To the Editor:
It is believed that one-third of the entire world population is latently infected with Mycobacterium tuberculosis, which has previously been attributed to the ability of the tubercle bacillus to survive for years without replication (1). The documented examples of tuberculosis reactivation after years of absence of disease illustrate the capacity of M. tuberculosis to reversibly switch from a nonreplicating state to active growth (2). The presence of M. tuberculosis in lungs of latently infected patients has been confirmed by molecular and immunological techniques (3), yet growth in culture of the bacteria associated with latent infection has not been demonstrated. Therefore, it has been hypothesized that during latent infection M. tuberculosis produces nonreplicating forms, which require resuscitation under specialized cultivation conditions to produce growth (4). However, nongrowing persister-like M. tuberculosis can also be detected in sputum collected from patients with active tuberculosis (5–7). The majority of these bacilli required specific growth conditions and could be cultivated only in the presence of recombinant resuscitation-promoting factor (Rpf) or culture supernatant (6). Moreover, these Rpf-dependent mycobacteria were more tolerant to rifampicin and accumulated during chemotherapy while other organisms were eliminated (6), confirming the production of physiologically distinct M. tuberculosis forms during tuberculosis infection or during transition to sputum. Because numbers of Rpf-dependent mycobacteria varied between patients (6), it is plausible to suggest the importance of specific host factors for the development of Rpf dependency.
The molecular mechanisms underlying the formation of Rpf-dependent bacteria recovered from sputum remain unknown. Rpf-dependent cells could be generated in loci of infection (e.g., lungs) in high numbers and subsequently gradually released into sputum; alternatively, mycobacteria may rapidly develop Rpf dependency during transition from lung to sputum under the influence of certain, but yet unknown, stimuli. Our previous identification of Rpf-dependent bacteria in patients with active tuberculosis points to the presence of a heterogeneity in growth states within the bacterial populations residing in sputum. However, how and when these adaptions arise remains unknown and in this regard we propose two possibilities: (1) Considering that tubercle bacilli traffic out of necrotic cavities and into the airways, the accompanying changes in the extracellular environment and short exposure to sputum may induce an adaptive response that results in Rpf dependency; (2) Rpf dependency may arise in response to stresses imposed by the immune system and longer stationary exposure to the tissue or cavity environment. We previously found that direct exposure of M. tuberculosis to sputum did not result in Rpf dependency (6), which suggested that the extracellular environment in sputum cannot be the sole inducer of this adaptive response in M. tuberculosis. Consequently, in this study we investigated whether Rpf-dependent bacilli are produced in pulmonary infection in an animal model.
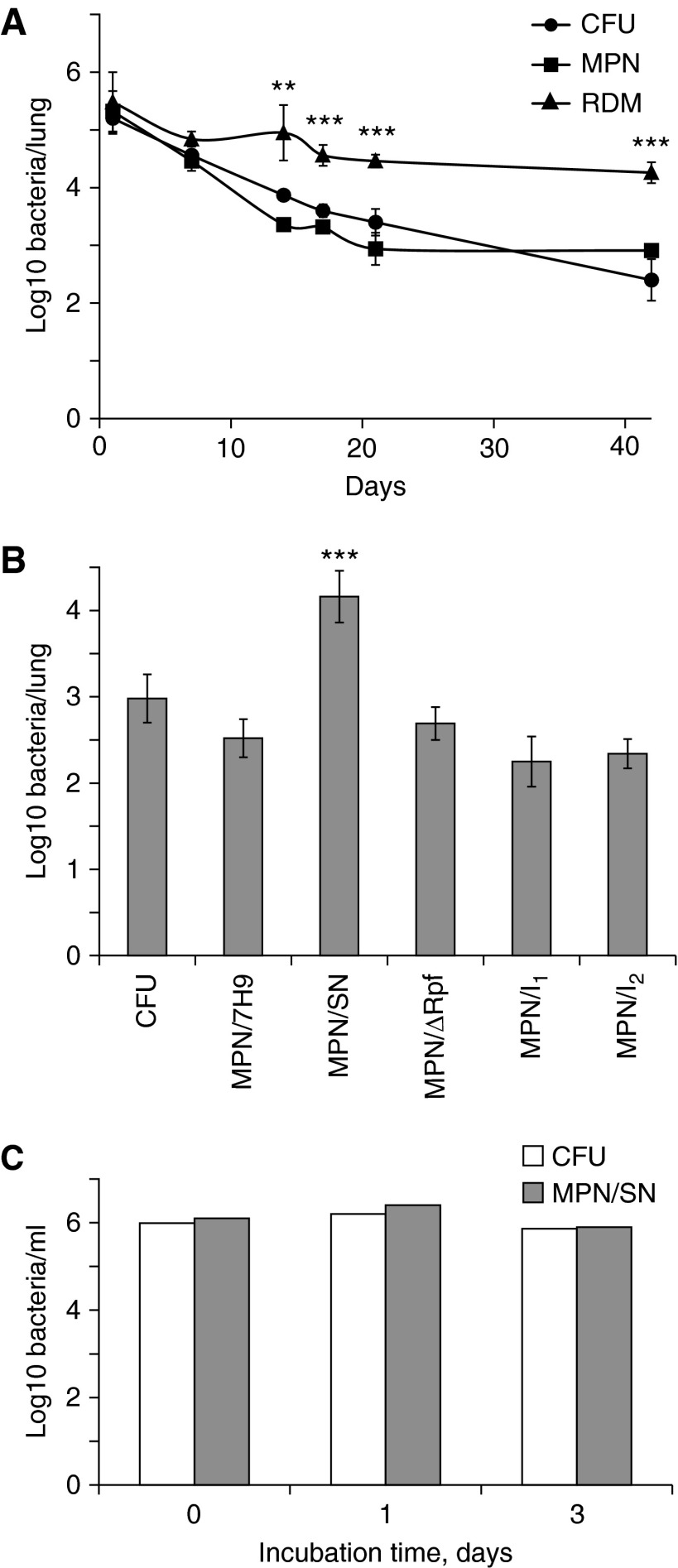
Nine-week-old BALB/c mice were infected intranasally with Mycobacterium bovis BCG Glaxo at a dose of 2 × 105 bacteria per mouse, and numbers of mycobacteria in lungs were monitored for 6 weeks. For this, we employed growth assays previously developed for investigation of mycobacterial populations in sputum (6). We quantified numbers of mycobacteria that were able to grow either on 7H10 agar (colony-forming unit [CFU] counts) or in liquid 7H9 medium (using the most probable number [MPN] assay). The numbers of Rpf-dependent mycobacteria (RDM) were assessed by MPN assay in liquid 7H9 medium, containing culture supernatant from growing bacteria. At 24 hours postinfection, CFU, MPN, and RDM counts of mycobacteria recovered from lungs of infected animals were not significantly different (P > 0.05, one-way analysis of variance), suggesting that the preparation of bacteria for infection and initial adaptation in vivo did not induce Rpf dependency. However, during the course of infection there was a dramatic 2.5 log10 reduction in CFU and MPN counts of mycobacteria in the lungs of infected animals (Figure 1A). These results are in good accordance with previously reported survival patterns of M. bovis BCG in BALB/c mice (8, 9). In contrast, the number of mycobacteria grown with culture supernatant changed only at the beginning of infection (a 0.5 log10 reduction 1 wk postinfection) and at later stages it remained constant, suggesting that more than 98% of mycobacteria recovered from lungs at 6 weeks postinfection required special conditions for cultivation (Figure 1A). To confirm that bacteria recovered in the presence of culture supernatant were indeed Rpf dependent, further experiments were performed. In these experiments numbers of mycobacteria grown in culture supernatant treated with specific inhibitors of Rpf (10), or in culture supernatant prepared from a quintuple M. tuberculosis mutant missing all five Rpfs (11), were assessed. As shown in Figure 1B, both Rpf inhibitors completely eliminated the resuscitation activity of culture supernatant, and Rpf-negative supernatant also failed to resuscitate nonculturable bacteria. Both of these control experiments confirm that the nonculturable mycobacteria recovered were indeed Rpf dependent.
Figure 1.
Generation of resuscitation-promoting factor (Rpf)-dependent Mycobacterium bovis (BCG) in murine lungs. (A and B) Nine-week-old BALB/c mice were infected intranasally with 2 × 105 bacterial colony-forming units (CFUs) per animal. (A) Homogenized tissue samples were used for assessment of CFU counts (circles) or most-probable number assay (MPN) counts in liquid 7H9 medium (squares) or Rpf-dependent mycobacteria (RDM) counts in 7H9 medium supplemented with culture supernatant (triangles). A PANTA antimicrobial mixture (Becton, Dickinson and Co., Franklin Lakes, NJ) was added to all cultivation media. Average values for four independent animals are presented; error bars indicate standard deviations. **RDM values were significantly different from CFU and MPN counts (P < 0.01, t test); ***RDM values were significantly different from CFU counts (P < 0.001, t test). (B) Control experiments. CFU and MPN counts were determined in lung homogenates from mice 3 weeks postinfection. The Rpf inhibitors NTB (3-nitro-4-thiocyanato-benzonitrile), designated as I1, and NTPPM [(3-nitro-4-thiocyanato-phenyl)-phenyl-methanone], designated as I2, did not inhibit growth of active M. bovis BCG in vitro at the concentration used in these experiments (5 μg/ml). SN = culture supernatant. (C) Effect of murine serum on M. bovis BCG viability. Bacteria from the logarithmic phase were exposed to 20% (vol/vol) murine serum in phosphate-buffered saline. CFU and RDM counts were determined after 1 and 3 days of exposure.
Incubation of mycobacteria in lung homogenates did not result in the development of Rpf dependency (data not shown). We therefore investigated whether exposure of mycobacteria to murine serum would stimulate production of Rpf-dependent forms. We incubated growing M. bovis BCG bacteria in phosphate-buffered saline (PBS) containing 25% (vol/vol), 50% (vol/vol), or undiluted murine serum, obtained from mice infected with M. bovis BCG for 24 hours, at 37°C without shaking. CFU and MPN counts were taken after 1 and 3 days of incubation. However, incubation of mycobacteria in PBS containing serum did not result in any statistically significant loss of culturability or generation of Rpf-dependent forms (Figure 1C). Sera from uninfected mice showed similar effects. This could be because cell-mediated immunity is essential for the generation of Rpf-dependent bacteria.
This study demonstrates that the in vivo environment changes mycobacterial physiological characteristics and accelerates the generation of Rpf-dependent mycobacteria. Our results suggest that Rpf-dependent mycobacteria are generated in murine lungs soon after infection and represent significant proportions of the bacteria present. Therefore it is plausible to suggest that Rpf-dependent mycobacteria recovered from the sputum of infected patients are generated in the lungs during infection rather than during the transition into sputum.
The precise factors responsible for the formation of Rpf-dependent bacteria remain unknown. Previously, it was demonstrated that M. tuberculosis bacilli developed Rpf dependency during prolonged incubation in the stationary phase (11) or on gradual acidification of medium (12). Nonculturable and Rpf-dependent cells of M. tuberculosis have been recovered from peritoneal macrophages of infected mice after several days of infection (13). However, exposure of mycobacteria to murine serum did not stimulate Rpf dependency, suggesting that a yet unknown combination of environmental conditions not identified (or present) in in vitro experiments or certain immune factors may trigger transition to the Rpf-dependent state. Our findings have important implications for the diagnosis of tuberculosis (14, 15) and development of models for testing the bactericidal activity of novel drugs. The presence of a high number of Rpf-dependent bacteria in vivo must be taken into account in the design of drugs for the treatment of tuberculosis, and compounds should also be tested for activity against Rpf-dependent forms. Our data suggest that significant proportions of mycobacteria may remain undetected in animal infection experiments and during clinical trials of new treatments.
Footnotes
Supported by grants from the Wellcome Trust and BBSRC.
Author Contributions: Conception, design, and experiments—O.T., S.G., and G.V.M.; analysis and interpretation—O.T., S.G., B.K., V.M., P.W.A., and G.V.M.; drafting the letter—G.V.M. and S.G.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.Lillebaek T, Dirksen A, Vynnycky E, Baess I, Thomsen VO, Andersen AB. Stability of DNA patterns and evidence of Mycobacterium tuberculosis reactivation occurring decades after the initial infection. J Infect Dis. 2003;188:1032–1039. doi: 10.1086/378240. [DOI] [PubMed] [Google Scholar]
- 3.Barrios-Payán J, Saqui-Salces M, Jeyanathan M, Alcántara-Vazquez A, Castañon-Arreola M, Rook G, Hernandez-Pando R. Extrapulmonary locations of Mycobacterium tuberculosis DNA during latent infection. J Infect Dis. 2012;206:1194–1205. doi: 10.1093/infdis/jis381. [DOI] [PubMed] [Google Scholar]
- 4.Chao MC, Rubin EJ. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol. 2010;64:293–311. doi: 10.1146/annurev.micro.112408.134043. [DOI] [PubMed] [Google Scholar]
- 5.Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med. 2010;181:174–180. doi: 10.1164/rccm.200905-0661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon J, Fourie PB, Mitchison DA. Persister populations of Mycobacterium tuberculosis in sputum that grow in liquid but not on solid culture media. J Antimicrob Chemother. 2014;69:437–440. doi: 10.1093/jac/dkt357. [DOI] [PubMed] [Google Scholar]
- 8.Tree JA, Williams A, Clark S, Hall G, Marsh PD, Ivanyi J. Intranasal bacille Calmette-Guérin (BCG) vaccine dosage needs balancing between protection and lung pathology. Clin Exp Immunol. 2004;138:405–409. doi: 10.1111/j.1365-2249.2004.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spratt JM, Britton WJ, Triccas JA. In vivo persistence and protective efficacy of the bacille Calmette Guérin vaccine overexpressing the HspX latency antigen. Bioeng Bugs. 2010;1:61–65. doi: 10.4161/bbug.1.1.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaprelyants AS, Mukamolova GV, Ruggiero A, Makarov VA, Demina GR, Shleeva MO, Potapov VD, Shramko PA. Resuscitation-promoting factors (Rpf): in search of inhibitors. Protein Pept Lett. 2012;19:1026–1034. doi: 10.2174/092986612802762723. [DOI] [PubMed] [Google Scholar]
- 11.Kana BD, Gordhan BG, Downing KJ, Sung N, Vostroktunova G, Machowski EE, Tsenova L, Young M, Kaprelyants A, Kaplan G, et al. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol Microbiol. 2008;67:672–684. doi: 10.1111/j.1365-2958.2007.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shleeva MO, Kudykina YK, Vostroknutova GN, Suzina NE, Mulyukin AL, Kaprelyants AS. Dormant ovoid cells of Mycobacterium tuberculosis are formed in response to gradual external acidification. Tuberculosis (Edinb) 2011;91:146–154. doi: 10.1016/j.tube.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Biketov S, Mukamolova GV, Potapov V, Gilenkov E, Vostroknutova G, Kell DB, Young M, Kaprelyants AS. Culturability of Mycobacterium tuberculosis cells isolated from murine macrophages: a bacterial growth factor promotes recovery. FEMS Immunol Med Microbiol. 2000;29:233–240. doi: 10.1111/j.1574-695X.2000.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 14.Kolwijck E, Friedrich SO, Karinja MN, van Ingen J, Warren RM, Diacon AH. Early stationary phase culture supernatant accelerates growth of sputum cultures collected after initiation of anti-tuberculosis treatment. Clin Microbiol Infect. 2014;20:O418–O420. doi: 10.1111/1469-0691.12441. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Qi Y, Diao Y, Yang F, Zha X, Ren C, Huang D, Franken KL, Ottenhoff TH, Wu Q, et al. Use of resuscitation-promoting factor proteins improves the sensitivity of culture-based tuberculosis testing in special samples. Am J Respir Crit Care Med. 2014;189:612–614. doi: 10.1164/rccm.201310-1899LE. [DOI] [PubMed] [Google Scholar]