Abstract
Replicating the group-based developmental trajectory methodology from our prior study (Patriquin, Lorenzi, Scarpa, & Bell, 2014), the current study examines the development of baseline respiratory sinus arrhythmia (RSA) across a new, larger cohort of typically developing children at 5, 10, 24, 36, and 48 months of age and examines the trajectory relationship with symptoms of childhood psychopathology. Group-based developmental trajectory modeling replicated our prior findings of a two-group model fit: a “High RSA” and “Low RSA” group. The “Low RSA” group, which demonstrated lower baseline RSA across all time points, had significantly more childhood problems at 48 months, namely increased withdrawal, aggressive behavior, pervasive developmental problems, and oppositional defiant problems. All participants for whom there were developmental or autism spectrum concerns (n = 6; based on maternal report at 48 months) were allocated to the Low RSA trajectory group. These results suggest that consistent developmental trajectories of RSA may point to protective factors (i.e., high RSA) against developing symptoms of childhood psychopathology.
Keywords: development, trajectory, longitudinal, respiratory sinus arrhythmia, childhood, psychopathology
Both past and current perspectives on psychopathology emphasize a multi-level conceptualization of transdiagnostic constructs in order to develop effective, empirically-based interventions (Cuthbert & Insel, 2010; Cuthbert & Kozak, 2013; Insel et al., 2010; Van Praag, Asnis, Kahn, & Brown, 1990). Our prior research highlights the significant variability in transdiagnostic constructs (i.e., social responsiveness) and physiological measures (i.e., respiratory sinus arrhythmia, RSA) in the general population; thus, providing empirical support for examining psychopathology, including the development of psychopathology, from a domain specific, biobehavioral perspective (Patriquin, Lorenzi, Scarpa, & Bell, 2014).
Herein, we extend our prior study that used group-based developmental trajectory modeling (Patriquin et al., 2014) to a new, larger cohort of children who also underwent baseline RSA measurement at 5, 10, 24, 36, and 48 months of age. In the current study, RSA trajectory groups were determined across 5–48 months of age and group differences were examined on a broadband measure of childhood competencies and problems at 48 months old. Unlike our prior cohort of children, parent-report data were available regarding the presence or suspicion of developmental delays (DD) and/or an autism spectrum disorder (ASD) at age 48 months. As such, additional analyses were completed to examine the accuracy of the RSA trajectory group allocation of children with (or suspected to have) ASD/DD.
A Broad Conceptualization of RSA
Although RSA has often been linked to social behavior empirically (Bal et al., 2010; Patriquin, Scarpa, Friedman, & Porges, 2013; Van Hecke et al., 2009) and within theoretical models (e.g., Porges, 2009), the current literature also suggests broader associations that includes cognitive development (Feldman & Eidelman, 2009; Patriquin, Lorenzi, & Scarpa, 2013; Patriquin, Scarpa, et al., 2013; Staton, El-Sheikh, & Buckhalt, 2008), emotion regulation (Gentzler, Santucci, Kovacs, & Fox, 2009), language (Patriquin, Lorenzi, et al., 2013), emerging symptoms of psychopathology (Gentzler, Rottenberg, Kovacs, George, & Morey, 2011; Patriquin et al., 2014), and internalizing/externalizing symptoms (Neuhaus, Bernier, & Beauchaine, 2014). Within typically developing populations, RSA has been identified as a indicator of overall functioning (Thayer & Lane, 2009) and may also be a metric of more general functioning in children with psychiatric symptoms.
When combined, multiple theories regarding autonomic function and behavior suggest a broad conceptualization of RSA: neurovisceral integration model (Thayer, Hansen, Saus-Rose, & Johnsen, 2009), polyvagal theory (Porges, 1995, 1998, 2001, 2003, 2007, 2009; Porges & Lewis, 2009), and the integration of the polyvagal theory and Gray’s motivational theory (Beauchaine, 2001). Across perspectives, the relations between social behavior, cognition, and/or emotion processes, and physiological processes (e.g., RSA) are hypothesized. The overlap among these three models provides a neurophysiological conceptualization of the physiological processes that may contribute to social, cognitive, and emotional development.
In particular, these three theoretical perspectives highlight the importance of a soothed physiological state on more positive functioning (e.g., better cognitive performance, social engagement, emotion regulation). For example, the neurovisceral integration theory hypothesizes the relationship between heart rate variability (HRV) and cognitive performance (Thayer et al., 2009). Thayer and colleagues suggest specific, top-down neurovisceral pathways that contribute to a soothed physiological state (increased HRV) that in turn promotes better prefrontal regulation and higher cognitive abilities. Similarly, the polyvagal theory outlines the relations between a soothed physiological state and the physiological mechanisms underlying social behavior. Within the polyvagal theory, it is theorized that activation of the vagus nerve promotes the coordination of five cranial nerves (i.e., Social Engagement System cranial nerves) in order to produce effective social engagement. The withdrawal of the vagus nerve (i.e., reduced RSA) may reduce coordination of the Social Engagement System cranial nerves and contribute to difficulties regarding social behavior, such as those characteristic of ASD (e.g., difficulties with communication, eye contact, joint attention; Bal et al., 2010; Patriquin, Scarpa, et al., 2013; Van Hecke et al., 2009).
Lastly, Beauchaine (2001) combines Gray’s motivational theory with Porges’ polyvagal theory to understand the behavioral and emotional processes associated with psychopathology. Beauchaine uses Gray’s behavioral activation system (BAS) and behavioral inhibition system (BIS) to describe the functioning of the sympathetic (‘fight-flight’) branch of the autonomic nervous system. Conversely, the parasympathetic branch is described as the regulation system (i.e., vagus nerve, RSA). Beauchaine emphasizes the importance of measuring the autonomic nervous system in understanding the physiological processes that underlie behavioral and emotional predispositions to psychopathology (Beauchaine, 2001). Anxiety, for example, is characterized within this model by low RSA (parasympathetic branch) and heightened BIS activity. This physiological state is hypothesized to contribute to passive and fearful behavioral tendencies, including the introversion characteristic of anxiety. On the other hand, heightened BAS activity and excessive vagal reactivity is hypothesized to contribute to appetitive behavioral tendencies that includes impulsive aggression.
Jointly, these three theories provide a theoretical lens for understanding the broad implications of RSA, including the relations with cognitive, social, and emotion regulation ability. Overall, a soothed physiological state – increased parasympathetic activity (i.e., increased RSA or HRV) – is theorized to promote higher cognitive abilities (via better prefrontal regulation; neurovisceral integration theory), effective social behavior (via coordination of Social Engagement System cranial nerves; polyvagal theory), and appropriate emotion regulation (via balanced sympathetic/parasympathetic functioning; Gray’s motivational theory & Porges’ polyvagal theory). Empirical findings appear congruent with these theories.
Childhood Psychopathology & RSA
Across early development, increases in baseline RSA parallel social-cognitive development, particularly during periods of rapid social, cognitive, and emotional changes (Feldman & Eidelman, 2009). More specifically, a child’s physiological and behavioral responses become more organized as RSA increases with development. In fact, a recent prospective study indicated that premature neonates who received a low-cost intervention (i.e., Kangaroo Care) within the first days of birth demonstrated higher baseline RSA, attenuated stress response, organized sleep, and better cognitive control at 10 years old compared to infants without the intervention (Feldman, Rosenthal, & Eidelman, 2014). Within this study, higher baseline RSA across development paralleled the child’s overall functioning. Thus, RSA may be a malleable metric (particularly at birth) that could be used to measure intervention effectiveness and impact on future developmental outcomes (e.g., sleep, stress), as well as serve as a marker of general functioning.
Higher baseline RSA is considered a positive marker of function. Higher baseline RSA is related to better emotion regulation (Beauchaine, 2001; El-Sheikh, 2005; Katz & Gottman, 1995; Scarpa, Tanaka, & Chiara Haden, 2008), higher cognitive ability (Blair, 2003; Morgan, Aikins, Steffian, Coric, & Southwick, 2007; Patriquin, Lorenzi, et al., 2013; Patriquin, Scarpa, et al., 2013; Staton et al., 2008; Watson, Baranek, Roberts, David, & Perryman, 2010), fewer internalizing symptoms (Neuhaus et al., 2014), and more effective social behavior (Bal et al., 2010; Patriquin, Scarpa, et al., 2013; Van Hecke et al., 2009). Individuals who display high tonic RSA and greater RSA suppression to attention-demanding stimuli are thought to engage and attend more effectively with stimuli, therefore producing higher cognitive performance and ability (Thayer, Friedman, Borkovec, Johnsen, & Molina, 2000). Conversely, low baseline RSA has been linked to conduct problems, trait hostility, anxiety disorders, and depression (for a comprehensive review see Beauchaine, 2001). Low baseline RSA also characterizes psychiatric disorders, such as ASD, and this low baseline RSA is significantly correlated with more difficulties with social interactions (Bal et al., 2010; Patriquin, Scarpa, et al., 2013; Van Hecke et al., 2009).
Predicting Symptoms of Psychopathology: Development of RSA
Findings from our recent study suggest that specific patterns in RSA development are related to atypical development that may presage psychopathology (Patriquin et al., 2014). In this prior study, we used group-based trajectory modeling (Jones, Nagin, & Roeder, 2001; Nagin, 2005) to identify two baseline RSA developmental trajectory groups in a sample of typically developing children. The two trajectories were referred to as “atypical” and “typical.” Similar to prior findings of RSA development (Porges, Doussard-Roosevelt, Lourdes Portales, & Suess, 1994), our “typical” RSA group had a linear increase in RSA from 5–48 months indicating an increase in regulation and self-soothing over time. Our “atypical” RSA trajectory group, however, demonstrated a steep RSA increase from 5–24 months and plateau between 24 and 48 months. We hypothesized that the “typical” RSA trajectory group would gradually increase in RSA until age 5 (Bornstein & Suess, 2000), potentially surpassing the “atypical” RSA trajectory group and suggesting a delay in RSA development (i.e., lower RSA) for the atypical group. Although not reaching clinical levels, the atypical group also had significantly more social symptoms characteristic of ASD. Lower and more unstable baseline RSA trajectories throughout early childhood may not only predict social difficulties but also broader childhood problems including aggression (Eisenberg et al., 2012) and emotional negativity and behavioral problems (Calkins & Keane, 2004).
In light of the results of our initial study, as well as other studies of baseline RSA development (Calkins & Keane, 2004; Eisenberg et al., 2012), we were interested in the relationship between child psychopathology and the developmental trajectories of RSA. As such, our objectives for this study were three-fold: 1) replicate group-based trajectory modeling of baseline RSA in a larger, new cohort of children from the community from 5–48 months old, 2) determine the relationship between baseline RSA trajectory groups and a broadband measure of early childhood problems at 48 months, and 3) examine the allocation of children with (or suspected to have) DD or ASD to RSA trajectory groups. The present study builds on our initial findings connecting early RSA developmental trajectories to social behavior and extends these findings to incorporate symptoms of broad childhood psychopathology (e.g., anxiety, depression). Due to the availability of maternal report of suspected or diagnosed DD/ASD, our results also provide deeper insight to the accuracy of the RSA trajectory group allocation.
We expected to replicate RSA trajectory patterns from our prior study (i.e., “atypical” participants demonstrating a plateau effect in RSA from 24–48 months that potentially signifies a ‘delay’ in RSA development) and that this “atypical” trajectory group would be associated with more childhood problems. We hypothesized that 100% of the children with suspected or diagnosed DD/ASD would be allocated to this “atypical” RSA trajectory group.
Method
Participants
Participants were part of a sample of children and mothers enrolled in a longitudinal study examining cognition and emotion integration across early development. The RSA data acquired during five research lab visits (5, 10, 24, 36, and 48 months) and maternal report of child problems at 48 months were the focus of the current study. The children in this analysis represent two cohorts of the children in the larger three-cohort longitudinal study and are the cohorts for which child problems data are available. Mothers of children in the remaining cohort reported on social responsiveness at 48 months, rather than child problems. Those data are published elsewhere (Patriquin, Lorenzi, Scarpa, & Bell, 2013).
For these two cohorts that are the focus on this study, 304 infants (153 girls, 151 boys; 7% Hispanic, 93% non-Hispanic; 16% African American, 77% Caucasian, 7% more than one race) were recruited by our two research locations (Blacksburg, VA; Greensboro, NC). The children were born full-term and had no diagnosed neurological or developmental problems at birth. All but 9 mothers and 10 fathers completed a high school education, with 62% of mothers and 54% of fathers having a college degree. Of 302 participants with at least one time point of usable RSA data, 143 participants had RSA data at all five time points, 65 at only four time points, 38 at only three time points, 28 at only two time points, and 28 at only one time point. Thus, a large majority of participants had at least three time points with usable RSA data. Further, with regard to attrition, an independent samples t-test indicated that participants with RSA data at 48 months (i.e., the oldest time point) did not differ significantly on baseline RSA at 5 months (i.e., the youngest time point) from participants without RSA data at 48 months, t(285) = 0.24, p = 0.81.
Data were collected in both research locations using identical protocols. Research assistants from both locations were trained together by the senior author on protocol administration, as well as on psychophysiological coding. To ensure that identical criteria were maintained between labs, the Blacksburg lab provided verification of artifact screening for psychophysiology data collected and coded by the Greensboro lab.
Procedures
Upon arrival at the research laboratory for each of the five visits, participants and their mothers were greeted, procedures were described, and signed consent was obtained from the mothers. Prior to the 24-, 36-, and 48-month visits, mothers were mailed the CBCL (Achenbach & Edelbrock, 1992) and the completed questionnaire was collected at the lab visit. This report focuses on the CBCL data collected at the 48-month lab visit. Families were paid for participation in the study and children were given a small gift at each visit.
RSA acquisition
After parental consent at each age, children were given an opportunity to acclimate to the research lab and the experimenter. Then electrocardiogram (ECG) electrodes were applied and a baseline recording procedure began. ECG was measured from two disposable electrodes using modified lead II alignment (right collarbone and lower left rib; Stern, Ray, & Quigley, 2001), grounded at the scalp near electrode site Fz. The cardiac electrical activity was amplified using a SA Instrumentation Bioamp (San Diego, CA) and bandpassed from 0.1 to 100 Hz. The QRS complex was displayed on the acquisition computer monitor and digitized at 512 samples per second. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later R-wave detection and RSA analyses.
Baseline electrophysiology was recorded for 1 minute during each of the infant lab visits (5 months, 10 months) while the infant sat on the mother’s lap. During the baseline recording, a research assistant manipulated a toy containing brightly colored balls on top of the testing table, 1.1 m in front of the infant. This procedure quieted the infant and yielded minimal gross motor movements. Mothers were instructed not to talk to infants during the baseline recording. This is our typical baseline for infant electrophysiology research (e.g., Bell, 2012; Cuevas & Bell, 2011)
During the toddler (24 months) and preschool (36 months, 48 months) visits, baseline electrophysiology was recorded as each child sat quietly for 2 minutes and watched a clip from the Disney film, Finding Nemo (sea turtles riding the East Australian Current). This procedure quieted the child and yielded minimal gross motor movements. Mothers sat in a chair beside the child and did not interact with the child during the recording. This is a typical baseline for toddler and preschooler electrophysiology research in our laboratory (e.g., Cuevas, Raj, & Bell, 2012; Morasch & Bell, 2012)
RSA analysis
ECG data were examined and analyzed using IBI Analysis System software developed by James Long Company (Caroga Lake, NY). First, R waves were detected offline with a four-pass peak detection algorithm, resulting in a data file with onset times for each detected R-wave. Next, the ECG signal was viewed on a computer monitor along with tick marks representing the onset times of the IBI software detected R-waves. For undetected visible and obscured R-waves, the tick marks were inserted manually. Movement artifact was designated by the absence of at least three consecutive R-waves. These artifact-scored epochs were eliminated from all calculations. The edited R-wave was converted to heart period (i.e., time between heart beats).
Spectral analysis was used to calculate high frequency variability (i.e., RSA) in the heart period data, using a discrete Fourier transform with a 16-second Hanning window and 50% overlap. The frequency band for quantification of RSA at each age was 0.24 – 1.04 Hz. This frequency band is appropriate for all age groups between infancy and early childhood (Bar-Haim, Marshall, & Fox, 2000). The RSA data were transformed using natural log to normalize the distribution.
Childhood problems
Childhood problems were measured at 48 months using maternal-report on the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, 1992) for children aged 1 ½ – 5 years old. Maternal ratings were obtained on 99 problem items, plus descriptions of problems, disabilities, what concerns the mother had most about the child, and the strengths of the child. Both syndrome and DSM-oriented scales were included: emotionally reactive, anxious/depressed, somatic complaints, withdrawn, sleep problems, attention problems, aggressive behavior (Syndrome Scales); affective problems, anxiety problems, pervasive developmental problems, attention-deficit/hyperactivity problems, and oppositional defiant problems (DSM-Oriented Scales). Ratings were obtained on a 3-point scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). The raw total scores for the scales were used in the present study.
Data analysis
Group-based trajectory analyses were conducted on baseline RSA data collected from the children at 5, 10, 24, 36, and 48 months using SAS macro PROC TRAJ (http://www.andrew.cmu.edu/user/bjones/; Jones et al., 2001; Nagin, 2005). Model selection involved a two-stage selection process of identifying both the optimal number of trajectory groups and the optimal order of polynomial that best described the trajectory of each group identified in the first step. Group differences, based on baseline RSA group assignments, on CBCL raw scores at 48 months were examined using a multivariate analysis of covariance (MANCOVA) and analysis of covariance (ANCOVA).
Results
Descriptive Statistics and Inter-Correlations
Table 1 presents means and standard deviations for key variables of interest (baseline RSA and CBCL raw scores), while Table 2 presents inter-correlations for the same variables. The inter-correlations revealed several significant positive correlations between baseline RSA at various time points, but there were few significant correlations between baseline RSA at any time point and CBCL scores at 48 months.
Table 1.
Means, Standard Deviations, and Ranges for Key Variables of Interest
| Variable | N | M | SD | Possible Range |
Observed Range |
|---|---|---|---|---|---|
| Baseline RSA, 5 months (ln(ms)2) | 287 | 4.02 | 1.27 | − | − |
| Baseline RSA, 10 months (ln(ms)2) | 261 | 4.74 | 1.16 | − | − |
| Baseline RSA, 24 months (ln(ms)2) | 228 | 5.44 | 1.23 | − | − |
| Baseline RSA, 36 months (ln(ms)2) | 207 | 6.49 | 1.53 | − | − |
| Baseline RSA, 48 months (ln(ms)2) | 190 | 6.80 | 1.38 | − | − |
| CBCL Emotionally reactive | 218 | 2.28 | 2.35 | 0–18 | 0–12 |
| CBCL Anxious/depressed | 218 | 2.12 | 2.04 | 0–16 | 0–11 |
| CBCL Somatic complaints | 216 | 1.63 | 1.96 | 0–22 | 0–11 |
| CBCL Withdrawn | 217 | 1.47 | 1.67 | 0–16 | 0–9 |
| CBCL Sleep problems | 217 | 2.95 | 2.44 | 0–14 | 0–12 |
| CBCL Attention problems | 218 | 2.52 | 1.75 | 0–10 | 0–10 |
| CBCL Aggressive behavior | 216 | 8.81 | 6.48 | 0–38 | 0–32 |
| CBCL Other problems | 212 | 7.56 | 5.82 | 0–66 | 0–34 |
| CBCL Internalizing problems | 215 | 7.50 | 6.31 | 0–72 | 0–37 |
| CBCL Externalizing problems | 216 | 11.34 | 7.67 | 0–48 | 0–42 |
| CBCL Total problems | 210 | 26.26 | 17.90 | 0–186 | 0–107 |
| CBCL Affective problems | 216 | 1.98 | 2.25 | 0–20 | 0–12 |
| CBCL Anxiety problems | 217 | 2.88 | 2.44 | 0–20 | 0–12 |
| CBCL Pervasive developmental problems | 216 | 3.02 | 2.58 | 0–26 | 0–15 |
| CBCL Attention-deficit/hyperactivity Disorder Problems | 217 | 4.34 | 2.60 | 0–12 | 0–12 |
| CBCL Oppositional defiant problems | 217 | 3.11 | 2.64 | 0–12 | 0–12 |
Table 2.
Inter-correlations for Key Variables of Interest
| (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | (15) | (16) | (17) | (18) | (19) | (20) | (21) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline RSA 5 months (1) | .29*** | .20** | .23** | .21** | −.07 | .04 | −.01 | .00 | .05 | .03 | −.03 | −.03 | −.02 | −.02 | −.02 | .04 | −.01 | −.08 | .05 | −.02 |
| Baseline RSA 10 months (2) | -- | .30*** | .31*** | .29*** | .05 | .04 | −.05 | .02 | .09 | .08 | .00 | .09 | .02 | .02 | .06 | .10 | .01 | .00 | .05 | −.01 |
| Baseline RSA 24 months (3) | -- | .36*** | .35*** | −.06 | −.02 | −.06 | −.15* | .00 | .03 | −.09 | .00 | −.07 | −.07 | −.05 | .00 | −.03 | −.19* | .03 | −.11 | |
| Baseline RSA 36 months (4) | -- | .40*** | −.07 | −.01 | −.10 | −.11 | −.06 | .02 | −.09 | −.03 | −.09 | −.08 | −.08 | −.06 | −.03 | −.13 | .00 | −.12 | ||
| Baseline RSA 48 months (5) | -- | .04 | .14 | .15* | −.04 | .02 | .16* | .02 | .09 | .10 | .05 | .09 | .00 | .14 | −.02 | .13 | −.03 | |||
| CBCL Emotionally Reactive (6) | -- | .63*** | .42*** | .54*** | .46*** | .44*** | .62*** | .75*** | .85*** | .62*** | .82*** | .65*** | .68*** | .65*** | .41*** | .61*** | ||||
| CBCL Anxious/Depressed (7) | -- | .43*** | .50*** | .37*** | .34*** | .47*** | .63*** | .82*** | .47*** | .71*** | .57*** | .82*** | .59*** | .33*** | .42*** | |||||
| CBCL Somatic Complaints (8) | -- | .38*** | .33*** | .29*** | .34*** | .45*** | .70*** | .36*** | .54*** | .56*** | .43*** | .49*** | .31*** | .33*** | ||||||
| CBCL Withdrawn (9) | -- | .30*** | .37*** | .52*** | .63*** | .75*** | .52*** | .71*** | .58*** | .47*** | .82*** | .30*** | .45*** | |||||||
| CBCL Sleep Problems (10) | -- | .40*** | .50*** | .52*** | .47*** | .51*** | .56*** | .66*** | .61*** | .29*** | .44*** | .46*** | ||||||||
| CBCL Attention Problems (11) | -- | .61*** | .64*** | .47*** | .74*** | .69*** | .41*** | .41*** | .37*** | .86*** | .53*** | |||||||||
| CBCL Agg. Behavior (12) | -- | .74*** | .64*** | .98*** | .88*** | .63*** | .49*** | .51*** | .70*** | .92*** | ||||||||||
| CBCL Other Problems (13) | -- | .79*** | .77*** | .93*** | .74*** | .69*** | .66*** | .65*** | .64*** | |||||||||||
| CBCL Internalizing (14) | -- | .65*** | .89*** | .75*** | .78*** | .80*** | .44*** | .59*** | ||||||||||||
| CBCL Externalizing (15) | -- | .90*** | .63*** | .51*** | .52*** | .79*** | .90*** | |||||||||||||
| CBCL Total Problems (16) | -- | .78*** | .72*** | .73*** | .70*** | .80*** | ||||||||||||||
| CBCL Affective (17) | -- | .63*** | .51*** | .44*** | .58*** | |||||||||||||||
| CBCL Anxiety (18) | -- | .55*** | .42*** | .44*** | ||||||||||||||||
| CBCL Pervasive Dev. (19) | -- | .33*** | .45*** | |||||||||||||||||
| CBCL ADHD (20) | -- | .57*** | ||||||||||||||||||
| CBCL Opp. Defiant (21) | -- |
Note. Pairwise deletion was used.
p < 0.05.
p < 0.01.
p < 0.001.
CBCL Agg. Behavior, CBCL Aggressive Behavior; CBCL Internalizing, CBCL Internalizing Problems; CBCL Externalizing, CBCL Externalizing Problems; CBCL Affective, CBCL Affective Problems; CBCL Anxiety, CBCL Anxiety Problems; CBCL Pervasive Dev., CBCL Pervasive Developmental Problems; CBCL ADHD, CBCL Attention-Deficit/Hyperactivity Disorder Problems; CBCL Opp. Defiant, CBCL Oppositional Defiant Problems
Trajectory Analysis of Baseline RSA
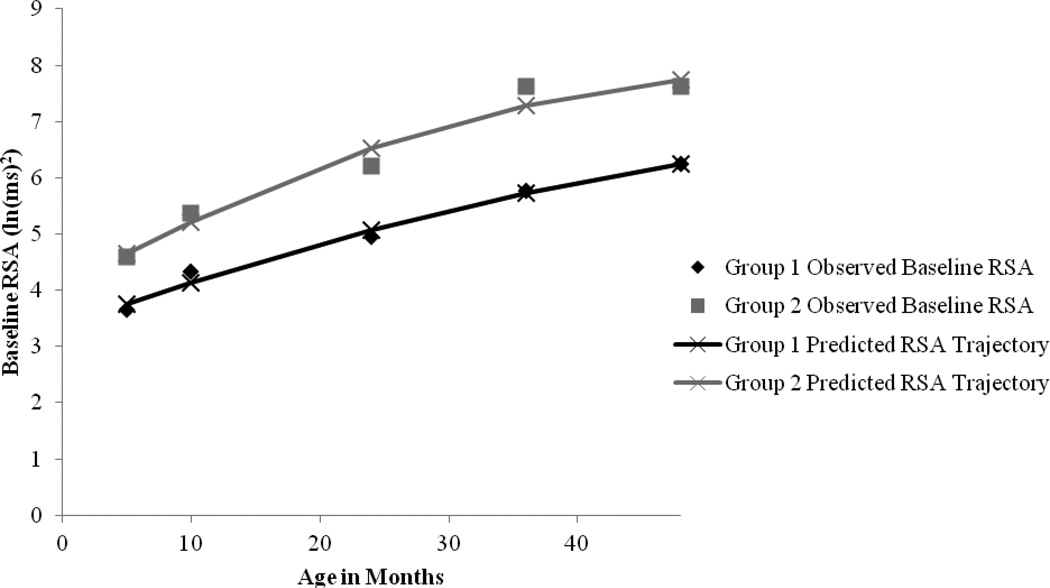
Results from the group-based trajectory analysis indicated that a two-group quadratic trajectory model best fit baseline RSA over the five time points (Figure 1; Table 3). This model was selected over a model with only one group, as well as over models with more than two groups. One factor that helped to inform this decision is the Bayesian Information Criterion (BIC), a common statistic that supports model selection. Table 4 presents BIC values for models with number of groups ranging from one to five, and statistics for model comparisons. An approximation for the BIC log Bayes factor, 2(ΔBIC) (where ΔBIC = BIC for more complex model - BIC for simpler model), supported the two-group model (http://www.andrew.cmu.edu/user/bjones/; Jones et al., 2001; Nagin, 2005). The two-group model also evidenced conceptual interpretability in terms of “high RSA” and “low RSA.”
Figure 1.
Baseline RSA on Age in Months: Observed and Predicted Trajectories.
Table 3.
Summary of Group-Based Trajectory Analysis on Baseline RSA
| Intercept B (SE) |
Linear B (SE) |
Quadratic B (SE) |
Predicted RSA 10 months (ln(ms)2) |
Predicted RSA 24 months (ln(ms)2) |
Predicted RSA 36 months (ln(ms)2) |
Predicted RSA 48 months (ln(ms)2) |
|
|---|---|---|---|---|---|---|---|
| Group 1 | 3.35 (0.13) | 0.08*** (0.01) | <0.01+ (<0.01) | 4.14 | 5.07 | 5.73 | 6.25 |
| Group 2 | 4.01 (0.51) | 0.13*** (0.06) | <0.01** (<0.01) | 5.22 | 6.52 | 7.29 | 7.74 |
Note. Linear and quadratic terms reflect change in baseline RSA (ln(ms)2) per month of age.
p < 0.1.
p < 0.05.
p < 0.01.
p < 0.001.
Table 4.
Bayesian Information Criterion (BIC) and Model Comparison Statistics for Determining Optimal Number of Groups
| Number of Groups | BIC (n = 1173) |
Null Model |
2(ΔBIC) |
|---|---|---|---|
| 1 | −2000.89 | ||
| 2 | −1962.59 | 1 | 76.60 |
| 3 | −1952.38 | 2 | 20.42 |
| 4 | −1958.88 | 3 | −13.00 |
| 5 | −1944.62 | 4 | −28.52 |
The two-group model performed best with a quadratic trajectory for both groups. The polynomial ordering selection process began at the lowest order for both groups; polynomial terms were added to each function while the coefficients continued to remain significant. For both groups, both the linear and quadratic terms were significant (for the Low RSA group, the quadratic term approached significance at p = 0.08), but the cubic term was significant for neither group, so it did not remain in the final model.
Estimated population group proportions for the two-group quadratic trajectory model were 60% for the Low RSA group (Group 1) whose average baseline RSA tended to increase gradually during the period from five months to 48 months of age, and an estimated 40% for a High RSA group (Group 2) whose average baseline RSA tended to increase gradually during the period from five months to 48 months of age, but at a level that was higher than Group 1 at each time point. In other words, the baseline RSA trajectory of Group 1 was overall reduced compared to that of Group 2. For this sample, of the 302 participants with usable baseline RSA data, 193 (63.9%) were assigned to Group 1, while 109 were assigned to Group 2 (36.1%). The estimated population proportions approximate sample membership proportions, which also supports good model fit. Finally, average posterior probabilities of group membership were high (.84 for membership in Group 1, .83 for membership in Group 2). These probabilities exceed Nagin’s (2005) suggested guideline of .7, indicating that the model with two distinct groups fits well. It should also be noted that a chi-square test revealed that group assignment was not significantly related to assessment location (i.e., Blacksburg and Greensboro), χ2(1, N = 302) = 0.15, p = .70.
Comparison of CBCL Scores by Trajectory Group
Based on the results of the trajectory analysis that identified two distinct groups, a MANCOVA was conducted to determine whether trajectory groups differed on subsequent CBCL scores at 48 months (raw scores for all subscales). Of the participants assigned to Group 1 (Low RSA) in the trajectory analysis, 127 had complete CBCL data at 48 months. Of the participants assigned to Group 2 (High RSA), 80 had complete CBCL data at 48 months. Table 5 presents the results of a MANCOVA comparing Group 1 and Group 2 on CBCL scores, covarying for maternal age at infant’s birth. According to these results, significant differences were found between trajectory group assignments on the dependent measures of CBCL scores, Wilks’ Λ = 0.89, F (13, 192) = 1.89, p = .03, partial eta2 = 0.11. Power to detect the effect was .91. Thus, the two distinct baseline RSA trajectory groups evidenced significantly different CBCL outcomes at 48 months (when taken as a set) after controlling for maternal age at infant’s birth.
Table 5.
Univariate Tests of MANCOVA Comparing CBCL Raw Scores Between Baseline RSA Trajectory Groups
| Group 1 M (SD) |
Group 2 M (SD) |
F | |
|---|---|---|---|
| Emotionally Reactive | 2.42 (2.47) | 2.11 (2.18) | 0.84 |
| Anxious/Depressed | 2.05 (2.02) | 2.29 (2.08) | 0.68 |
| Somatic Complaints | 1.66 (1.80) | 1.59 (2.24) | 0.08 |
| Withdrawn | 1.72 (1.85) | 1.14 (1.32) | 6.33* |
| Sleep Problems | 2.92 (2.57) | 3.04 (2.25) | 0.10 |
| Attention Problems | 2.42 (1.76) | 2.60 (1.61) | 0.56 |
| Aggressive Behavior | 9.40 (6.52) | 7.71 (6.09) | 3.58+ |
| Other Problems | 7.77 (6.16) | 7.21 (5.38) | 0.49 |
| Internalizing Problems | 7.85 (6.61) | 7.13 (6.00) | 0.69 |
| Externalizing Problems | 11.82 (7.65) | 10.31 (7.30) | 2.08 |
| Total Problems | 27.44 (18.64) | 24.65 (16.86) | 1.29 |
| Affective Problems | 2.06 (2.33) | 1.93 (2.15) | 0.18 |
| Anxiety Problems | 2.86 (2.44) | 3.00 (2.51) | 0.15 |
| Pervasive Developmental Problems | 3.39 (2.83) | 2.51 (2.14) | 5.82* |
| Attention-Deficit/Hyperactivity Disorder Problems | 4.23 (2.40) | 4.39 (2.74) | 0.18 |
| Oppositional Defiant Problems | 3.38 (2.68) | 2.58 (2.37) | 4.91* |
Note. Group 1 n = 127; Group 2 n = 80.
p < 0.05.
p < 0.1.
Given the significance of the overall MANCOVA, follow-up univariate main effects were then examined using ANCOVA. Significant univariate main effects for trajectory group assignment were obtained for CBCL raw scores for the subscales of Withdrawn, Pervasive Developmental Problems, and Oppositional Defiant Problems, and with a trend in the direction of a significant univariate main effect for trajectory group assignment for the subscale of Aggressive Behavior. For all these subscales, Group 1 (Low RSA) evidenced significantly higher scores (i.e., more symptoms reported by mothers). This suggests that the two distinct baseline RSA trajectory groups evidenced significant differences in these CBCL subscales at the age of 48 months.
Trajectory Group Assignments and Parent-Reported Concerns on CBCL
In addition to completing the rating scale items of the CBCL, parents also responded to an open-ended question that requested additional information regarding any developmental concerns about their child. Of the 302 participants with useable baseline RSA data, six parents reported concerns that related to ASD or a developmental delay (n = 3 with parent-reported developmental delay diagnoses; n = 1 with parent-reported Pervasive Developmental Disorder – Not Otherwise Specified diagnosis; n = 2 with parent-reported speech delays and both being tested for autism). All six children (100%) with parental concerns relating to ASD or a developmental delay were assigned to the low baseline RSA group in the trajectory analyses. Thus, the group-based trajectory analysis identified all six children whose parents had concerns about their development.
Discussion
The present study examined the developmental trajectories of baseline RSA in a new, larger cohort of typically developing children across 5, 10, 24, 36, and 48 months of age and the association with childhood behavior problems. Additionally, the accuracy of the allocation of ASD/DD participants to the predicted RSA group was examined. Our findings provide novel insight to the relations between developmental trajectories of baseline RSA and broad symptoms of childhood psychopathology. To our knowledge, this is the first study to examine this relationship.
Using group-based trajectory modeling based on Nagin (2005), we replicated our prior two-group quadratic model fit across all ages: 1) “High RSA group” – consistently demonstrated higher baseline RSA at each age, and 2) “Low RSA group” – consistently demonstrated lower baseline RSA relative to the High RSA group at each age. We did not replicate the prior plateau effect of RSA across 24–48 months (Patriquin et al., 2014). In our prior study, we hypothesized this plateau in baseline RSA reflected a developmental ‘delay’ in the autonomic nervous system (i.e., vagal development), however, the current paper finds consistently low (Low RSA group) and high (High RSA group) trajectories of RSA rather than a plateau effect.
Due to the significant increase of participants in the current paper (i.e., N = 218 with CBCL data at 48 months) compared to our prior paper (i.e., N = 62 with social responsiveness data at 48 months), these current group-based trajectory analyses improve on our prior findings and may reflect more accurate developmental trajectories of baseline RSA across early childhood. The higher baseline RSA trajectory group demonstrated significantly lower childhood problems in multiple domains on the CBCL at 48 months: Withdrawn, Aggressive Behavior, Pervasive Developmental Problems, and Oppositional Defiant Problems. Conversely, the Low RSA group demonstrated significantly more childhood problems in these domains. These findings are congruent with the literature that suggests that higher RSA predicts more positive emotional, social, and cognitive outcomes (Bal et al., 2010; Beauchaine, 2001; Blair, 2003; Calkins & Keane, 2004; Eisenberg et al., 2012; El-Sheikh, 2005; Katz & Gottman, 1995; Morgan et al., 2007; Neuhaus et al., 2014; Patriquin, Lorenzi, et al., 2013; Patriquin, Scarpa, et al., 2013; Scarpa et al., 2008; Staton et al., 2008; Van Hecke et al., 2009; Watson et al., 2010). Along the same vein, maternal-report data of DD/ASD indicated that 100% of DD/ASD participants were allocated to the Low RSA trajectory group. As low RSA is associated with difficulties across multiple behavioral and developmental areas, it is unsurprising that children who present with the complex symptoms associated with DD/ASD (e.g., language delays, difficulty with social engagement) were all assigned to the low RSA group.
In combination with prior findings that highlight the utility of RSA as a marker of social, cognitive, and emotional outcomes, this study plays an important role in demonstrating that consistent developmental trajectories of RSA (i.e., high or low RSA across early childhood) may point to protective factors (i.e., high RSA) against developing symptoms of childhood psychopathology. Further, the distinct advantage of the current longitudinal study over cross-sectional studies or longitudinal studies with fewer time points (e.g., RSA measured at only 2 or 3 time points) is the ability of group-based trajectory modeling to amalgamate a large longitudinal dataset of baseline RSA; thus, reducing the instability evident in RSA across early development (e.g., the current dataset demonstrates only moderate correlations between baseline RSA at various time points, r = .20–.40) and providing a clearer picture of the behavioral outcomes relevant to baseline RSA. Since the present study has identified the broad behavioral outcomes in childhood related to early RSA development, future studies should now begin to map relations between these relevant outcomes and individual baseline RSA trajectories.
Limitations were present in the current study. Childhood problems were reported by one parent at 48 months on the CBCL and were not collected from multiple informants (e.g., teacher-report) or using more comprehensive assessment measures (e.g., structured clinical interviews). Future research should utilize multiple informants, structured clinical interviews, and other standardized clinical assessment measures to provide a more complete clinical picture. Lastly, due to the longitudinal nature of the paper there were missing CBCL and RSA data because of participant dropout or ECG artifact.
This study replicated the two-group fit that was demonstrated in our prior study and similar to this study, the current findings demonstrated that higher RSA was associated with better outcomes. As this study was conducted in typically developing participants, it also stresses the variability within the community sample and highlights the importance of studying developmental psychopathology from a biobehavioral perspective that allows for the prospective identification (and possible targets) for intervention and objective measurement of improvements. Across studies, RSA has emerged as an economically acquired metric of function that provides valuable insight into the physiological processes that underlie the development of both typical and atypical behavior in early childhood.
Acknowledgments
This research was supported by grants HD049878 and HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research. The assistance of our Blacksburg and Greensboro research assistants with data collection and coding is greatly appreciated.
References
- Achenbach TM, Edelbrock C. Manual for the child behavior checklist. Department of Psychiatry, University of Vermont Burlington; 1992. [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders. 2010;40(3):358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall P, Fox N. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37(1):44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective on working memory performance at 8 months of age. Child Development. 2012;83(1):251–265. doi: 10.1111/j.1467-8624.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Blair C. Behavioral inhibition and behavioral activation in young children: Relations with self regulation and adaptation to preschool in children attending Head Start. Developmental Psychobiology. 2003;42(3):301–311. doi: 10.1002/dev.10103. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36(1):54. [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45(3):101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80:119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Raj V, Bell MA. A frequency band analysis of two-year-olds memory processes. International Journal of Psychophysiology. 2012;83:315–322. doi: 10.1016/j.ijpsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. The data of diagnosis: New approaches to psychiatric classification. Psychiatry: Interpersonal and Biological Processes. 2010;73(4):311–314. doi: 10.1521/psyc.2010.73.4.311. [DOI] [PubMed] [Google Scholar]
- Cuthbert B, Kozak M. Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology. 2013;122(3):928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Sulik MJ, Spinrad TL, Edwards A, Eggum ND, Liew J, Hart D. Differential susceptibility and the early development of aggression: Interactive effects of respiratory sinus arrhythmia and environmental quality. Developmental Psychology. 2012;48(3):755. doi: 10.1037/a0026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology. 2005;46(1):66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Biological and environmental initial conditions shape the trajectories of cognitive and social-emotional development across the first years of life. Developmental Science. 2009;12(1):194–200. doi: 10.1111/j.1467-7687.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry. 2014;75(1):56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, Morey JN. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology. 2011 doi: 10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–83. [Google Scholar]
- Morasch KC, Bell MA. Self-regulation of negative affect at 5 and 10 months. Developmental Psychobiology. 2012;54:215–221. doi: 10.1002/dev.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, Aikins DE, Steffian G, Coric V, Southwick S. Relation between cardiac vagal tone and performance in male military personnel exposed to high stress: Three prospective studies. Psychophysiology. 2007;44(1):120–127. doi: 10.1111/j.1469-8986.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Neuhaus E, Bernier R, Beauchaine T. Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders. 2014;44:730–737. doi: 10.1007/s10803-013-1923-7. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A. Relationship between respiratory sinus arrhythmia, heart period, and caregiver-reported language and cognitive delays in children with autism spectrum disorders. Applied Psychophysiology and Biofeedback. 2013;38:203–207. doi: 10.1007/s10484-013-9225-6. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, Bell MA. Developmental trajectories of respiratory sinus arrhythmia: Associations with social responsiveness. Developmental Psychobiology. 2014;56(3):317–326. doi: 10.1002/dev.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, Porges SW. Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology. 2013;55(2):101–112. doi: 10.1002/dev.21002. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A Polyvagal Theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23(8):837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment. Annals of the New York Academy of Sciences. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2009;76(Suppl 2):S86. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Lourdes Portales A, Suess PE. Cardiac vagal tone: Stability and relation to difficultness in infants and 3-year-olds. Developmental Psychobiology. 1994;27(5):289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Porges SW, Lewis GF. The polyvagal hypothesis: Common mechanisms mediating autonomic regulation, vocalizations and listening. Handbook of Behavioral Neuroscience. 2009:255–264. [Google Scholar]
- Scarpa A, Tanaka A, Chiara Haden S. Biosocial bases of reactive and proactive aggression: The roles of community violence exposure and heart rate. Journal of Community Psychology. 2008;36(8):969–988. [Google Scholar]
- Staton L, El-Sheikh M, Buckhalt JA. Respiratory sinus arrhythmia and cognitive functioning in children. Developmental Psychobiology. 2008;51(3):249. doi: 10.1002/dev.20361. [DOI] [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological record. 2nd ed. Ney York: Oxford; 2001. [Google Scholar]
- Thayer J, Friedman B, Borkovec T, Johnsen B, Molina S. Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology. 2000;37:361–368. [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Van Hecke AV, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development. 2009;80(4):1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Van Praag HM, Asnis GM, Kahn RS, Brown S. Nosological tunnel vision in biological psychiatry: A plea for a functional psychopathology. Annals of the New York Academy of Sciences. 1990;600:501–510. doi: 10.1111/j.1749-6632.1990.tb16905.x. [DOI] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Roberts JE, David FJ, Perryman TY. Behavioral and physiological responses to child-directed speech as predictors of communication outcomes in children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2010;53(4):1052. doi: 10.1044/1092-4388(2009/09-0096). [DOI] [PMC free article] [PubMed] [Google Scholar]