Abstract
Introduction: Biomarkers represent a promising adjunct to clinical techniques in the diagnosis of Alzheimer’s Disease (AD) and other neurodegenerative diseases. At present, the potential of cerebrospinal fluid (CSF) biomarkers in diagnosing AD has been suggested but the degree of clinical utility is yet to be defined due to variability between studies. In this paper we compare the performance of two cerebrospinal fluid assay methods in predicting clinically diagnosed AD. Methods: CSF biomarker concentrations for Aβ1-42, P-tau181P and T-tau were analysed using INNOTEST (ELISA) and INNO-BIA AlzBio3 (Luminex) assay methods from Innogenetics, Belgium. Patients were clinically diagnosed based on NINCDS-ADRDA criteria supplemented with structural MRI, 18F-fluorodeoxy-glucose positron emission tomography (FDG-PET) and cognitive profiling. Results: An abnormally low Aβ1-42 was the most useful biomarker in predicting clinical AD. Depending on the assay method, the predictive accuracy remained constant or improved slightly when abnormalities in P-tau181P and T-tau were considered in addition to Aβ1-42. The Luminex method with our optimised reference concentrations performed best for patients ≤ 65 years with sensitivity = 1 and a specificity = 0.60 for both Aβ1-42 and when one or more abnormal biomarkers were considered. Conclusion: Given accurate, robust and reproducible CSF analytical methods, of which the Luminex method seems the most useful and practicable, our investigation suggests that measuring CSF Aβ1-42, P-tau and T-tau has utility in the diagnosis of probable AD and, when used with clinical diagnostic techniques, seems especially helpful in the diagnosis of AD with onset prior to the age of 65 years.
Keywords: Alzheimer’s disease, cerebrospinal fluid, amyloid beta, tau, P-tau, T-tau, biomarkers, clinical diagnosis
Introduction
Alzheimer’s Disease (AD) is the most common cause of dementia and is therefore a major social and health concern in an aging population [1]. Cerebrospinal fluid (CSF) biomarker analysis provides the potential to facilitate accurate and rapid diagnosis of clinical AD [2,3]. In addition, CSF biomarker research has shown the potential to diagnose pre-symptomatic and prodromal states important for early intervention, developing therapeutics [4], and to investigate the pathophysiology of AD [5].
AD is characterised pathologically by extracellular Aβ plaques, intracellular neurofibrillary tangles (NFTs) and neuronal loss [2]. However, it is not the only cause of dementia that expresses these pathological hall marks, making differentiation between neurodegenerative diseases difficult. Other neurodegenerative pathologies such as Lewy-body dementia (LBD) and Parkinson’s Disease dementia (PDD) also can exhibit Aβ plaques and NFTs [6]. In addition, the specificity of Aβ plaques and NFTs for AD decreases with age due to their increasing prevalence in asymptomatic individuals [7,8]. Whether this is due to compensatory cognitive capacity or an undetected pathology unique to AD is yet to be determined [8]. Diagnosis of AD is obscured further by coexistent non-Alzheimer’s disease (NAD), which is present in a significant proportion of dementia patients [9], making it difficult to differentiate clinically [10]. This clinical and pathological overlap of neurodegenerative disease is a problem for clinicians as accurate and early diagnosis is necessary for appropriate management and recommendation of symptomatic treatment. In addition, accurate diagnosis will help to reduce the costs and burden of unnecessary investigations, the risks of medication errors [11], and alleviate patient concerns with regard to prognosis [12]. Currently, clinical work-up combined with imaging techniques diagnoses AD dementia with an accuracy of 80-90% [12,13].
Non-clinical techniques such as CSF analysis and neuroimaging have been shown to improve diagnostic accuracy of AD dementia and other neurodegenerative diseases when used in addition to clinical diagnosis [14,15]. The attention given to CSF biomarkers relates to the highly communicable and rapid exchange between brain interstitial fluid and the CSF [15], and the high association with neuronal pathology [2]. The characteristic CSF biomarkers of low amyloid-β1-42 (Aβ1-42), high phosphorylated-tau at the threonine 181 position (P-tau181P), and high total-tau (T-tau) are highly associated with AD pathology [2], and have been found to be the most consistent diagnostic markers of AD [1,6,16,17], their predictive accuracy increasing when used in combination [2,12]. On confirming dementia via autopsy, combinations of these markers have been shown to have sensitivities and specificities consistently > 80% in discriminating AD dementia from controls and NAD dementia [13].
The potential of biomarkers in diagnosing the prodromal stages of AD has also been investigated [18-20]. CSF Aβ1-42 and tau concentrations have been shown to reliably reflect prodromal AD pathology [20] and predict conversion from mild cognitive impairment (MCI) to AD dementia [21]. MRI has also been shown to detect prodromal AD [22], and when used in association with CSF biomarkers and neuropsychological evaluation, has been shown to predict the conversion of MCI to AD dementia more accurately than each modality in isolation [23].
Diagnosing pre-symptomatic and prodromal AD will be critical once disease modifying therapeutics are developed to prevent irreversible disease and neuronal loss [12,24]. Although Aβ1-42, P-tau181P and T-tau have been found to be associated with presymptomatic disease [20], the low prevalence of AD in the presymptomatic population will limit the predictive values and therefore the clinical value of such a diagnostic test [18].
The diagnostic abilities of CSF biomarkers are widely accepted. However, the accuracy of CSF biomarkers and other biomarkers in predicting AD has been variable amongst studies [15], making their absolute utility uncertain [15]. Lack of standardized laboratory technique and inconsistencies in assay kit performance between studies has resulted in the variability of absolute values of CSF analytes and contributed to study comparability issues [2,3]. Inter-study comparison has been obscured further by variability in ages and characteristics of patients enrolled in studies, variability in clinical and pathological diagnostic criteria [24,25], and the presence of other diseases possessing pathological overlap with AD [9]. Efforts continue to investigate the underlying issues creating these discrepancies and to standardise sample handling, laboratory methods and vendor calibration, in order to harmonise clinical data [2,26].
Noting the degree of inter-study variability in biomarker performance, we set out to investigate the utility of two CSF analysis methods at our Neurodegenerative Disorders Research clinic and associated laboratory. Using ELISA and Luminex methods, we investigated the accuracy of Aβ1-42, P-tau181P and T-tau CSF concentrations in predicting clinical AD in a population of AD and NAD dementia patients. For the ELISA method, we employed the manufacturer’s reference concentrations. For the Luminex method we calculated our own reference concentrations: first, via linear regression using the manufacturer’s values for the ELISA method (Luminex with linear regression derived reference concentrations, LLRRC) and secondly, via optimisation of the reference concentrations to provide the greatest test sensitivity in predicting clinical AD (Luminex with optimised reference concentrations, LORC). To our knowledge, no previous studies have compared the Luminex and ELISA methods in the diagnosis of AD.
Methods and materials
AD and NAD dementia patients from our neurology research clinic were diagnosed using accepted NINCDS-ADRDA criteria supplemented with structural MRI, (18)F-fluorodeoxy-glucose positron emission tomography (FDG-PET) and cognitive profiling. Following Institutional Review Board approval, lumbar punctures were requested in 73 patients. Figure 1 outlines the enrolment of the final 54 patients used for CSF analysis. Of the 54 patients, 44 patients had a clinical diagnosis of AD and 10 control patients had various NAD neurodegenerative disorders including MCI (n = 2), fronto-temporal dementia (FTD; n = 3), LBD (n = 1), posterior-cortical atrophy syndrome (PCAS; n = 1) and vascular cognitive impairment (VCI; n = 1) (Figure 1). There were 31 patients with AD and 5 without at > 65 years, and 13 with AD and 5 without at ≤ 65 years. For the Luminex analysis there were two non-results in the AD group, both over 65 years, reducing the number of AD patients to n = 42. Patient demographics are shown in Table 1.
Figure 1.
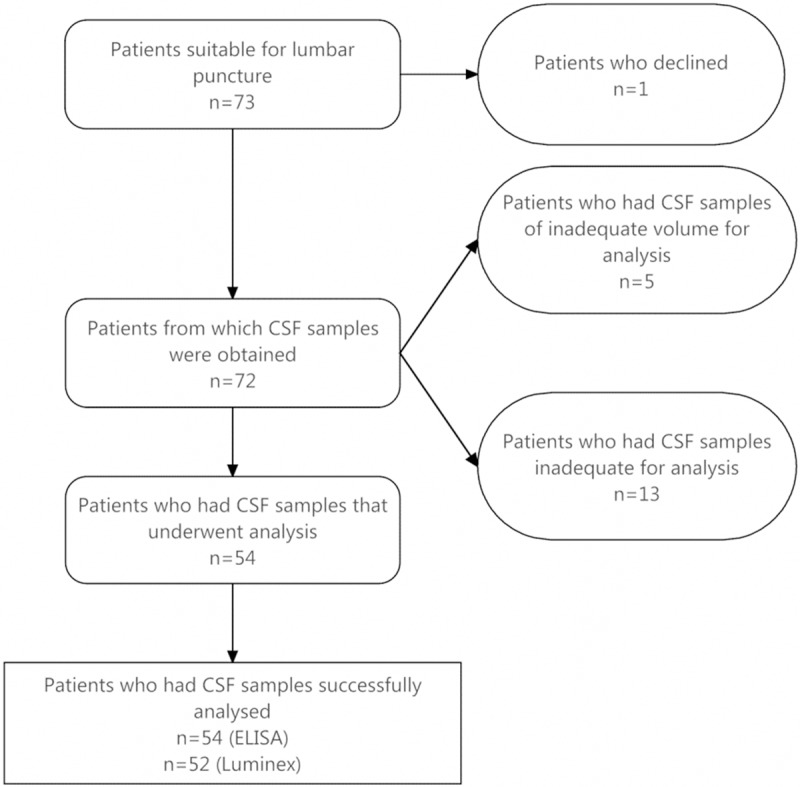
Flowchart of patient enrolment for CSF analysis.
Table 1.
Patient demographics
| Age of Diagnosis | N | N (male:female) | Mean (yrs) | Std. Dev. (yrs) | Range (yrs) |
|---|---|---|---|---|---|
| AD ≤ 65 | 13 | 7:6 | 60.3 | 4.4 | 51-65 |
| AD > 65 | 31 | 22:9 | 74.2 | 7.2 | 66-101 |
| Non-AD | 10 | 5:5 | 68.1 | 8.3 | 52-99 |
The two CSF biomarker analysis methods were the ELISA and Luminex assay methods both from Innogenetics (Gent, Belgium). The ELISA method uses a separate assay for each of the CSF biomarkers: Aβ1-42, P-tau181P and T-tau. The Luminex is a single assay, measuring all three biomarkers using colour coded microsphere beads bound to monoclonal antibodies.
Various combinations of the three biomarkers and each biomarker individually were analysed in their ability to predict the clinical diagnosis of AD. Combinations have been designated A, B and C. Combination A consists of a combination of one or more abnormal biomarkers; Combination B consists of 2 or 3 abnormal biomarkers; Combination C consists of 3 abnormal biomarkers. The performance of each biomarker profile was investigated for all patients and also for patients aged ≤ 65 years and > 65 years.
All statistics and linear regression models were calculated using Graphpad Prism 6.0. In comparing clinical diagnosis and abnormal biomarker concentrations, 2x2 analyses were conducted with two tailed Fisher’s tests rather than Chi-squared due to the small sample sizes. T-tests with Welch’s correction (variances assumed unequal) were used in comparing the mean CSF protein levels in AD and NAD patients.
The conduct of this study was approved by Neurodegenerative Disorders Research Ethics Advisory Council.
CSF biomarker concentrations for ELISA and Luminex assay methods
The CSF biomarker concentrations for the ELISA and Luminex analyses are shown in Figure 2. Differences in mean biomarker concentrations for AD and NAD groups for the entire patient sample were most statistically significant for Aβ1-42: the ELISA and Luminex methods had P-values of 0.06 and 0.08 respectively. This was mirrored in the strong correlation of Aβ1-42 with clinical AD. For P-tau181P and T-tau, statistical significance between the difference of means for AD and NAD patients was weaker (p > 0.20) for both ELISA and Luminex methods, reflected by the reduced accuracy of these markers in predicting clinical AD and the limited improvement in diagnostic accuracy when used in combination with Aβ1-42. Mean ages in AD and NAD groups were assumed equivalent with p > 0.35 for all age groups. Age dependent variations in biomarker concentrations were therefore assumed to be minimal.
Figure 2.
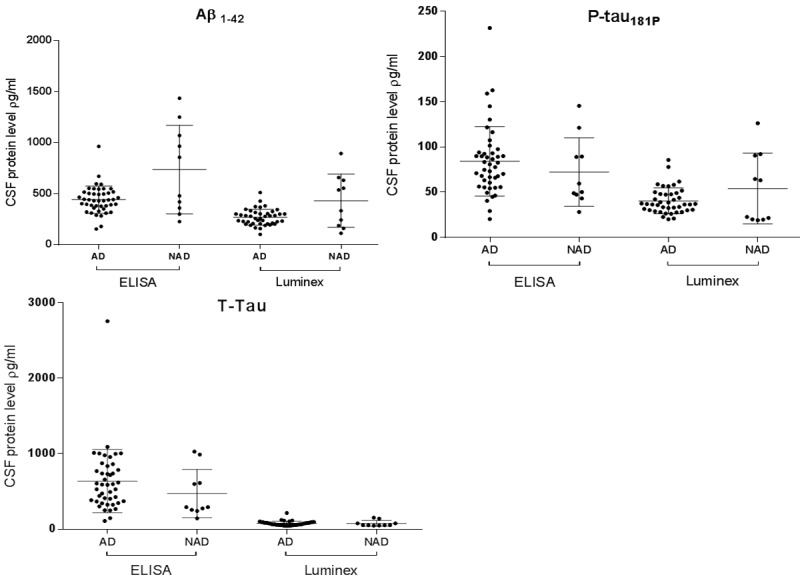
CSF biomarker concentrations for ELISA and Luminex assay methods for all patients. Horizontal lines represent mean ± standard deviation.
Calculation of biomarker reference concentrations
For the ELISA method the reference concentrations used were based on manufacturer’s values (Table 2). For the Luminex method, values were not provided by the manufacturer so were deduced via linear regression (LLRRC) (Figure 3). In addition, optimising the Luminex reference concentrations to produce the greatest sensitivity in predicting clinical AD were then carried out (LORC). A summary of the reference concentrations used is outlined in Table 2. Below the lower limit for Aβ1-42 and above the upper limits for P-tau181P and T-tau were considered abnormal and characteristic of AD pathology [12].
Table 2.
CSF reference concentrations ρg/mL
| Method | Age (years) | Aβ1-42 Lower limit | T-tau Upper limit | P-tau181P Upper limit |
|---|---|---|---|---|
| ELISA | 51-70 | 562 | 370 | 66.3 |
| > 71 | 567 | 512 | ||
| 45-77 | 66.3 | |||
| > 77 | 66.3† | |||
| LLRRC | All ages‡ | 332 | 65 | 39 |
| LORC | All ages‡ | 513 | 55 | 23 |
Estimated value as manufacturer only provided reference concentration for 45-77 years.
Reference concentrations approximated for all ages.
Figure 3.
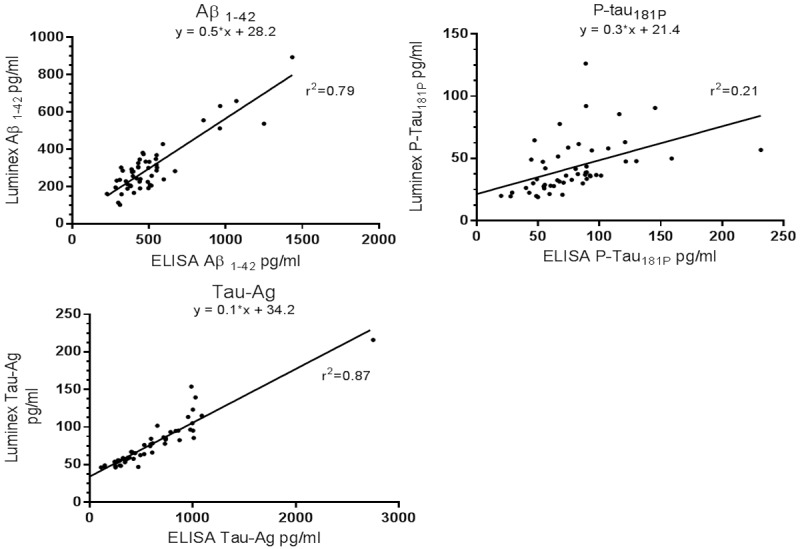
Linear regression: relationship between ELISA and Luminex CSF biomarker concentrations.
Results
Of the 44 clinically diagnosed AD and 10 NAD patients of which CSF analysis was appropriate, CSF samples were analysed using the ELISA and Luminex assays for Aβ1-42, P-tau181P and T-tau concentrations. The ability of a combination of abnormal biomarker concentrations and abnormal single biomarker concentrations in predicting clinical AD was calculated by 2x2 analyses for ELISA, LORC and LLRRC methods. This was conducted for all patients and then for patients ≤ 65 and > 65 years.
Biomarker performance in predicting clinical AD
For all patients, LORC performed best of the three methods, and equally for both Combination A (Table 3, Row 3) and a single low Aβ1-42 (Table 4, Row 3), while a single high P-tau181P performed slightly less in predicting clinical AD (Table 4, Row 3). The performance of T-tau was poorer in predicting clinical AD (Table 4, Row 3). Predictive accuracy decreased for Combination B, and then further for Combination C for all three methods (Table 3, Rows 1, 2 and 3).
Table 3.
Performance of a biomarker panel in predicting AD. Sn, sensitivity; Sp, specificity; +LR, positive likelihood ratio; p, P-value
| Biomarker combination | A | B | C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Row | Sn | Sp | +LR | p | Sn | Sp | +LR | p | Sn | Sp | +LR | p | |
| All patients | |||||||||||||
| 1 | ELISA | 0.95 | 0.50 | 1.90 | * | 0.68 | 0.60 | 1.70 | 0.57 | 0.60 | 1.43 | ||
| 2 | LLRRC | 0.88 | 0.50 | 1.76 | * | 0.65 | 0.50 | 1.30 | 0.24 | 0.70 | 0.80 | ||
| 3 | LORC | 1 | 0.50 | 2.00 | * | 0.98 | 0.50 | 1.96 | * | 0.76 | 0.60 | 1.90 | * |
| Patients < 65 yrs | |||||||||||||
| 4 | ELISA | 1 | 0.60 | 2.50 | * | 0.62 | 0.60 | 1.55 | 0.62 | 0.60 | 1.55 | ||
| 5 | LLRRC | 0.92 | 0.60 | 2.30 | * | 0.70 | 0.60 | 1.75 | 0.23 | 0.60 | 0.58 | ||
| 6 | LORC | 1 | 0.60 | 2.50 | * | 1 | 0.60 | 2.50 | * | 0.77 | 0.60 | 1.93 | |
| Patients > 65 yrs | |||||||||||||
| 7 | ELISA | 0.94 | 0.40 | 1.57 | 0.70 | 0.60 | 1.75 | 0.55 | 0.60 | 1.38 | |||
| 8 | LLRRC | 0.86 | 0.40 | 1.43 | 0.59 | 0.40 | 0.98 | 0.24 | 0.80 | 1.20 | |||
| 9 | LORC | 1 | 0.40 | 1.67 | * | 0.97 | 0.40 | 1.62 | * | 0.76 | 0.60 | 1.90 | |
p < 0.05.
Table 4.
Performance of a single biomarker in predicting AD
| Single biomarker | Aβ1-42 | P-tau | T-tau | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Sn | Sp | +LR | p | Sn | Sp | +LR | p | Sn | Sp | +LR | p | ||
| Row | |||||||||||||
| All patients | |||||||||||||
| 1 | ELISA | 0.91 | 0.50 | 1.80 | * | 0.68 | 0.60 | 1.70 | 0.61 | 0.60 | 1.53 | ||
| 2 | LLRRC | 0.81 | 0.60 | 2.02 | * | 0.38 | 0.50 | 0.76 | 0.46 | 0.60 | 1.14 | ||
| 3 | LORC | 1 | 0.50 | 2.00 | * | 0.95 | 0.50 | 1.90 | * | 0.79 | 0.56 | 1.77 | |
| Patients < 65 yrs | |||||||||||||
| 4 | ELISA | 1 | 0.60 | 2.50 | * | 0.62 | 0.60 | 1.54 | 0.62 | 0.60 | 1.54 | ||
| 5 | LLRRC | 0.92 | 0.60 | 2.30 | * | 0.31 | 0.60 | 0.77 | 0.62 | 0.60 | 1.54 | ||
| 6 | LORC | 1 | 0.60 | 2.50 | * | 1 | 0.60 | 2.50 | * | 0.77 | 0.60 | 1.92 | |
| Patients > 65 yrs | |||||||||||||
| 7 | ELISA | 0.87 | 0.40 | 1.45 | 0.71 | 0.60 | 1.77 | 0.61 | 0.60 | 1.53 | |||
| 8 | LLRRC | 0.76 | 0.60 | 1.90 | 0.41 | 0.40 | 0.69 | 0.52 | 0.60 | 1.29 | |||
| 9 | LORC | 1 | 0.40 | 1.67 | * | 0.93 | 0.40 | 1.55 | 0.79 | 0.60 | 1.98 | ||
p < 0.05.
For patients ≤ 65 years, clinical AD was predicted most accurately. Aβ1-42 (Table 4, Row 6) and Combination A (Table 3, Row 6) for both ELISA and LORC and p-tau (Table 4, Row 6) for LORC were equivalent in predicting clinical AD and performed best overall in this study. A high P-tau was significantly weaker in predicting clinical AD for ELISA and LLRRC (Table 4, Rows 4 and 5). High T-tau performed weakest out of the three biomarkers (Table 4, Rows 4, 5 and 6). Combinations B and C generally had reduced predictive accuracy for all methods (Table 3, Rows 4, 5 and 6). An exception was Combination B using LORC (Table 3, Row 6), performing equivalently to Combination A, due to the high correlation of both Aβ1-42 and P-tau181P for LORC (Table 4, Row 6).
The methods evaluated in the present study were least accurate for patients > 65 years, as shown in Tables 3 and 4. However, using LORC, a single abnormally high T-tau performed at its best in the study (Table 4, Row 9).
In summary, LORC predicted clinical AD equivalently or more accurately than the ELISA method for all biomarker combinations and single biomarkers. LLRRC was the least accurate in predicting clinical AD. Overall, Combination A was equivalent or slightly better than a single low Aβ1-42 in predicting clinical AD, performing the most accurately out of all the biomarker combinations considered. An exception was a high P-tau181P, using LORC for patients ≤ 65 years, which performed equivalently. Overall, an abnormally high P-tau181P or high T-tau predicted clinical AD less accurately, and had a relatively small contribution to predictive accuracy when in combination with Aβ1-42. Predictive accuracy was reduced for Combinations B and C as the requirement for two or more and three abnormal biomarkers respectively restricted the biomarker profiles that could be considered abnormal. The predictive accuracy of clinical AD was greatest for patients ≤ 65 years, the accuracy decreasing when the entire patient sample was considered, the predictive accuracy poorest for patients > 65 years.
Discussion
Within the present study we identify low Aβ1-42 as the most useful biomarker in predicting clinical AD, the predictive accuracy remaining constant or increasing only slightly when the other biomarkers and their possible permutations were considered. However, the various degrees of tauopathy and amyloidopathy that constitute the heterogeneous nature of AD [10,20,27-29] will not be accounted for by a single CSF biomarker. The superior performance of Aβ1-42 in predicting clinical AD in our sample should not discourage measuring P-tau and T-tau, as it might help in understanding the heterogeneity of AD and response to treatment [16]. The reference concentrations and assay method were also responsible for the diagnostic findings: ELISA showed a high correlation of Aβ1-42 with clinical AD; while LORC, in addition to a high correlation with abnormal Aβ1-42, had a more significant correlation with the other biomarkers. Multiple biomarkers that account for the CSF correlates of the amyloidopathy and tauopathy of AD will not only increase the ability to detect various pathological phenotypes of AD but also distinguish between AD and other neurodegenerative disease having pathological overlap. CSF biomarkers such as α-synuclein and neurofilament light chain, when used in addition to Aβ1-42, P-tau181P and T-tau, and determination of the tau/Aβ ratio might further facilitate differentiation between AD and other neurodegenerative diseases such as Parkinson’s Disease (PD), PDD, LBD and Parkinsonian disorders [6,16].
The Luminex method has been used in CSF analyses to understand its diagnostic usefulness [2,30]. The performance of the Luminex technique in this study revealed an increased speed of laboratory turn-around time of 2 days (as compared to 6 days for the ELISA method), and a reduced sample volume both of which make the Luminex method, in our experience, the forerunner in CSF analysis for the diagnosis of AD.
Our study found a better correlation between biomarkers and clinical AD in patients ≤ 65 years, which is in line with the increase of these biomarkers with age in normal adults [7,8] and are thus more favourable in younger populations for ruling in AD [8], where the diagnosis of AD can be more difficult and challenging [31]. However, careful selection of age dependent reference concentrations may improve clinical utility in older patients [8].
There were other limitations in this study. Specificities in predicting the clinical diagnosis of AD may have been underestimated if there was a failure to detect co-existent or incipient AD in the NAD group. The small sample size of this study, particularly the NAD group must be considered when interpreting our findings and will affect the generalizability of test accuracy and reference concentrations. The potential for inter-assay variability must also be taken into account when considering these results. A larger population of AD and NAD patients needs to be studied to confirm our results, without selection bias, and confirmed by post-mortem analysis.
Conclusion
Since clinical diagnosis is not definitive for AD, this study does not give an absolute performance of these biomarkers in predicting AD but confirms their utility in supporting a diagnosis of probable AD, especially in younger adults with onset of AD prior to 65 years of age. Given robust and reliable CSF analysis, these CSF biomarkers will probably be helpful in achieving a more accurate diagnosis in uncertain clinical situations [13] and to enhance our understanding of the causes of dementia in individual patients. CSF proteomic studies in dementia might be useful in predicting responses to particular therapies like monoclonal antibodies to Aβ.
Acknowledgements
This research was funded by Neurodegenerative Disorders Research Pty Ltd.
Disclosure of conflict of interest
The authors declare that they have no competing interests.
References
- 1.Hampel H, Lista S, Khachaturian ZS. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 2012;8:312–36. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 2.Kang JH, Vanderstichele H, Trojanowski JQ, Shaw LM. Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer’s disease. Methods. 2012;56:484–93. doi: 10.1016/j.ymeth.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Mattsson N, Zetterberg H. Alzheimer’s disease and CSF biomarkers: key challenges for broad clinical applications. Biomark Med. 2009;3:735–7. doi: 10.2217/bmm.09.65. [DOI] [PubMed] [Google Scholar]
- 4.Perrin RJ. Cerebrospinal fluid biomarkers for clinical trials: why markers for differential diagnosis are important. Arch Neurol. 2012;69:1407–8. doi: 10.1001/archneurol.2012.2353. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Lista S. Use of biomarkers and imaging to assess pathophysiology, mechanisms of action and target engagement. J Nutr Health Aging. 2013;17:54–63. doi: 10.1007/s12603-013-0003-1. [DOI] [PubMed] [Google Scholar]
- 6.Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Håkan W, Decraemer H, Någga K, Minthon L, Londos E, Vanmechelen E, Holmberg B, Zetterberg H, Blennow K, Hansson O. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–52. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 7.Paternicò D, Galluzzi S, Drago V, Bocchio-Chiavetto L, Zanardini R, Pedrini L, Baronio M, Amicucci G, Frisoni GB. Cerebrospinal fluid markers for Alzheimer’s disease in a cognitively healthy cohort of young and old adults. Alzheimers Dement. 2012;8:520–7. doi: 10.1016/j.jalz.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Mattsson N, Rosén E, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek MM, Olde Rikkert M, Tsolaki M, Mulugeta E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Wallin A, Eriksdotter-Jönhagen M, Minthon L, Winblad B, Blennow K, Zetterberg H. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–76. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger KA. Can neuropathology really confirm the exact diagnosis? Alzheimers Res Ther. 2010;2:11. doi: 10.1186/alzrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, GomezTortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 12.Lewczuk P, Kornhuber J. Neurochemical dementia diagnostics in Alzheimer’s disease: where are we now and where are we going? Expert Rev Proteomics. 2011;8:447–58. doi: 10.1586/epr.11.37. [DOI] [PubMed] [Google Scholar]
- 13.Engelborghs S, De Vreese K, Van de Casteele T, Vanderstichele H, Van Everbroeck B, Cras P, Martin JJ, Vanmechelen E, De Deyn PP. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29:1143–59. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Lista S, Garaci FG, Ewers M, Teipel S, Zetterberg H, Blennow K, Hampel H. CSF Aβ1-42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer’s disease. Alzheimers Dement. 2014;10:381–92. doi: 10.1016/j.jalz.2013.04.506. [DOI] [PubMed] [Google Scholar]
- 15.Gaugler JE, Kane RL, Johnston JA, Sarsour K. Sensitivity and specificity of diagnostic accuracy in Alzheimer’s disease: a synthesis of existing evidence. Am J Alzheimers Dis Other Demen. 2013;28:337–47. doi: 10.1177/1533317513488910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, Waligórska T, Taylor P, Pan S, Frasier M, Marek K, Kieburtz K, Jennings D, Simuni T, Tanner CM, Singleton A, Toga AW, Chowdhury S, Mollenhauer B, Trojanowski JQ, Shaw LM Parkinson’s Progression Markers Initiative. Association of Cerebrospinal Fluid β-Amyloid 1-42, T-tau, P-tau181, and α-Synuclein Levels With Clinical Features of Drug-Naive Patients With Early Parkinson Disease. JAMA Neurol. 2013;70:1277–87. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson N, Zetterberg H. Future screening for incipient Alzheimer’s disease: the influence of prevalence on test performance. Eur Neurol. 2009;62:200–3. doi: 10.1159/000228591. [DOI] [PubMed] [Google Scholar]
- 19.Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr, Feldman HH, Bokde AL, Alexander GE, Scheltens P, Vellas B, Dubois B, Weiner M, Hampel H North American Alzheimer’s Disease Neuroimaging Initiative (ADNI) Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33:1203–14. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–16. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 22.Adaszewski S, Dukart J, Kherif F, Frackowiak R, Draganski B Alzheimer’s Disease Neuroimaging Initiative. How early can we predict Alzheimer’s disease using computational anatomy? Neurobiol Aging. 2013;34:2815–26. doi: 10.1016/j.neurobiolaging.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Cui Y, Liu B, Luo S, Zhen X, Fan M, Liu T, Wanlin Zhu, Park M, Jiang T, Jin JS. Alzheimer’s Disease Neuroimaging Initiative. Identification of conversion from mild cognitive impairment to Alzheimer’s disease using multivariate predictors. PLoS One. 2011;6:e21896. doi: 10.1371/journal.pone.0021896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, elacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 25.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–73. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, Parnetti L, Perret-Liaudet A, Shaw LM, Teunissen C, Wouters D, Blennow K. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 28.Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113:107–17. doi: 10.1007/s00401-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 29.Tiraboschi P, Sabbagh MN, Hansen LA, Salmon DP, Merdes A, Gamst A, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Alzheimer disease without neocortical neurofibrillary tangles: “a second look”. Neurology. 2004;62:1141–7. doi: 10.1212/01.wnl.0000118212.41542.e7. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira D, Perestelo-Pérez L, Westman E, Wahlund LO, Sarria A, Serrano-Aguilar P. Meta-review of CSF core biomarkers in Alzheimer’s disease: the state-of-the-art after the new revised diagnostic criteria. Front Aging Neurosci. 2014;6:47. doi: 10.3389/fnagi.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panegyres PK, Frencham K. The course and causes of suspected dementia in young adults: A longitudinal study. Am J Alzheimers Dis Other Demen. 2007;22:48–56. doi: 10.1177/1533317506295887. [DOI] [PMC free article] [PubMed] [Google Scholar]


