, Lancaster L, Noble PW, Sahn SA, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184:1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt SL, Tayob N, Han MK, Zappala C, Kervitsky D, Murray S, Wells AU, Brown KK, Martinez FJ, Flaherty KR. Predicting pulmonary fibrosis disease course from past trends in pulmonary function. Chest. 2014;145:579–585. doi: 10.1378/chest.13-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 37.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, Elicker BM, Wolters PJ, Koth LL, King TE, Jr, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145:723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]