Abstract
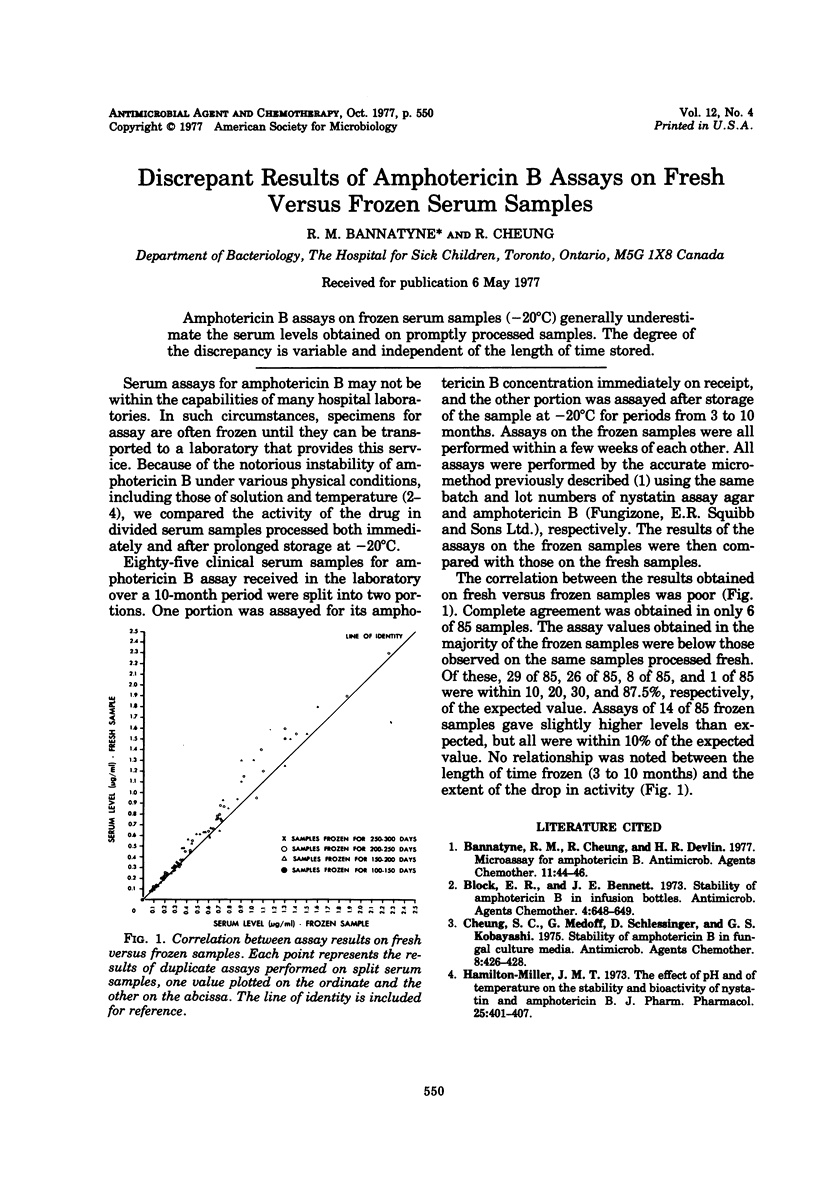
Amphotericin B assays on frozen serum samples (−20°C) generally underestimate the serum levels obtained on promptly processed samples. The degree of the discrepancy is variable and independent of the length of time stored.
Full text
PDFPage 550
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannatyne R. M., Cheung R., Devlin H. R. Microassay foramphotericin B. Antimicrob Agents Chemother. 1977 Jan;11(1):44–46. doi: 10.1128/aac.11.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block E. R., Bennett J. E. Stability of amphotericin B in infusion bottles. Antimicrob Agents Chemother. 1973 Dec;4(6):648–649. doi: 10.1128/aac.4.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S. C., Medoff G., Schlessinger D., Kobayashi G. S. Stability of amphotericin B in fungal culture media. Antimicrob Agents Chemother. 1975 Oct;8(4):426–428. doi: 10.1128/aac.8.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. The effect of pH and of temperature on the stability and bioactivity of nystatin and amphotericin B. J Pharm Pharmacol. 1973 May;25(5):401–407. doi: 10.1111/j.2042-7158.1973.tb10035.x. [DOI] [PubMed] [Google Scholar]


