Abstract
Many brain structures project to both the anteroventral thalamic nucleus and the anteromedial thalamic nucleus. In the present study, pairs of different tracers were placed into these two thalamic sites in the same rats to determine the extent to which these nuclei receive segregated inputs. Only inputs from the laterodorsal tegmental nucleus, the principal extrinsic cholinergic source for these thalamic nuclei, showed a marked degree of collateralisation, with approximately 13% of all cells labelled in this tegmental area projecting to both nuclei. Elsewhere, double labelled cells were very scarce, comprising ~1% of all labelled cells. Three general patterns of anterior thalamic innervation were detected in these other areas. In some sites, e.g., prelimbic cortex, anterior cingulate cortex, and secondary motor area, cells projecting to the anteromedial and anteroventral thalamic nuclei were closely intermingled, with often only subtle distribution differences. These same projections were also often intermingled with inputs to the mediodorsal thalamic nucleus, but again there was little or no collaterisation. In other sites, e.g., the subiculum and retrosplenial cortex, there was often less overlap of cells projecting to the two anterior thalamic nuclei. A third pattern related to the dense inputs from the medial mammillary nucleus, where well defined topographies ensured little intermingling of the neurons that innervate the two thalamic nuclei. The finding, that a very small minority of cortical and limbic inputs bifurcate to innervate both anterior thalamic nuclei, highlights the potential for parallel information streams to control their functions, despite arising from common regions.
Keywords: cingulate cortex, hippocampus, mediodorsal thalamic nucleus, prelimbic cortex, retrosplenial cortex, subicular cortex
Introduction
The rat anterior thalamic nuclei (ATN) principally comprise the anterodorsal (AD), anteroventral (AV), and anteromedial (AM) thalamic nuclei. There is considerable evidence from both lesion and electrophysiological studies that these same thalamic nuclei are vital for navigation and spatial memory in rodents (Sutherland and Rodriguez, 1989; Taube, 1995; Aggleton et al., 1996; Byatt and Dalrymple-Alford, 1996; Vertes et al., 2001; Warburton et al., 2001). Furthermore, there is direct evidence that this role in spatial learning is directly linked with hippocampal function (Aggleton and Brown, 1999; Warburton et al., 2000, 2001; Vann and Albasser, 2009). At the same time, there is also growing evidence that in humans the anterior thalamic nuclei, in concert with the mammillary bodies, are required for normal episodic memory (Harding et al., 2000; Van der Werf et al., 2000; Tsivilis et al., 2008; Carlesimo et al., 2011), and that these functions are again closely linked to hippocampal function (Aggleton and Saunders, 1997; Tsivilis et al., 2008; Aggleton et al., 2010). These findings raise the question of why might these hippocampal – thalamic interactions prove so important for memory.
One potentially important clue in answering this question is that the anterior thalamic nuclei probably support learning in multiple, complementary ways (Taube, 2007; Aggleton et al., 2010). Evidence from lesion studies in rats shows that surgeries targeted at specific nuclei within the ATN result in only partial spatial learning deficits, and that when these lesion are combined there is an additive effect (Aggleton et al., 1996; Byatt and Dalrymple-Alford, 1996; Van Groen et al., 2002). Likewise, electrophysiological studies often emphasise the different properties of the major anterior thalamic nuclei (Vertes et al., 2001, 2004; Albo et al., 2003; Vann and Aggleton, 2004; Tsanov et al. 2011a). Consequently, it is important to identify those connections of the individual nuclei that are unique and those that are shared.
In the case of the anterodorsal thalamic nucleus (AD) there is considerable evidence for a specific role in navigation as it is a key component of the ‘head-direction’ system (Taube, 1995, 2007; Taube et al., 1996), with a unique set of connections (Hopkins, 2005; Yoder and Taube, 2011). It is, therefore, most likely that the principal role of this nucleus is different from that of the other anterior thalamic nuclei (Vann and Aggleton, 2004). The situation for AV and AM is, however, far less clear. While electrophysiological studies suggest that AV shows a greater frequency of theta firing cells than AM and may contain a population of head-direction cells (Vertes et al. 2004; Tsanov et al. 2011a), both nuclei receive inputs from many of the same sources. Furthermore, lesion studies indicate a shared involvement in spatial learning, such that lesions combining both nuclei have an additive effect (Aggleton et al., 1996; Byatt and Dalrymple-Alford, 1996; Van Groen et al., 2002). Consequently, the extent to which these two adjacent anterior thalamic nuclei retain distinct functions remains unresolved.
Some ATN inputs have clear topographies, making it likely that the projections to AV and AM arise from distinct cell populations and, thereby, have potentially different properties. An example concerns the dense inputs from the mammillary bodies that have complex topographies such that different subregions within the medial mammillary nucleus project to distinct, non-overlapping subregions within AV and AM (Seki and Zyo, 1984; Hayakawa and Zyo, 1989; Shibata, 1992; Hopkins, 2005). For many other ATN inputs, however, the extent of segregation is far less clear. Examples include the inputs to ATN from the subiculum, anterior cingulate cortex, retrosplenial cortex, and prelimbic cortex. All of these areas not only project to AV and AM, but the sources of their inputs often appear to overlap (Sikes et al., 1977; Seki and Zyo, 1984; van Groen and Wyss, 1990a,b,c; Shibata, 1998; Ishizuka, 2001; Van Groen and Wyss, 2003; Shibata and Naito, 2005; Wright et al., 2010). The only direct test of the extent to which the projections to AV and AM are truly segregated comes from a single case (Seki and Zyo, 1984). It was reported that a rat with an injection of Evans Blue in AM and bisbenzimide in AV had numerous intermingled labelled cells across the limbic cortices, with many double labelled cells in the prelimbic, anterior cingulate, and dysgranular retrosplenial cortices (Seki and Zyo, 1984). The authors, however, acknowledge that the injection into AM may have been taken up by fibers of passage, so limiting the ability to interpret this experiment.
The question, therefore, remains whether those projections to AV and AM that arise from overlapping cell populations retain underlying evidence of topographical segregation. A closely related issue is whether inputs to both nuclei often arise from the same individual neurons (Seki and Zyo, 1984). To address both questions, the present study placed different pairs of retrograde tracers into either AV or AM in the same hemisphere of a series of rats. A small number of injections were also targeted at the mediodorsal thalamic nucleus (MD), as well as the ATN. These additional experiments were in response to the large numbers of retrogradely labelled cells found in the prelimbic and anterior cingulate regions after injections into either AV or AM, cortical regions that also project to the mediodorsal thalamic nucleus (Groenewegen, 1988). Once again, the question was whether the sources of the various cortical-thalamic projections have distinct origins from within frontal areas and whether there is any collateralisation in the projections to these various thalamic nuclei.
Materials and Methods
Subjects
The experiments involved 38 male Lister Hooded rats weighing 270-300g (Harlan, U.K.). All animals were housed in pairs under diurnal light conditions (14h light/10h dark). Animal weight was monitored so that all rats remained at 85% or more of their free feeding weight. All experiments were carried out in accordance with UK Animals (Scientific Procedures) Act, 1986 and associated guidelines.
Materials
A selection of retrograde fluorescent tracers, which comprised diamidino yellow (Sigma-Aldrich, Gillingham, UK), fast blue (Polysciences Inc, Eppelheim, Germany), cholera toxin subunit B (CTB) conjugated to either Alexa Fluor 488 (CTB488) or Alexa Fluor 594 (CTB594) (Life Technologies Ltd, Paisley, UK), and wheat germ agglutinin (WGA) conjugated to either Alexa Fluor 488 (WGA488) or Alexa Fluor 594 (WGA594) (Life Technologies Ltd, Paisley, UK), were used to allow double fluorescent labelling in the same animal.
General surgical methods
Anaesthesia was induced and maintained by gaseous Isoflurane (IVAX Pharmaceuticals, London, UK). A subcutaneous injection of Metacam (1mg/kg body weight, Boehringer Ingelheim, Germany) was given prior to surgery to provide initial pre- and post-operative analgesia. Chloramphenicol eye ointment (Martindale Pharmaceuticals, Romford, UK) was topically applied to the eyes to protect the cornea. Animals were then placed in a stereotaxic frame (Kopf, Tujunga, CA, USA), with the mouthbar set at +0.5mm. Small openings were made in the skull and dura to allow access for the 0.5 mm gauge needle of 0.5μl Hamilton syringes (Hamilton, Bonaduz, Switzerland).
Pairs of single injections, each using a different tracer, were made unilaterally in 27 rats. In eleven rats, bilateral injections of four different tracers were made into the anterior thalamic nucleus (ATN) of the same animal. Those injections that targeted the anteroventral nucleus (AV) were centered around the coordinates AP −0.4, ML +/− 1.5, DV −6.2 from bregma. Those injections that targeted the anteromedial nucleus (AM) were centered around AP −0.2, ML +/− 0.7, DV −6.8 from bregma. In the six cases where the medial dorsal thalamic nucleus (MD) was targeted, a single injection was centered around AP −0.4, ML +/− 1.5, DV −6.2 from bregma, with accompanying injections in both AV and AM. The majority of experiments used the combination of the fast blue and diamidino yellow tracers, which require viewing with the same fluorescent filter cube. Fast blue containing cells display label throughout the cytoplasm and nucleus, while the diamidino yellow label is restricted to the nucleus. This difference, along with the emission wavelength difference, allows double labelled cells to be accurately identified.
Both fast blue and diamidino yellow were made up as 3% solutions in sterile phosphate buffer saline (PBS). The tracers CTB488 and CTB594 were made up as 0.5% solutions in sterile PBS, while WGA488 and WGA594 were made up as 5% solutions in sterile PBS. The syringe was lowered to the target location and left in place for three minutes prior to injecting the tracer gradually over ~10 seconds, after which it was left in place for a further seven minutes to help limit any tracer travelling back up the syringe tract. Typically, injection volumes at each site were 0.03μl for fast blue, 0.05-0.06μl for diamidino yellow and 0.05μl for all the CTB and WGA tracers.
After surgery, animals received a 5 ml subcutaneous injection of 5% glucose in 0.9% saline (Baxter Healthcare Ltd, Norfolk, UK), and clindamycin hydrochloride antibiotic powder (Pharmacia Ltd, Sandwich, UK) was applied over the closed sutured scalp. Animals were then allowed to recover in a thermostatically controlled container before returning to individual housing with ad lib food and water. Each animal’s health was monitored daily.
Following a postoperative period of 3-4 days (except WGA488 and WGA594 cases where the postoperative period was 48 hours), the animals were deeply anesthetized with sodium pentobarbital (Euthatal, Merial, Harlow, UK). This survival time was selected on the basis of previous studies of these tracers that examined a variety of survival times from 6h to 6 days, showing an asymptote in retrograde label after three days (Amin et al., 2010)
The rats were perfused intracardially with 0.1M PBS at room temperature followed by 4% paraformaldehyde in 0.1M PBS at ~4°C. Brains were removed and placed in the dark for 4 hours in 4% paraformaldehyde and then transferred to 25% sucrose solution in 0.1M PBS for 24 hours in the dark to cyroprotect the tissue before cutting. Brains were placed on a freezing platform, and 40μm coronal sections were cut on a sledge microtome (Leica 1400). Two 1-in-3 series of sections were mounted directly onto gelatine-subbed slides, and then allowed to dry in the dark at room temperature. One series was stained with cresyl violet to allow localisation of injection sites and provide anatomical boundaries for comparison with images from the second series of adjacent fluorescent sections. A Leica DM5000B microscope with a Leica DFC310FX digital camera and Leica Application Suite image acquisition software was used for brightfield and fluorescence microscopy. The microscope used Leica fluorescence filter cubes A (Fast Blue and Diamidino Yellow), L5 (CTB488 and WGA488) and TX2 (CTB594 and WGA594). Photoshop (Adobe Systems Incorporated) was used to enhance the sharpness and contrast of the photomicrographs, e.g., for figure preparation, and so match the appearance of sections when viewed on the microscope. No other image processing was involved although individual labelled cells were marked up manually on the digitised image.
Anatomical nomenclature
In the rat brain, the borders of the three major anterior thalamic nuclei (anterodorsal, anteromedial, and anteroventral) are widely agreed (Swanson, 1992). An additional nucleus, the interanteromedial thalamic nucleus, is often placed at the midline, between the medial borders of the more caudal left and right anteromedial thalamic nuclei in the rat brain (e.g., Shibata, 1992; Swanson, 1992), although this same nucleus is not typically recognised in the monkey thalamus (e.g., Olszewski, 1952). In the present study, the anatomical names, borders, and lamination of most cortical and subcortical areas follow Swanson (1992). Any exceptions are specified in the text.
Cell counting
Although systematic cell counts were not made of entire cases, estimates of the proportion of cells that were double labelled were obtained from representative sections of each of three highlighted cases (45_14, 45_15, and 45_11), all of which involved joint AV and AM injections. The sections were selected to include all of the regions of interest (i.e., prelimbic, cingulate, retrosplenial cortex, subiculum, dorsal tegmental nucleus and the mammillary bodies) and to be comparable across these cases. Microscopic examination identified those cells labelled by either a specific tracer (e.g., diamidino yellow and fast blue) or by both tracers (double labelled). Each cell was manually marked up with a colour-coded dot, and subsequently, the number of dots for each colour was obtained using an automated counting algorithm within the Image J software (NIH). As only the percentages of cells labelled for each (or both) tracers were calculated, a more stereological approach was not deemed necessary. This percentage was calculated by taking the number of single or double labelled cells and dividing that number by the total counts of all labelled cells (i.e., cells labelled by one of the two tracers, along with any double labelled cells) in the same site, and then multiplying by 100.
Results
Case selection
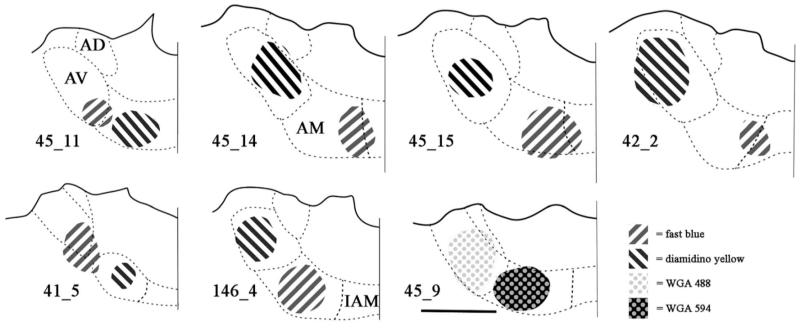
From the total of 38 animals used for this experiment, seven cases where the pairs of unilateral injections in ATN appeared largely confined to the target nuclei (Fig. 1, Table 2) were identified and examined in more detail. These seven cases did not include any of the six animals with additional medial dorsal thalamic injections. The written descriptions often highlight four representative examples (cases 45_11, 45_14, 45_15, and 146_4). These four cases were selected as each injection was centered within either AV or AM, with no evident spread to the other nucleus. These highlighted cases also helped to reflect the range of results as both AM and AV have their own cytoarchitectonic subfields, and some projections are known to have topographies within AM and within AV, i.e., they only project to particular subareas within these nuclei. Examples include projections from the retrosplenial cortex and mammillary bodies (Shibata 1992, 1993). As a consequence, the pattern of findings will inevitably vary from case to case. Additional variability will also arise from any spread into the neighboring anterodorsal and interanteromedial thalamic nuclei (Fig. 1). For two of the seven cases (45_14 and 45_15), photomicrographs of the label in various sites are presented to convey the pattern of results (Figs. 2-5).
Fig. 1.
Line drawings constructed from photomicrographs of the injection sites located in the anteroventral (AV) and anteromedial thalamic (AM) nuclei from selected cases. Fast blue is indicated by diagonal stripes to the right. Diamidino yellow is indicated by diagonals to the left. WGA488 is indicated by grey dots on a white background. WGA594 is indicated by pale dots on a black background. Scale bars = 1 mm. All sections are in the coronal plane, though it should be noted that the injections were spherical in shape remaining within the target nuclei. For case 41_5, the injection into AM is located approximately 280 μm posterior to the injection in AV. Abbreviations as Table 1.
Table 2.
Summary information from the seven cases with separate, paired injections into the anteroventral (AV) and anteromedial (AM) thalamic nuclei that form the core of the present study. The table lists the type of tracer (DY, diamidino yellow; FB, fast blue; WGA, wheat germ agglutin) with brief notes about location (e.g., +IAM indicates AM injections involving the interanteromedial nucleus). An indication is given of the relative density of retrogradely labelled cells (+, ++, +++, or ++++) while a ‘0’ signifies the lack of any labelled cells. A * indicates the presence of at least some double labelled cells, while ** indicates plentiful (>10%) double label. Separate information is given for the medial division of the medial mammillary nucleus (mMM) and the lateral division of the medial mammillary nucleus (lMM). All other abbreviations, see Table 1.
| Target | Tracer | Cases | PrL | ACA | Rga/b | Rdg | SUB | POST | mMM/lMM | LM | RTN | LDTg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV | FB Close to AM | 45_11 | ++ * | ++ * | +++ * | ++ * | ++ * | 0 | +++ lMM ++ mMM * |
+ | ++ * | ++ * |
| DY | 45_14 | +++ * | +++ * | ++++ * | ++ * | +++ * | +++ | ++++ lMM + mMM * |
+++ * | +++ | ++ ** | |
| DY | 45_15 | + | ++ * | ++++ * | + * | +++ * | ++ | ++++ lMM + mMM * |
++ * | + | +++ ** | |
| DY | 146_4 | 0 | + | ++ * | + | ++ | ++ * | ++ lMM 0 mMM |
0 | + | + ** | |
| FB Close to AM | 41_5 | +++ | +++ * | + | ++ | + | ++ | +++ lMM + mMM |
++++ | ++ | ++ | |
| DY | 42_2 | +++ * | +++ * | +++ | ++ | +++ | ++ | ++++ lMM 0 mMM |
+ | ++ | ++ | |
| WGA488 | 45_9 | + | +++ * | +++ * | + | ++ * | + | ++ lMM * + mMM * |
++ | + | ++ ** | |
| AM | DY | 45_11 | +++ * | ++++ * | ++ * | ++ * | ++ * | 0 | ++++ mMM * 0 lMM |
+++ | +++ * | ++ * |
| FB +IAM | 45_14 | +++ * | +++ * | + * | ++ * | ++ * | 0 | ++++ mMM * 0 lMM |
+ * | +++ | + ** | |
| FB +IAM | 45_15 | ++++ | ++++ * | ++ * | +++ * | +++ * | + | ++++ mMM * + lMM |
++ * | ++ | ++ ** | |
| FB | 146_4 | ++++ | +++ | +++ * | +++ | +++ | ++ * | ++++ mMM ++ lMM |
++++ | +++ | ++ ** | |
| DY | 41_5 | ++ | ++ * | 0 | + | 0 | 0 | +++ mMM 0 lMM |
0 | 0 | 0 | |
| FB +IAM | 42_2 | ++++ * | +++ * | + | + | + | 0 | +++ mMM 0 lMM |
++ | ++ | + | |
| WGA594 Close to AV | 45_9 | ++++ | ++++ * | + * | +++ | ++ * | 0 | ++++ mMM * +++ lMM * |
+ | ++ | ++ ** |
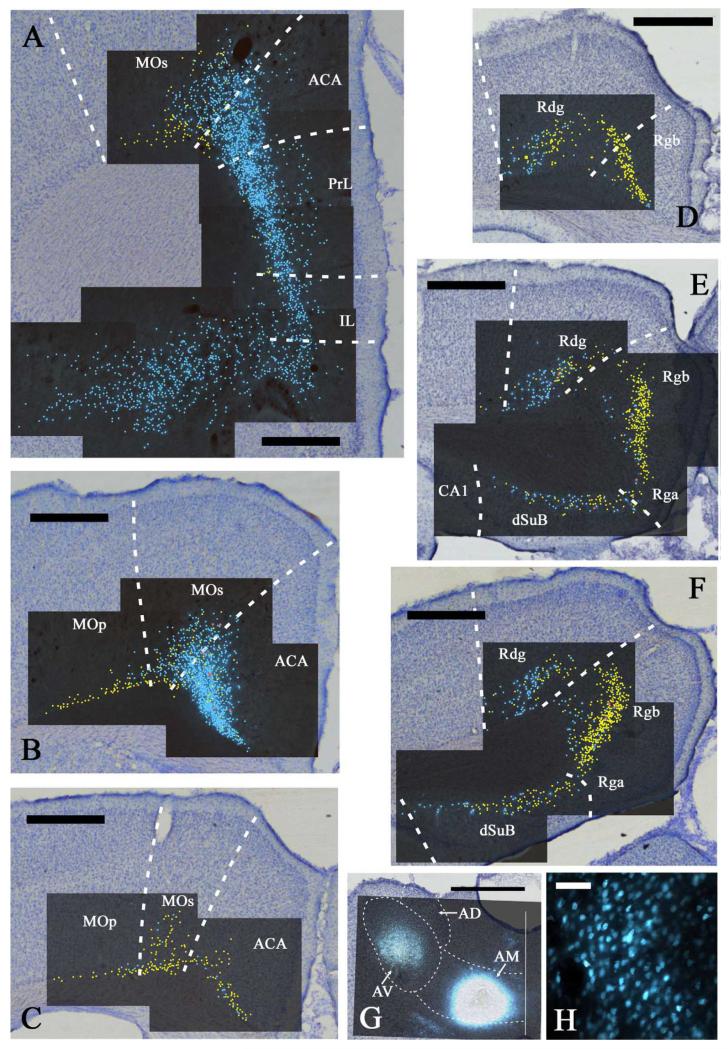
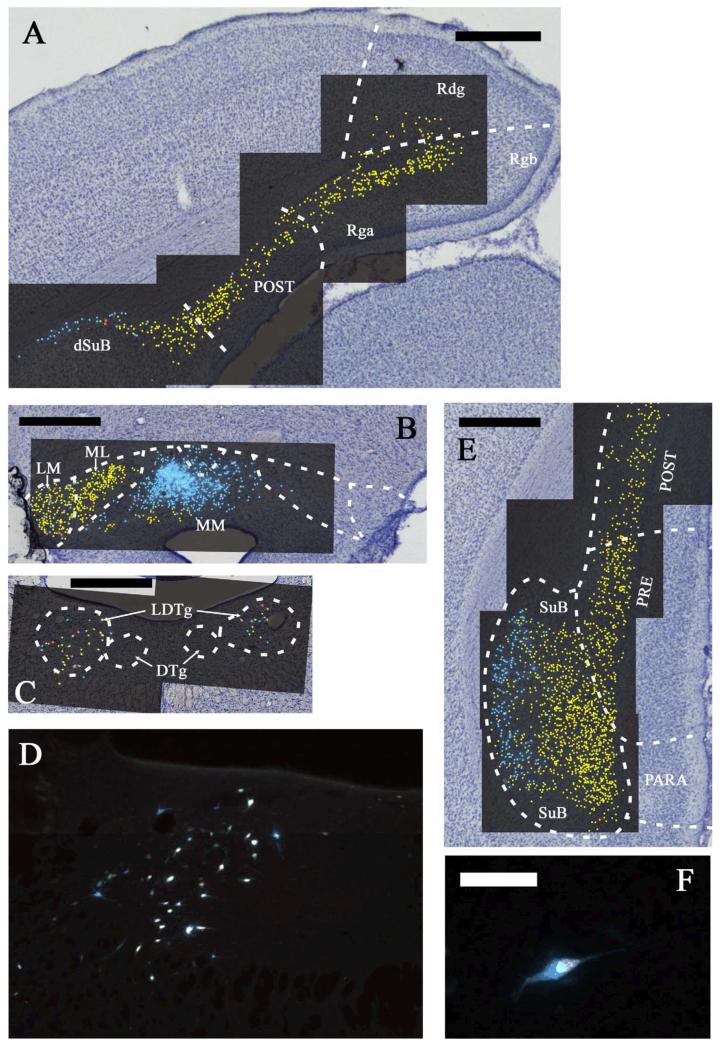
Fig. 2.
A-F: Distribution of labelled cells in case 45_15 following injections of fast blue into the anteromedial (AM) and diamidino yellow into the anteroventral (AV) thalamic nuclei. Fast blue is indicated by the blue labelled cells that have been highlighted further with blue dots over the fluorescence photomicrographs. Diamidino yellow is indicated by the yellow labelled cell nuclei that have been highlighted further with yellow dots over the fluorescence photomicrographs. Double labelled cells are highlighted in red. Scale bars = 1 mm. G: Combined line drawing and photomicrograph of the injection sites within the anterior thalamic nuclei. Scale bar = 1 mm. H: Fast blue labelled cells in the prelimbic cortex. Scale bar = 50 μm. All sections are reconstructed by realignment of the montages of the fluorescence images positioned over an image of the adjacent cresyl violet stained section to allow cytoarchitectural boundaries to be identified. All sections are in the coronal plane. Abbreviations as Table 1.
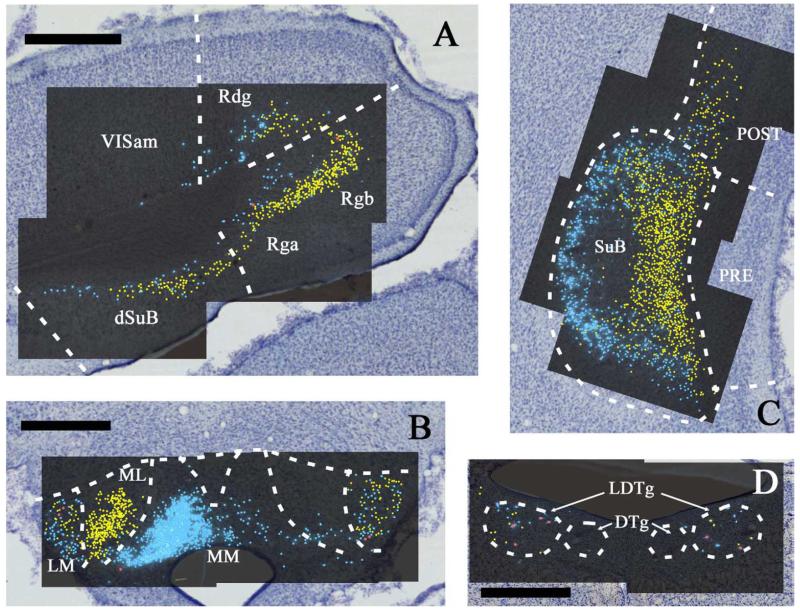
Fig. 3.
A-D: Continuation of the distribution of labelled cells in case 45_15 following injections of fast blue into the anteromedial (AM) and diamidino yellow into the anteroventral (AV) thalamic nuclei. Fast blue is indicated by the blue labelled cells that have been highlighted further with blue dots over the fluorescence photomicrographs. Diamidino yellow is indicated by the yellow labelled cell nuclei that have been highlighted further with yellow dots over the fluorescence photomicrographs. Double labelled cells are highlighted in red. Scale bars = 1 mm. All sections are reconstructed by realignment of the montages of the fluorescence images positioned over an image of the adjacent cresyl violet stained section to allow cytoarchitectural boundaries to be identified. All sections are in the coronal plane. Abbreviations as Table 1.
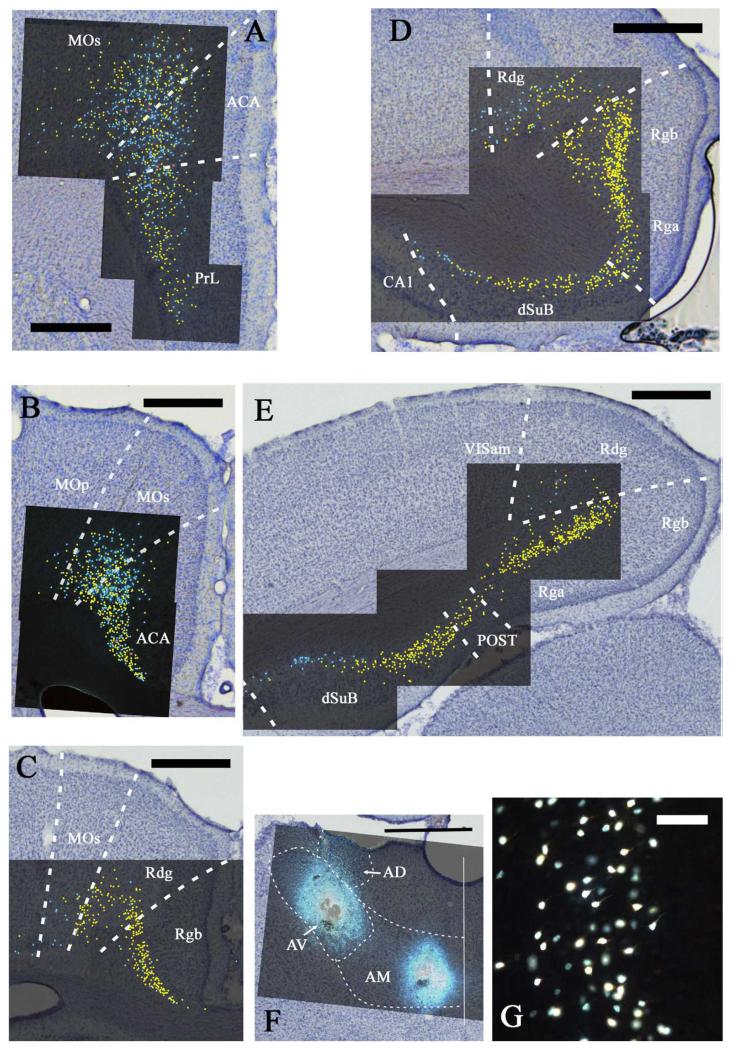
Fig. 4.
A-E: Distribution of labelled cells in case 45_14 following injections of fast blue into the anteromedial thalamic (AM) nucleus and diamidino yellow into the anteroventral (AV) thalamic nucleus. Fast blue is indicated by the blue labelled cells that have been highlighted further with blue dots over the fluorescence photomicrographs. Diamidino yellow is indicated by the yellow labelled cell nuclei that have been highlighted further with yellow dots over the fluorescence photomicrographs. Double labelled cells are demoted in red. Scale bars = 1 mm. F: Combined line drawing and photomicrograph of the injection sites within the anterior thalamic nuclei. Scale bar = 1 mm. G: Diamidino yellow labelled cells in the granular retrosplenial cortex. Scale bar = 50 μm. All sections are reconstructed by realignment of the montages of the fluorescence images positioned over an image of the adjacent cresyl violet stained section to allow cytoarchitectural boundaries to be identified. All sections are in the coronal plane. Abbreviations as Table 1.
Fig. 5.
A-F: Continuation of the distribution of labelled cells in case 45_14 following injections of fast blue into the anteromedial (AM) and diamidino yellow into the anteroventral (AV) thalamic nuclei. Fast blue is indicated by the blue labelled cells that have been highlighted further with blue dots over the fluorescence photomicrographs. Diamidino yellow is indicated by the yellow labelled cell nuclei that have been highlighted further with yellow dots over the fluorescence photomicrographs. Double labelled cells in highlighted in red (except for 5D and 5F). Figure 5D shows ipsilateral label in the laterodorsal tegmental nucleus from Figure 5C. Figure 5F shows a single double labelled cell (tegmentum) that clearly illustrates how the diamidino yellow label is more confined within the cell body than the fast blue label. Scale bars = 1 mm except for Figure 5F where the scale bar = 50 μm. All sections are reconstructed by realignment of the montages of the fluorescence images positioned over an image of the adjacent cresyl violet stained section to allow cytoarchitectural boundaries to be identified. All sections are in the coronal plane. Abbreviations as Table 1.
Of the six additional cases with MD injections, there was evidence that in three cases the MD tracer injection zone may have reached the anterior thalamic nuclei. For this reason, only the remaining three MD injection cases are described (Table 3).
Table 3.
Summary information from the three described cases with separate injections into the anteroventral (AV) and anteromedial (AM) thalamic nuclei, along with the mediodorsal thalamic nucleus (MD). The table lists the tracers (CTB, cholera toxin subunit B; FB, fast blue; WGA, wheat germ agglutin). An indication is given of the density of retrogradely labelled cells (+, ++, +++, or ++++) while a ‘0’ signifies the lack of any labelled cells. A * indicates the presence of at least some double labelled cells, while ** indicates plentiful (>10%) double label. Separate information is given for the medial division of the medial mammillary nucleus (mMM) and the lateral division of the medial mammillary nucleus (lMM). Note, the findings from the AV injections are not depicted on the table as the label from these injections did not co-occur in the same regions as the label from the MD injections, i.e., the label was only present in separate areas. All other abbreviations, see Table 1.
| Target | Tracer | Cases | PrL | ACA | Rga/b | Rdg | SUB | POST | mMM/lMM | LM | RTN | LDTg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD | FB | 154_5 | ++ | +++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + * |
| FB | 154_3 | +++ * | ++ * | 0 | 0 | 0 | 0 | 0 | 0 | ++ | ++ * | |
| FB | 47_1 | ++ | +++ * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ * | |
| AM | WGA488 | 154_5 | ++++ | +++ + | +++ | + | ++ | 0 | ++++ mMM + lMM |
++ | +++ | ++ * |
| WGA594 | 154_3 | +++ * | +++ + * | +++ | ++ | ++ | + | ++++ mMM + lMM |
+++ | + | +++ * | |
| CTB488 | 47_1 | +++ | +++ + * | ++ | + | ++ | 0 | +++ mMM ++ lMM |
0 | + | + * |
Figure 1 depicts the injection sites of the seven cases that form the core of the present study. The injection sites of the fluorescent tracers were fairly well defined as they consistently appeared compact with clear borders. The WGA injections were more diffuse, and for these cases the injection site was defined using the outer limit of continuous fluorescence, i.e., the definition may, if anything, have overestimated the active extent of the injection site. In all of these cases, one injection was centered in AV, and the other in AM (Fig. 1). The unilateral injections often resulted in some label in both hemispheres, though the ipsilateral labelling was always considerably more numerous. The results described here always refer to the ipsilateral patterns of label, unless otherwise stated.
CASES WITH PAIRED ANTEROVENTRAL AND ANTEROMEDIAL NUCLEI INJECTIONS
Prelimbic cortex
All seven cases with injections centered in AM had large numbers of labelled cells in the prelimbic cortex (e.g., Fig. 2A). In almost all cases, this prelimbic label was dense, with only case 41_5 having appreciably fewer labelled cells than the other AM injection cases. This case (41_5) had one of the smallest AM injections. The prelimbic label was typically restricted to cells in layer VI, but in case 45_15, a few additional labelled cells were present in layer V (Fig. 2A). Labelled cells after AM injections were typically found throughout the dorso-ventral and anterior posterior limits of the prelimbic cortex. A common feature following AM injections was that the labelled prelimbic cells became more numerous closer to the dorsal border with the anterior cingulate cortex (Figs. 2A, 4A). Here, the labelled prelimbic cells spread more superficially with the widening of layer VI. In the most rostral prelimbic cortex, the labelling almost entirely consisted of projections to AM, i.e., far fewer inputs to AV, and in cases 41_5 and 146_4, the most rostral prelimbic cortex showed only projections to AM.
Almost all of the seven cases with an AV injection contained some retrogradely labelled cells in the prelimbic cortex, although in the majority of these cases the AV label was appreciably less than that seen following the injection into AM. At one extreme, e.g., cases 45_15 (Figs. 2A), 146_4 and 45_9, there were almost no prelimbic cells labelled after the AV injection (Table 2). In contrast, other cases such as 45_14 had considerable amounts of prelimbic label, which appeared comparable to that associated with the AM injection in the same case (Fig. 4A). Labelled cells were located mainly in layer VI, but as with the AM, labelled cells following AV injections were also located to a lesser extent in layer V. Again, the prelimbic label in 45_14 after the AV injection appeared to increase moving dorsally towards the anterior cingulate cortex. It is notable that cases with appreciable prelimbic label after an AV injection (Table 2) included examples (e.g., case 42_2) where the AV injection site appeared to be distant from the AM border, i.e., it is unlikely to be due to spread into AM. Indeed, despite the variability in the quantity of retrograde label in the prelimbic cortex after injections into AV, it was not possible to discern a topographical feature to explain these individual case differences.
Despite the substantial inputs to AM and AV from the prelimbic cortex (especially to AM), double labelled cells were either completely absent (four of the seven cases) or, in cases such as 45_11 comprised a tiny number (~1%) of cells in each section (Table 2). These rare examples of double labelled cells included cases where the AV and AM injections were well separated, e.g., cases 45_14, 42_2, making it most unlikely that the occasional double labelled cell arose from any injection overlap. This scarcity of double labelled cells was particularly striking as the cell populations projecting to AM and AV appeared completely intermingled and often no obvious topography could distinguish those cells that projected to AV from those that projected to AM (e.g. Fig. 4A).
Infralimbic and orbital cortices
Label in the infralimbic cortex after an injection into AM was typically a little less numerous than that found in the prelimbic cortex, e.g., cases 45_11, 45_14, 45_15 (Fig. 2A). There was, however, only a gradual change in frequency at the border between these two cortical areas. The retrograde label seen after AM injections not only continued throughout the infralimbic region (mainly in layer VI and also to a lesser extent in layer V) but also extended across layers V and VI of the adjacent orbital cortex (especially prominent in case 45_15). The infralimbic label stopped at the border with the taenia tecta.
Injections of tracer into AV produced far less label in the infralimbic cortex than that associated with AM injections, and little, or none, in the orbital cortex. When label following AV injections was present in the infralimbic cortex, it was intermixed among the layer VI label from the AM injection, but double labelled cells were never found.
Anterior cingulate cortex and secondary motor area
The anterior cingulate cortex (ACA) extends rostro-caudally, starting from near the frontal pole to the border with the retrosplenial cortex (Swanson, 1992). Along this extent, the secondary motor area (MOs) occupies its dorsal and lateral border.
Rostral from the genu of the corpus callosum
In front of the genu of the corpus callosum, i.e., above the prelimbic cortex, a great many labelled ACA cells were seen after injections into AM (Figs. 2A, 4A). This region of dense label was found in all cases although, again, the fewest cells were in case 41_5 (the case with the smallest AM injection). There was no obvious change in the frequency of label at the border with the prelimbic cortex. The ACA label spread continuously across both the ventral and dorsal portions of the ACA and continued into the adjacent MOs. A few labelled cells were also present in layer V, but the large majority were in layer VI. The label in MOs was most dense close to the border with ACAd, and then rapidly diminished in frequency more laterally (Figs. 2A, 4A).
While injections into AV also resulted in retrogradely labelled cells in ACA, these cells were typically fewer in number than after AM injections. There was, however, much variation as some AV injections (e.g., case 45_14) still resulted in consistent, appreciable numbers of ACA labelled cells (Fig. 2A). The distribution of labelled cells in ACA was very similar after AV and AM injections, and so the label was intermingled mainly in layer VI, with some labelling present in layer V.. Only a few double labelled cells were seen (<1% in case 45_14, ~1% in case 45_15, and ~2% in case 45_11, see Table 2). In a few cases, the AV label extended into layer VI of MOs (cases 45_15 and 42_2). In case 45_15, this AV label became more frequent than the AM label in the more lateral parts of MOs. Only case 45_11 appeared to have double labelled cells in MOs, but even in this case they were very rare (~1%).
Above the corpus callosum (behind the genu)
Label continued in both ACA and MOs above the corpus callosum. The label associated with the AM injections was often particularly dense, occupying layer VI and extending into layer V in dorsal ACA and MOs (e.g., Figs. 2B,C, 4B,C). Cells projecting to the AV region were intermingled with those projecting to the AM area, though the former were typically fewer than those that projected to AM. In most cases, a considerable number of both cell types were labelled in the same section in ACA and MOs, but the percentage of double labelled cells per section remained extremely low (e.g., ACA; 0% in 45_15, <0.5% in 45_14, and ~1% in 45_11: MOs 0% in 45_15 and 45_14, and ~1.5% in 45_11). The AV and AM label in MOs often extended far enough laterally to include just a few cells in the layer VI of MOp. This was most evident in case 45_15 as label from the AV injection continued laterally along the deepest cortical layer (VIb) of medial MOp, while the AM label only reached the border of MOs with MOp (Fig. 2B).
Across the rostral – caudal extent of the ACA, there appeared to be a topographic shift in inputs such that cells projecting to AM were most numerous anterior to the genu. In contrast, cells projecting to AV became more numerous posterior to the genu. While the majority of cases showed this overall pattern, in case 146_4 there were still obvious regions of AM inputs from the more posterior ACA, while in case 45_11 few inputs to AV were found from the more caudal ACA.
Retrosplenial cortex
Using the nomenclature of Van Groen and Wyss (2003), the rat retrosplenial cortex contains two major subdivisions, the granular and dysgranular cortices. These two subdivisions have also been designated area 29 and area 30, respectively (Vogt et al., 2004). The granular cortex is further subdivided into the subregions Rga and Rgb. Subregion Rgb occupies most of the granular retrosplenial cortex rostral to the splenium, while caudal to the splenium, Rga expands so the granular retrosplenial cortex is more evenly divided between Rga and Rgb. Area Rga is positioned ventral to Rgb so that it borders the subiculum and postsubiculum.
Rostral retrosplenial cortex: above the corpus callosum
The retrosplenial cortex contained substantial numbers of retrogradely labelled cells in every case (Table 2). These labelled cells were associated with both AM and AV injections, but very few double labelled cells were present (Figs. 2D, 4C). The large majority of retrogradely labelled cells in the granular cortex (Rgb) were typically associated with the injections into AV, although some label was always also present after AM injections. In the dysgranular cortex (Rdg), the label from the AM injections often dominated although there were also appreciable numbers of cells associated with the AV injections. Despite regions of intermingling, there was also evidence of some separation within the retrosplenial subfields (e.g., Fig. 2D and Fig. 4C). Despite the areas of overlap in Rdg and Rgb, most cases typically displayed <1% doubled labelled cells, which were completely absent in some animals (cases 41_5 and 42_2).
Two cases (45_14 and 45_15) that produced remarkably similar patterns of retrograde label across the retrosplenial region (Figs. 2, 4) are described first. In both cases, the granular retrosplenial cortex (Rgb) contained numerous labelled cells following injections into AV (Figs. 2D, 4C). Consequently, both rats contained a great many labelled cells in layer VI (especially VIa), with a very occasional labelled cell in layer V. The Rga label associated with the AM injections in these two cases was far less frequent but, where present, remained intermingled with the label from the AV injection. A slightly different result was found in case 45_11, where in rostral Rgb the label density from the AM injection appeared more comparable to that after the AV injection. Case 146_4 showed a more separated arrangement of inputs as the label from the AM injection was a little more concentrated in ventral Rgb, i.e., close to the corpus callosum, while the AV associated label in Rgb was strongly concentrated close to the Rdg border. As a consequence, there was a clear, overall difference in the source of the projections to the AV and AM nuclei in this case, despite the intermingling of label in more dorsal Rgb. For both injections in case 146_4, the label still remained largely confined to layer VI.
The dysgranular retrosplenial cortex (Rdg) consistently contained labelled cells associated with both the AV and AM injections, though typically more from the AM injection (Table 2). In cases 45_14 and 45_15, moderate numbers of labelled cells from the AV injection were present in Rdg (layer VIa), but these were largely concentrated close to the its medial border, i.e., adjacent to Rgb (Figs. 2D, 4C). In the more lateral part of Rdg (i.e., adjacent to visual cortex), the cells labelled by the AM injection predominated. Consequently, there were opposing gradients of label in the medial – lateral plane within Rdg. The adjacent part of MOs often contained a few cells, mainly from the AM injection. In case 146_4, this medial-lateral change in Rdg was particularly exaggerated as only the most medial Rdg contained any labelled cells from the AV injection, while the more lateral Rdg and the adjacent MOs contained appreciable number of labelled cells from the AM injection. Case 45_11 appeared somewhat different as Rdg showed fairly even numbers of labelled cells from both the AV and AM injection, these cells were highly intermingled and did not display any separate distributions. In both cases 45_14 and 45_15, most sections of the Rdg contained no double labelled cells, however, in one section from each case 3.7% and 1%, respectively, double labelled cells were observed. In case 45_11, very few (<0.5%) double labelled cells occurred, and in case 146_4, no double labelled cells were present.
Caudal retrosplenial cortex: posterior to the corpus callosum
In three of the highlighted cases (45_11, 45_14, 45_15), the label in Rga and Rgb predominantly arose from the AV injection, with the cells projecting to the AM injection site being less frequent and often having a slightly different laminar distribution (Figs. 2-5). The numerous labelled cells following the AV injections were found across Rga and Rgb in layer VI. The large majority were in the more superficial portions of layer VIa. The labelled cells following the AM injections were both intermingled with those labelled by the AV injections and also included more cells that were found in deeper parts of VIa (and VIb) and, hence, were often distinct from the AV label in Rga and Rgb (Figs 2, 4). The fourth highlighted case (146_4) differed as it was label from the AM injection that predominantly filled up Rga and Rgb, although once again the AM labelled cells were more deeply positioned within the cortex. In this case (146_4), the Rgb cells labelled by the AV injection were largely concentrated close to the Rdg border. In these four cases, just a few double labelled cells were consistently present in layer VI of granular retrosplenial cortex. A maximum of 1.1% of double labelled cells was seen in a section from case 45_11, while in the other cases that were counted the individual sections contained <1% double labelled cells and quite often none at all.
The label in Rdg was strikingly similar in cases 45_14 and 45_15 (Figs. 2-5). In both rats, Rdg contained labelled cells from both the AM and AV injections, but the AV cells predominated close to the border with Rgb while the AM cells predominated close to its lateral border, i.e., with the parietal and visual cortices. The labelled cells following the AV and AM injections were in layer VI, where the label from the AM injections tended to be a little deeper (although there was much overlap). Even where these two populations of cells intermingled, there were very few double labelled cells in cases 45_14 and 45_15, resulting in solitary double labelled cells being found in a few sections in the Rdg. This same distribution pattern was exaggerated in case 146_4 as the AM labelled cells were clearly the more numerous in lateral Rdg, where many layer VI cells were labelled. In contrast, the AV labelled cells (superficial layer VI) were far fewer and concentrated close to the Rgb border. Finally, in case 45_11, Rdg contained numerous, intermingled cells from the AM and AV injections, although again the AM injection cells tended to be located more deeply in Rdg. As before, an occasional double labelled cell was found in the Rdg of case 45_11, but none of the other cases contained double labelled cells.
Hippocampal formation
The hippocampal formation designations follow those used by Swanson (1992). The term postsubiculum is used for that caudal subicular region adjacent to the retrosplenial cortex (Swanson, 1992). While some authors regard this region in the rat brain as being part of the presubiculum (e.g., Kloosterman et al., 2003), the present study retains the use of the term postsubiculum based on its connectivity and its distinctive cytoarchitecture (van Groen and Wyss, 1990b,c).
While some authors described the rat subiculum as having three laminae, others (e.g,. Kloosterman et al., 2003) only describe two laminae. These two laminae consist of a superficial molecular layer and a deeper, thick layer of pyramidal cells. In this study, we will adopt the latter practice, even though the cells within this deeper lamina are not entirely homogeneous. The lamination in the postsubiculum follows that used by Van Groen and Wyss (2003). We also use the term ‘intermediate subiculum’ (Groenewegen et al., 1987) to describe that region of the subiculum at the caudal extent of the hippocampal flexure where the dorsal (septal) subiculum and ventral (temporal) subiculum converge.
Subiculum
There was a very consistent pattern of label within the subiculum in six of the seven highlighted cases, with only case 41_5 differing due to an absence of label from the AM injection, presumably due to reduced uptake at the smaller injection site. In these six cases, considerable label from both the AM and AV injections was found along the length of the septal (dorsal) subiculum, continuing caudally to reach the flexure at the intermediate hippocampus (e.g., Figs. 2F, 3A, 4E, 5A). This label was found in the deepest, cellular part of the subiculum. While labelled cells from both the AV and AM injections were found across the proximal (near to CA1) to distal (furthest from CA1) plane of the subiculum, the label associated with AM injections was consistently concentrated in the proximal subiculum while the label associated with AV injections was concentrated distal to CA1. As a consequence, most of the intermingling between the labelled cells from the AV and AM injections occurred midway along the proximal-distal plane of the subiculum. The dorsal subiculum sections with the maximum numbers of double labelled cells contained 1.6% such cells in case 45_14, 1.2% in case 45_15, and 3.8% in case 45_11. In cases 45_11 and 45_15, the label from the AV and AM injections often appeared intermixed, but it separated out more caudally. Case 45_14 differed slightly as the label from the two injections remained separate throughout the subiculum, i.e., there was less intermingling. Cells in the CA1 field were not labelled after an injection in either anterior thalamic nucleus, although the most proximal labelled subicular cells (after AM injections) were located immediately adjacent to the most distal CA1 region.
At the level of the intermediate subiculum, the numbers of labelled cells from both injection sites increased further, but the two sets of label maintained their separate locations. Because of the flexure of the hippocampus, it becomes very difficult to make a clear proximal versus distal distinction within the subiculum, and so the cell populations are described by their medial or lateral placement (see Fig. 3C, Fig. 5E). The three highly similar cases from the four highlighted cases (45_11, 45_14, 45_15) contained large numbers of cells associated with the AM injection that were matched by adjacent cells labelled by the AV injection (especially in cases 45_14 and 45_15). As a consequence, in coronal sections, the label associated with AV and AM injections forming two very densely filled but discrete populations at the flexure of the subiculum, with the AV injection label appearing medial to the AM injection label (see Fig. 3C, Fig. 5E). Going further ventral and rostral, i.e., into the temporal parts of the hippocampus, only very limited label was present in the ventral subiculum. In case 45_14, only label from the AV injection was seen and this was restricted to that part of the ventral subiculum immediately adjacent to the intermediate subiculum. In almost all of the other highlighted cases, retrograde label was not found in the ventral subiculum, with the single exception of case 146_4.
Postsubiculum
Once again, the pattern of label was very similar in cases 45_14 and 45_15. Considerable label was present along the length of the postsubiculum deep to the lamina dissecans following the injections into AV, but the injection in AM resulted in very few cells in this area. In case 45_15, the very limited AM label was just found close to the border with the subiculum. No double-labelled cells were seen in either case. From Table 2 it can be seen that this general pattern was repeated, with only one case (146_4) having any double labelled cells in the postsubiculum. It should be noted that, in cases 45_11 and 45_9, there was very little label in the postsubiculum from either injection. In view of the well known projection from the postsubiculum to the anterior dorsal thalamic nucleus (van Groen and Wyss, 1990b; Yoder and Taube, 2011), caution should be taken when interpreting the presence of labelled cells in the postsubiculum after an AV injection as the injection tract to AV would often pass through AD.
Mammillary bodies
The dense projections from the mammillary bodies to the anterior thalamic nuclei have been described on numerous occasions (e.g., Seki and Zyo, 1984; Hayakawa and Zyo, 1989; Shibata, 1992), and it is known that these connections maintain a complex topography. The goal here was not to re-examine this topography, rather it was to determine whether any individual mammillary body cells project to more than one of the anterior thalamic nuclei, i.e., whether this topography is sufficient to ensure the separation of projections to AM and AV.
Starting with the two cases depicted in Figures 3 and 5 (cases 45_15 and 45_14), a great many mammillary body cells were labelled by both the AV (DY) injection and the AM (FB) injection. These cells were tightly grouped in separate regions within the medial mammillary nucleus. Table 2 also shows how in the remaining cases both the AV and AM injections again resulted in a great many labelled cells in the medial mammillary nucleus. Perhaps more surprising was that in five of the seven highlighted cases the injections placed in AM and AV led to intermingled labelled cells in the lateral mammillary nuclei (LM). This LM label from both the AM and AV injections was bilateral. Of the two illustrated cases, 45_15 had a much lower number of labelled cells in the lateral mammillary nucleus after the AV injection. Double labelled cells were occasionally seen in the mammillary bodies, but they still remained very infrequent, and in cases 45_14 and 45_15, they were restricted to the LM (0.4-0.7% of the total cells within MB were double labelled in these two cases). In view of the route taken by the fibers that link the lateral mammillary nucleus to the anterodorsal thalamic nucleus, it is possible that some, or even all, of this label arose from uptake by fibers of passage.
In both cases 45_14 and 45_15, most of the labelling from the AM injection (FB) occurred in the medial part of the ipsilateral medial mammillary nucleus (mMM) while the label from the AV injection (DY) occurred in the lateral part of the ipsilateral medial mammillary bodies (lMM) (Figs. 3B, 5B). Double labelling was almost non-existent in both cases 45_14 and 45_15, with just solitary cells located in the ventral mMM. The only case to show any difference in the number of double labelled cells within the mammillary bodies was 45_9, where ~5% of the labelled cells in mMM and 8.5% in lMM were double labelled, although still a small proportion. This increase in double label raises the possibility of uptake by fibres of passage, which is feasible given the proximity on the two injections sites (Fig. 1). Finally, some retrograde label was found in nucleus medianus of the mammillary bodies in six of the seven cases (not in case 45_9), although appreciably fewer cells were labelled than that in the adjacent medial mammillary nucleus (e.g., Figs. 3B, 5B). This label suggests some possible additional uptake by the interanteromedial thalamic nucleus (Hopkins, 2005).
Laterodorsal tegmental nucleus
As shown in Figures 3D and 5C,D, occasional labelled cells were scattered across the laterodorsal tegmental nucleus (LDTg) after either AM or AV injections (cases 45_15 and 45_14). An appreciable percentage of the total number of labelled cells was double labelled (Fig. 5D). An example of a double labelled cell in LDTg is shown in Figure 5F. The mean percentage of double labelled cells (from the total of all labelled cells in this nucleus) in these two cases came to 13% when the results were compiled across a series of sections. The highest percentage on any individual section was 43% (from case 45_15). In case 45_14, the most abundant LDTg label was from the AV injection and, as a consequence, approximately 50% of the less-frequent cells projecting to AM were double-labelled, i.e., they also projected to AV. Again, many more of the labelled cells in case 45_15 were from the AV injection, with approximately 68% of the labelled cells that projected to AM in this case also projecting to AV. An additional feature of this input in both cases was that it was bilateral, with approximately 5% for case 45_14 and 20% for case 45_15 of the total projections arising from the contralateral hemisphere being double labelled.
The same pattern was repeated in the remaining cases. That is, a clear bilateral input to both anterior thalamic nuclei from the LDTg nucleus was found in nearly all cases, with double labelled cells present in both hemispheres. In the majority of cases (Table 2), the input to AV appeared to involve slightly more cells than the input to AM.
Reticular thalamic nucleus
Labelled cells were found in nearly all of the seven cases in the reticular nucleus (Table 2), but the very close proximity of this nucleus to the injection sites warrants some caution. For example, in case 45_15, more labelled cells were found in the reticular thalamic nucleus after the injection in AM (fast blue) compared to the injection in AV (diamidino yellow). These labelled cells were in the more ventral part of the rostral, reticular nucleus and, while there was some intermingling of label, there were no double labelled cells. Of the seven cases (Table 2), double labelled cells were only seen in case 45_11 (where the injections were in close proximity). The implication is that the large majority of inputs from the reticular thalamic nucleus terminate in either AV or AM but not in both.
CASES WITH MEDIODORSAL NUCLEUS INJECTIONS, ALONG WITH ANTEROVENTRAL AND ANTEROMEDIAL NUCLEI INJECTIONS
Mediodorsal and anteromedial thalamic nuclei case
To investigate the projections into MD, AV and AM, six animals were injected with three different tracers into each of these thalamic nuclei. Attention focussed on three of these cases (Table 3), as in these cases there was least evidence that the tracer injection in MD had spread into the anterior thalamic nuclei.
Injection locations
A representative case, JAR 154_5, is described first. The WGA594 injection into the AV subdivision was fairly extensive (Fig. 6), with a bias towards occupying more lateral AV. It did not appear to involve the neighboring AD and AM nuclei. The WGA488 injection into AM was again fairly large, and appeared centered slightly dorsolaterally within AM (Fig. 6). This AM injection spread right up to the border with AV. Both of these injections were located around 1.9mm behind bregma (see Swanson, 1992). The injection of fast blue into MD was centered in a more ventral portion of the central part of the mediodorsal thalamic nucleus and, to a lesser extent, involved the medial part of the mediodorsal thalamic nucleus. This injection was located about 2.8 behind bregma (Fig. 6), and was well separated from the ATN injections. Of the other two rats, the MD injection in case 47_1 was not only the largest, with the possible risk of rostral spread into AM, but the AM injection was also larger than that in case 154_5. In case 154-3, the injections into AV and AM partially overlapped and so the focus is on the presence of any double label from these injections with the MD injection. The AM injection in this case (154_3) was the largest of the three highlighted animals.
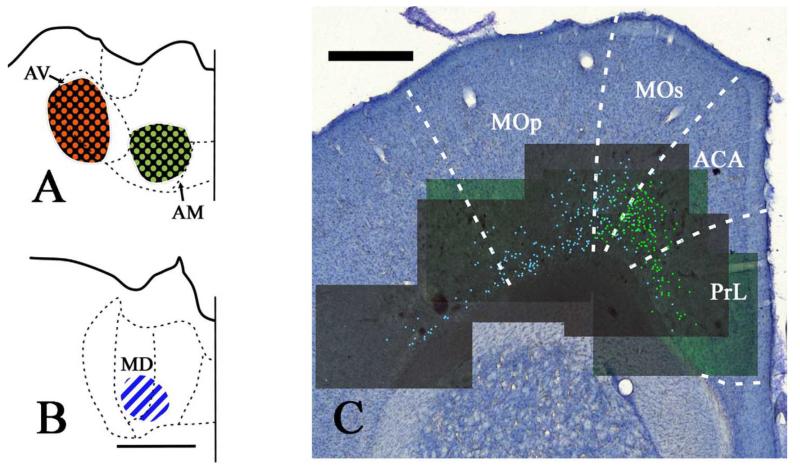
Fig. 6.
A, B: Line drawings constructed from photomicrographs of the injection sites located in the anteroventral (AV), anteromedial (AM) and mediodorsal (MD) thalamic nuclei in case 154_5. Fast blue (MD) is indicated by diagonally striped blue and white regions. CTB488 (AM) is indicated by green dots on a black background. CTB594 (AV) is indicated by red dots on a black background. All sections are in the coronal plane. C: Distribution of labelled cells in case 154_5 following injections of fast blue into MD and CTB488 into AM. Fast blue label (MD) is indicated by the blue labelled cells that have been highlighted further with blue dots over the fluorescence photomicrographs. CB488 label (AM) is indicated by the green labelled cell nuclei that have been highlighted further with green dots over the fluorescence photomicrographs. There were no double labelled cells in this section. Scale bars = 1 mm.
Prelimbic
Labelling from the AM injection in case 154-5 was present in the prelimbic cortex, where it was more abundant in dorsal prelimbic cortex bordering the anterior cingulate cortex and almost entirely absent in ventral prelimbic cortex. This labelling was located in layer VI. The labelling in the prelimbic cortex from the MD injection was intermingled with that from the AM injection in layer VI of the cortex, but fewer cells were labelled. No double labelled cells were seen. Case 154-3 had more labelled cells within the prelimbic cortex after the AM and MD injections than the previous case. In addition, there was a scattering of double labelled cells (~3% of all cells labelled after by respective AM and MD injections). Finally, in case 47_1, there were many prelimbic labelled cells after the AM injection but far less following the MD injection. In this case, <1% of all labelled cells (AM and MD) were double labelled. The injections into AV resulted in labelled cells that were not intermingled with those after the MD injections as the AV cells were concentrated in the anterior cingulate cortex rather than prelimbic cortex (for other examples see Table 2) and the MD labelled cells in prelimbic cortex were rostral to the relatively few from AV in case 154-5. Consequently there were no double labelled cells associated with AV and MD.
Anterior cingulate cortex and secondary motor area
In agreement with the other injections into AM, numerous labelled cells were located within the anterior cingulate cortex. In case JAR 154_5, the cells were distributed more abundantly in dorsal cingulate cortex up to the border with the secondary motor cortex. These cells in ACA projecting to AM were located within layer VI. The injection into MD resulted in the majority of labelled cells occurring throughout the primary and secondary motor cortices, but also a few labelled cells in the anterior cingulate cortex. Labelled cells were most abundant in the secondary motor cortex and less so in the primary motor cortex. The labelling in the anterior cingulate cortex was far more diffuse and intermingled with the labelling from AM. In this case, there was no evidence of double labelled cells across these regions.
While case 154_3 showed many more labelled projections from the anterior cingulate cortex to AM than case 154_5, there were far fewer labelled projections to MD than in case 154_5. There was a small number of double labelled (MD plus AM) cells in ACA (~1.3%) and the secondary motor area (~0.4%) in case 154_3. In case 47_1, there were almost twice as many labelled cells after the AM injection than the MD injection, and this case also contained the highest proportion of double labelled cells in ACA, ~4.5% of total labelled cells. While the labelled cells in the secondary motor area mainly reflected the MD injection, ~2.3% of the total cells in this area were double labelled. While the injections into AV resulted in appreciable numbers of labelled cells, these were separate from those after the MD injections, and so there were no double labelled cells.
Discussion
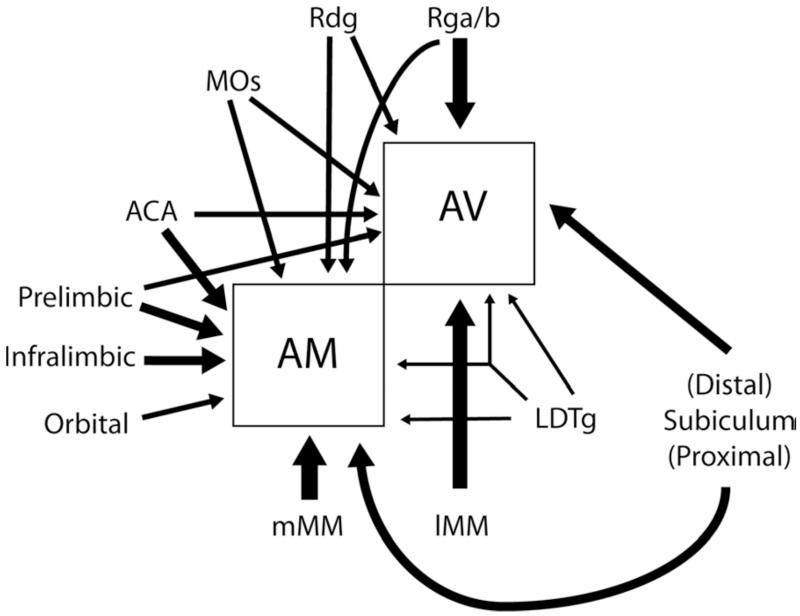
The present study addressed the question of whether the anteroventral and anteromedial thalamic nuclei receive afferent information from distinct populations of neurons, even when they appear to arise from the same region within a given site. The answer, as repeatedly found in the present experiments, is that only a very small minority of cortical and limbic inputs to AV and AM arise from neurons that bifurcate to innervate both thalamic nuclei. The only exception was the laterodorsal tegmental nucleus (LDTg), which contained an appreciable number of individual cells that project to both nuclei (Fig. 7). Many sites were found to have seemingly intermingled cells that projected to just one of the two anterior thalamic nuclei, with individual cells projecting to both nuclei remaining a rarity. This pattern was extended in the case of the frontal inputs to AV and AM, as these inputs were also intermingled with cells projecting to the mediodorsal thalamic nucleus (MD). Only occasional frontal cells that innervated both ATN and MD were observed. In other cortical and subcortical sites the sources of the projections to AV and AM overlapped, but there were subtle topographic differences in their distributions. Finally, in structures such as the medial mammillary nucleus, the inputs to AV and AM appeared largely segregated (see also Seki and Zyo, 1984; Shibata, 1992).
Fig. 7.
Schematic summary figure depicting the principal inputs to the anteromedial (AM) and anteroventral (AV) thalamic nuclei. Cortical inputs are shown to the left and above the two nuclei. The thickness of the arrows indicates the relative scale of the thalamic inputs. Only the arrow from the laterodorsal tegemental nucleus (LDTg) is depicted as bifurcating as this appeared to be the only input with appreciable numbers of neurons that project to both nuclei. Abbreviations as Table 1.
Before discussing the findings in more detail, including their possible functional implications, it is advisable to first consider whether the present results provide a true picture of the pattern of dual connections. The need to address this issue stems from the fact that the only previous study to have reported the outcome of paired injections of retrograde tracers into AV and AM in the rat (Seki and Zyo, 1984) stated that there were many double labelled cells in the prelimbic and cingulate cortices, a pattern never found in the present study. One important issue is whether there was any spread from one injection site to the other in our study, so inadvertently leading to double labelled cells. In fact, several lines of evidence strongly indicate that there was typically no active overlap across the injection sites within the present cases. One source of evidence comes from the patterns of mammillary body label. The various anterior thalamic injections led to dense, discrete clusters of labelled cells within different parts of the medial mammillary nucleus (see also Seki and Zyo, 1984; Shibata 1992), but within this same nucleus, double labelled cells were rare. Had pairs of injections spread into each other then there would have been obvious groups of double labelled cells within the medial mammillary nucleus. [In one case where the thalamic injections were inadvertently too close and there was considerable overlap of the tracers (not described), the medial mammillary nuclei contained up to 30% double labelled cells.] Instead, the general scarcity of double labelled cells across multiple sites, with the single exception of the laterodorsal tegmental nucleus, further shows the lack of injection overlap. A related issue concerns the potential spread of an injection into adjacent thalamic nuclei other than the target. It is undoubtedly the case that some of the AM injections will have involved the lateral parts of the interanteromedial nucleus (IAM, Table 2), an assumption supported by the presence of label in nucleus medianus of the mammillary bodies in some cases (see Vertes et al., 2004; Hopkins, 2005). It is also the case that some AV injections may have extended into adjacent parts of the anterodorsal thalamic nucleus.
A specific concern is whether there was any uptake of tracer by fibers of passage that might lead to misleading patterns of label, given that this is a property of many fluorescent tracers. Evidence that some fiber uptake may have occurred comes from the finding that almost all injections resulted in some labelled cells in the lateral mammillary nucleus, of which a small percentage were double labelled. It is known that the lateral mammillary nucleus specifically targets the anterodorsal nucleus, a projection that is bilateral (Cruce, 1975; Seki and Zyo, 1984; Shibata, 1992). Consequently, there may have been some limited tracer uptake as fibers reaching the anterodorsal nucleus pass by and through AM and AV. Likewise, the projection from the postsubiculum to the anterior thalamic nuclei preferentially targets the anterodorsal nucleus (van Groen and Wyss, 1990b; Yoder and Taube, 2011), yet some retrograde tracer was found in the postsubiculum after many of the present injections, especially those to the adjacent anteroventral nucleus (Table 2). This postsubicular label could, therefore, include uptake by fibers or spread into the anterodorsal nucleus from the injection tract. Even so, any contributions from tracer uptake by fibers of passage does not alter the main findings given the overall lack of double labelled cells for the combined AV and AM injections.
From the present study, the conclusion is that AV and AM (IAM) standardly receive inputs from different neurons, so maintaining a potential segregation of information (Fig. 7). To reach this conclusion, it was necessary to restrict the injection sites so that they do not overlap. As a consequence, there was no attempt to fill the two thalamic nuclei (AV, AM) in the same case. Thus, any cell counts will be an underestimation. Despite this caveat, the low percentages of double labelled cells in nearly all regions (around 1%, with the exception of LDTg) strongly indicates that, while the true proportion may be a little higher, it is still remarkably low. This overall pattern of results contradicts the single case described by Seki and Zyo (1984). These authors injected bisbenzimide and Evans Blue into AV and AM, respectively, and stated that many double labelled cells were found in preagranular (anterior cingulate and MOs), anterior limbic (prelimbic and anterior cingulate) and the cingular (dysgranular cingulate) cortices. This pattern of results was not found in any of our cases. Unfortunately, in their figure showing the distribution of double labelled cells across the cortex (Seki and Zyo, 1984) the same symbol was used for cells containing just Evans blue and those that were double labelled, i.e., they cannot be distinguished. The same authors were also concerned about possible tracer uptake by fibers of passage. The resultant inability to determine the precise findings from that study, which was only based on one case, added to the perceived need for the present series of experiments.
The segregation of inputs to AV and AM partially stems from the various topographic differences in the sources of afferents to these thalamic nuclei. While these topographic differences were perhaps most evident for the thalamic projections from the medial mammillary nucleus (see Cruce, 1975; Seki and Zyo, 1984; Shibata 1992, Vann et al., 2007), it was also the case that the cells projecting to AV and AM from the dorsal subiculum showed clear evidence of different topographies, despite some overlap. The resultant lack of double labelled cells in the subiculum is consistent with previous studies (Namura et al., 1984; Naber and Witter, 1998; but see Donovan and Wyss, 1983), which emphasise how subicular outputs can predominantly be seen as parallel, and often columnar (Witter et al., 1990; Naber and Witter, 1998; Witter, 2006). The columnar organisation of many hippocampal connections along the proximal – distal plane has been repeatedly described (Naber and Witter, 1998; Witter et al., 1990; Witter et al., 2000), and some previous studies indicate that inputs to the anteroventral thalamic nucleus arise principally from the distal subiculum and adjacent presubiculum while those to the interanteromedial nucleus derive from the proximal subiculum (Swanson and Cowan, 1977; Witter et al., 1990; Ishizuka, 2001). These descriptions were broadly supported by the present results as the inputs to AM (plus IAM) arose predominantly from the proximal subiculum while the inputs to AV arose predominantly from the distal subiculum. These findings build on the notion that there are different information streams within the hippocampus formation, with the potential to segregate different classes of hippocampal interconnections (Witter et al., 1990; Witter, 2006; Aggleton, 2012). Consequently, AV and AM could largely receive qualitatively different forms of hippocampal information.
The present study found large numbers of labelled cells in frontal areas, with the clear majority in the prelimbic and anterior cingulate cortices. Indeed, it was the density of the frontal inputs that prompted the additional experiments involving injections into the mediodorsal thalamic nucleus. Despite the many intermingled cells within the prelimbic and anterior cingulate cortices that project to these three thalamic nuclei, the overwhelming majority of labelled neurons only projected to a single thalamic nucleus. To this pattern can be added the inputs from the infralimbic cortex and the secondary motor area (MOs), although in both of these areas there was more evidence that the distribution of inputs to AV and AM differed. The sources of these frontal inputs matched previous descriptions (e.g., Groenewegen, 1988; Vertes, 2004; Shibata and Naito, 2005), which all indicate that, of the three major anterior thalamic nuclei, AM is most heavily innervated by frontal areas. Although this pattern was consistently supported by the current study, the present cases did indicate that parts of AV can receive moderately dense frontal inputs. In contrast, previous studies report that AV (medial) receives only light inputs from the anterior cingulate cortex, prelimbic cortex, and MOs (Vertes, 2004; Shibata and Naito, 2005). In the present study, the density of these frontal inputs to AV proved to be quite variable across cases, which might suggest differential involvement of medial AV (Vertes, 2004; Shibata and Naito, 2005), although it has proved difficult to make this precise association with the apparent injection locations (Fig.1; Table 2).
As expected, the retrosplenial cortex contained a great many inputs to AV and AM, with a mixture of intermingled cells of origin set against broader topographic changes. It was found that Rgb and Rga predominantly, but not solely, innervat AV while Rdg projected more equally to both AM and AV. While Rdg projected to both nuclei, there were different gradients of inputs as more medial Rdg (i.e., adjacent to Rgb) preferentially projected to AV while more lateral Rdg preferentially projected to AM. This pattern fits previous descriptions of these projections (van Groen and Wyss, 1992, 2003; Shibata, 1998), but by using two tracers in the same case, the shifting gradients of inputs to AM and AV from across the medial – lateral plane of Rdg become unusually clear. In addition, the use of multiple tracers showed that the inputs to AM were often a little deeper within the caudal retrosplenial cortex than those to AV. This subtle lamination difference is interesting as the different outputs from the retrosplenial cortex appear to be partially segregated by layer (Sripanidkulchai and Wyss, 1987). Even so, there are many retrosplenial locations where the inputs to the two thalamic nuclei appear intermingled, and cannot be distinguished by features such as cell type or lamina.
The sole exception to the pattern of separated inputs to AV and AM came from the laterodorsal tegmental nucleus (LDTg). Previous studies have reported how this nucleus projects to the anterior thalamic nuclei (Shibata, 1992) and that it is the key source of extrinsic cholinergic innervation to ATN (Sofroniew et al., 1985). The present study found that approximately 13% of the LDTg cells that project to the ATN project to both nuclei. As noted above, this proportion is almost certainly an underestimation as it would be necessary to fill both AV and AM with tracer in order to visualise the full extent of any projections that collateralise to reach both nuclei. Previous descriptions of the projections from LDTg indicate that AV is the preferred target, with appreciably lighter inputs to AM (Shibata, 1992). The same relative distribution was found in the present study (Table 2), although the numbers of double labelled cells shows that the AM input is not insignificant, and of these AM inputs approximately 50% also project to AV. While many of the inputs to the ATN have a weak contralateral contribution, the LDTg input was distinctive as the contralateral input was unusually prominent, though still less than the ipsilateral input (see also Shibata, 1992). All of these properties suggest that this cholinergic input does not convey high resolution information, but rather has a nonselective role across ATN that presumably involves modulating its activity and plasticity (Tsanov et al., 2011c,d). The importance of the cholinergic projections from LDTg for ATN function is underlined by the finding that infusions of scopolamine into the AV region are sufficient to impair radial-arm maze performance (Mitchell et al., 2002), i.e., these LDTg inputs are vital for normal spatial memory.
With the exception of LDTg, these anatomical findings support the notion of parallel, segregated functions across the rodent anterior thalamic nuclei. This conclusion is, however, an oversimplification. The present study has treated AV and AM as unified nuclei, yet there is both cytoarchitectonic and connectional evidence to signify distinct subregions within each of these thalamic nuclei (Cruce, 1975; Seki and Zyo, 1984; Shibata, 1992, 1993). Consequently, there is the potential for further levels of system separation. The potential levels of separation become all the more complex given that those projections to the anterior thalamic nuclei and the mammillary bodies that arise from the same structures are also segregated, with cells that terminate in both structures being very rare (Ishizuki, 2001; Wright et al., 2010). This arrangement is informative given the density of the mammillary body inputs to the anterior thalamic nuclei and their complex topography. One source of evidence concerning the extent to which AV and AM process distinct information types comes from electrophysiological studies. Here, different properties are observed in the two nuclei but these differences are rarely absolute. Responsiveness to head direction is a prominent electrophysiological phenotype of some cells in AV (Tsanov et al., 2011a). Furthermore, units in AV spike rhythmically in the theta frequency range (Vertes et al., 2001; Tsanov et al., 2011b). Spectral power analyses of activity in AV coheres with hippocampal theta rhythm (Albo et al., 2003; Tsanov et al., 2011b), suggesting that the anterior thalamus is a functional component of limbic theta processing.
The functional implications of the present findings derive from the ways in which AV and AM share both partially overlapping inputs, e.g., from frontal and cingulate cortices, along with inputs that are more topographically segregated, e.g., from the subiculum and mammillary bodies (Fig. 7). The latter inputs, from the subiculum and mammillary bodies, stand out as the most directly linked to memory processes (Delay and Brion, 1969; Vann and Aggleton, 2004; Tsivilis et al., 2008; Vann, 2010; Carlesimo et al., 2011). The subiculum has dense projections to both the anterior thalamic nuclei and mammillary bodies, but the cells of origin are largely segregated by laminar (Ishizuki, 2001; Aggleton et al., 2005) and so do not arise from common cells (Wright et al., 2010). As a consequence, there is the potential for direct, segregated information streams to AV and AM from the subiculum that remain in step with indirect streams that are also segregated (via the mammillary bodies). This apparent duplication might be explained by the contrasting electrophysiological properties within the anterior thalamic nuclei associated with the direct and indirect input routes from the hippocampus (Aggleton et al., 2010; Tsanov et al., 2011c). One possible consequence is that the slight lag in processing created by the mammillary body relay might help to mark or enhance any coincident signal from both sites (hippocampus and mammillary bodies). Such a mechanism could also be linked to the presence of theta in the anterior thalamic nuclei, which is most evident within AV (Vertes et al., 2001; Albo et al., 2003; Tsanov et al., 2011b), along with the inputs to the mammillary bodies from the ventral tegmental nucleus of Gudden (Vann, 2009). The net result might be a regulation of consolidation.
The properties of these more separated inputs to the anterior thalamic nuclei (from the mammillary bodies and hippocampus) can be contrasted with those from more intermingled cortical inputs (frontal and cingulate areas). The functions of these cortical areas in rats have been linked to more general modes of interaction involving central set, response flexibility, and temporal processing (e.g., Birrell and Brown, 2000; Dias and Aggleton, 2000; Barker et al., 2007). Behavioral studies have shown that anterior thalamic lesion in rats can impair tasks that tax these same attributes (Beracochea et al., 1989; Aggleton et al., 1991; Wolff et al., 2006; but see Chudasama et al., 2001), pointing to ways in which these cortico-thalamic interconnections might be explored in future studies. The more specific proposal that the anterior medial nucleus is part of a ‘feed-forward’ system focussed on frontal interactions while the anterior ventral nucleus is part of a ‘return-loop’ system that regulates hippocampal function (Aggleton et al., 2010), is clearly supported by the arrangement of respective connections, although it is also evident that any such distinction is very unlikely to be absolute.
In summary, a consideration of the present findings shows that while individual neurons do not typically project to both AV and AM, there are inputs to these thalamic nuclei from some sites (e.g., frontal cortices) that appear highly intermingled. Such areas could, but need not, exert common influences across both nuclei. In contrast, other inputs are more clearly separated e.g., those from the hippocampal and mammillary bodies, and so suggest more overt information separation (Fig. 7). The scarcity of cortical or limbic inputs that innervate both AV and AM underlines the potential for these nuclei to have distinct roles in supporting learning and memory. The presence of direct inputs to the anterior thalamic nuclei that are not shared by the hippocampus proper or subiculum, e.g., from the mammillary bodies, prefrontal and cingulate cortex, also highlights ways in which the anterior thalamus might modify and enhance hippocampal processes involved in memory.
Table 1.
List of abbreviations
| ACA - anterior cingulate cortex |
| AD - anterodorsal thalamic nucleus |
| AM - anteromedial thalamic nucleus |
| AV - anteroventral thalamic nucleus |
| CA1 - hippocampus field CA1 |
| CTB – cholera toxin B |
| dSuB - dorsal subiculum |
| DTg - dorsal tegmental nucleus of Gudden |
| DY – diamidino yellow |
| FB – fast blue |
| IAM - interanteromedial nucleus |
| IL - infralimbic cortex |
| LDTg - laterodorsal tegmental nucleus |
| LM - lateral mammillary nucleus |
| MD - mediodorsal thalamic nucleus |
| lMM - lateral part of the medial mammillary nucleus |
| mMM - medial part of the medial mammillary nucleus |
| MOp - primary motor cortex |
| MOs - secondary motor cortex |
| PARA - parasubiculum |
| POST - postsubiculum |
| PRE - presubiculum |
| PrL - prelimbic cortex |
| Rdg - dsygranular retrosplenial cortex |
| Rgb - granular retrosplenial cortex b |
| Rga - granular retrosplenial cortex a |
| RTN – reticular nucleus of the thalamus |
| SUB - subiculum |
| VISam - anteromedial visual cortex |
| WGA – wheat germ agglutin |
Acknowledgments
Grant Sponsor: Wellcome Trust; Grant number #092480
References
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res. 1996;81:189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Keith AB, Sahgal A. Both fornix and anterior thalamic, but not mammillary, lesions disrupt delayed nonmatching-to-position memory in rats. Behav Brain Res. 1991;44:151–161. doi: 10.1016/s0166-4328(05)80020-8. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Saunders RC. The relationships between temporal lobe and diencephalic structures implicated in anterograde amnesia. Memory. 1997;5:49–71. doi: 10.1080/741941143. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Albo Z, Viana Di Prisco G, Vertes RP. Anterior thalamic unit discharge profiles and coherence with hippocampal theta rhythm. Thalamus Related Systems. 2003;2:133–144. [Google Scholar]
- Amin E, Wright N, Poirier GL, Thomas KL, Erichsen JT, Aggleton JP. Selective lamina dysregulation in granular retrosplenial cortex (area 29) after anterior thalamic lesions: An in situ hybridization and trans-neuronal tracing study in rats. Neuroscience. 2010;169:1255–1267. doi: 10.1016/j.neuroscience.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beracochea DJ, Jaffard R, Jarrard LE. Effects of anterior or dorsomedial thalamic ibotenic lesions on learning and memory in rats. Behav Neural Biol. 1989;51:364–376. doi: 10.1016/s0163-1047(89)91000-5. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byatt G, Dalrymple-Alford JC. Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav Neurosci. 1996;110:1335–1348. doi: 10.1037//0735-7044.110.6.1335. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia. 2011;49:777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Cruce JA. An autoradiographic study of the projections of the mammillothalamic tract in the rat. Brain Res. 1975;85:211–219. doi: 10.1016/0006-8993(75)90072-4. [DOI] [PubMed] [Google Scholar]
- Delay J, Brion S. Le Syndrome de Korsakoff. Masson; Paris: 1969. [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Wyss JM. Evidence for some collateralization between cortical and diencephalic efferent axons of the rat subicular cortex. Brain Res. 1983;259:181–192. doi: 10.1016/0006-8993(83)91249-0. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123:141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Retrograde double-labeling study of the mammillothalamic and the mammillotegmental projections in the rat. J Comp Neurol. 1989;284:1–11. doi: 10.1002/cne.902840102. [DOI] [PubMed] [Google Scholar]
- Hopkins DA. Neuroanatomy of head direction cell circuits. In: Wiener SI, Taube JS, editors. Head direction cells and the neural mechanisms of spatial orientation. MIT Press; Cambridge, MA: 2005. pp. 17–44. [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol. 2001;435:89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Witter MP, Van Haeften T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J Comp Neurol. 2003;455:156–171. doi: 10.1002/cne.10472. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC, Christie MA. Spatial working memory and the brainstem cholinergic innervation to the anterior thalamus. J Neurosci. 2002;22:1922–1928. doi: 10.1523/JNEUROSCI.22-05-01922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Namura S, Takada M, Kikuchi H, Mizuno N. Topographical organization of subicular neurons projecting to subcortical regions. Brain Res Bull. 1994;35:221–231. doi: 10.1016/0361-9230(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Olszewski I. The thalamus of Macaca mulatta. S.Karger; Basel: 1952. [Google Scholar]
- Seki M, Zyo K. Anterior thalamic afferents from the mamillary body and the limbic cortex in the rat. J Comp Neurol. 1984;229:242–256. doi: 10.1002/cne.902290209. [DOI] [PubMed] [Google Scholar]
- Shibata H. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J Comp Neurol. 1993;337:431–445. doi: 10.1002/cne.903370307. [DOI] [PubMed] [Google Scholar]
- Shibata H. Organization of projections of rat retrosplenial cortex to the anterior thalamic nuclei. Eur J Neurosci. 1998;10:3210–3219. doi: 10.1046/j.1460-9568.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- Shibata H. Topographic organization of subcortical projections to the anterior thalamic nuclei in the rat. J Comp Neurol. 1992;323:117–127. doi: 10.1002/cne.903230110. [DOI] [PubMed] [Google Scholar]
- Shibata H, Naito J. Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Res. 2005;1059:93–103. doi: 10.1016/j.brainres.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Chronister RB, White LE., Jr. Origin of the direct hippocampus-anterior thalamic bundle in the rat: a combined horseradish peroxidase-Golgi analysis. Exp Neurol. 1977;57:379–395. doi: 10.1016/0014-4886(77)90074-7. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Priestley JV, Consolazione A, Eckenstein F, Cuello AC. Cholinergic projections from the midbrain and pons to the thalamus in the rat, identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry. Brain Res. 1985;329:213–223. doi: 10.1016/0006-8993(85)90527-x. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. The laminar organization of efferent neuronal cell bodies in the retrosplenial granular cortex. Brain Res. 1987;406:255–269. doi: 10.1016/0006-8993(87)90790-6. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rodriguez AJ. The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behav Brain Res. 1989;32:265–277. doi: 10.1016/s0166-4328(89)80059-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW. Processing the head direction cell signal: a review and commentary. Brain Res Bull. 1996;40:477–484. doi: 10.1016/0361-9230(96)00145-1. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Vann SD, Reilly RB, Erichsen JT, Aggleton JP, O’Mara SM. Theta-modulated head direction cells in the rat anterior thalamus. J Neurosci. 2011a;31:9489–9502. doi: 10.1523/JNEUROSCI.0353-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Wright N, Vann SD, Reilly R, Erichsen JT, Aggleton JP, O’Mara SM. Oscillatory entrainment of thalamic neurons by theta rhythm in freely moving rats. J Neurophysiol. 2011b;105:4–17. doi: 10.1152/jn.00771.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Vann SD, Erichsen JT, Wright N, Aggleton JP, O’Mara SM. Differential regulation of synaptic plasticity of the hippocampal and the hypothalamic inputs to the anterior thalamus. Hippocampus. 2011c;21:1–8. doi: 10.1002/hipo.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Wright N, Vann SD, Erichsen JT, Aggleton JP, O’Mara SM. Hippocampal inputs mediate theta-related plasticity in anterior thalamus. Neuroscience. 2011d;187:52–62. doi: 10.1016/j.neuroscience.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Michael Wyss J. Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behav Brain Res. 2002;132:19–28. doi: 10.1016/s0166-4328(01)00390-4. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol. 1990a;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Res. 1990b;529:165–177. doi: 10.1016/0006-8993(90)90824-u. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990c;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Vann SD. Gudden’s ventral tegmental nucleus is vital for memory: re-evaluating diencephalic inputs for amnesia. Brain. 2009;132:2372–2384. doi: 10.1093/brain/awp175. [DOI] [PubMed] [Google Scholar]
- Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010;48:2316–2327. doi: 10.1016/j.neuropsychologia.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vann SD, Albasser MM. Hippocampal, retrosplenial, and prefrontal hypoactivity in a model of diencephalic amnsesia: Evidence towards an interdpendent subcortical-cortical memory network. Hippocampus. 2009;19:1090–1102. doi: 10.1002/hipo.20574. [DOI] [PubMed] [Google Scholar]
- Vann SD, Saunders RC, Aggleton JP. Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys. The Eur J Neurosci. 2007;26:1575–1586. doi: 10.1111/j.1460-9568.2007.05773.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Albo Z, Viana Di Prisco G. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez’s circuit. Neuroscience. 2001;104:619–625. doi: 10.1016/s0306-4522(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP. Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci. 2000;12:1714–1726. doi: 10.1046/j.1460-9568.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird A, Morgan A, Muir JL, Aggleton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. J Neurosci. 2001;21:7323–7330. doi: 10.1523/JNEUROSCI.21-18-07323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174:251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. Heterogeneity in the Dorsal Subiculum of the Rat. Distinct Neuronal Zones Project to Different Cortical and Subcortical Targets. Eur J Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Dalrymple-Alford JC. Beyond spatial memory: the anterior thalamus and memory for the temporal order of a sequence of odour cues. J Neurosci. 2006;26:2907–2913. doi: 10.1523/JNEUROSCI.5481-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Erichsen JT, Vann SD, O’Mara SM, Aggleton JP. Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. J Comp Neurol. 2010;518:2334–2354. doi: 10.1002/cne.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Projections to the anterodorsal thalamus and lateral mammillary nuclei arise from different cell populations within the postsubiculum: implications for the control of head direction cells. Hippocampus. 2011;21:1062–1073. doi: 10.1002/hipo.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]