Abstract
Context:
Fatigue is suggested to be a risk factor for anterior cruciate ligament injury. Fatiguing exercise can affect neuromuscular control and laxity of the knee joint, which may render the knee less able to resist externally applied loads. Few authors have examined the effects of fatiguing exercise on knee biomechanics during the in vivo transition of the knee from non–weight bearing to weight bearing, the time when anterior cruciate ligament injury likely occurs.
Objective:
To investigate the effect of fatiguing exercise on tibiofemoral joint biomechanics during the transition from non–weight bearing to early weight bearing.
Design:
Cross-sectional study.
Setting:
Research laboratory.
Patients or Other Participants:
Ten participants (5 men and 5 women; age = 25.3 ± 4.0 years) with no previous history of knee-ligament injury to the dominant leg.
Intervention(s):
Participants were tested before (preexercise) and after (postexercise) a protocol consisting of repeated leg presses (15 repetitions from 10°–40° of knee flexion, 10 seconds' rest) against a 60% body-weight load until they were unable to complete a full bout of repetitions.
Main Outcome Measure(s):
Electromagnetic sensors measured anterior tibial translation and knee-flexion excursion during the application of a 40% body-weight axial compressive load to the bottom of the foot, simulating weight acceptance. A force transducer recorded axial compressive force.
Results:
The axial compressive force (351.8 ± 44.3 N versus 374.0 ± 47.9 N; P = .018), knee-flexion excursion (8.0° ± 4.0° versus 10.2° ± 3.7°; P = .046), and anterior tibial translation (6.7 ± 1.7 mm versus 8.2 ± 1.9 mm; P < .001) increased from preexercise to postexercise. No significant correlations were noted.
Conclusions:
Neuromuscular fatigue may impair initial knee-joint stabilization during weight acceptance, leading to greater accessory motion at the knee and the potential for greater anterior cruciate ligament loading.
Key Words: knee, anterior cruciate ligament, axial loading
Key Points
After closed chain exercise, participants demonstrated an increase in anterior tibial translation during simulated lower extremity weight acceptance.
Observed alterations of knee biomechanics in a fatigued state may suggest increased anterior cruciate ligament strain during the latter part of the competition.
The anterior cruciate ligament (ACL) is one of the most commonly injured ligaments in the knee.1–4 Injuries to the ACL frequently result from noncontact mechanisms, occurring when the knee is near full extension at the time of foot strike during activities such as landing, cutting, and deceleration-type maneuvers.5 Neuromuscular fatigue has been defined as any exercise-induced loss in the ability to produce force with a muscle or muscle group, involving processes at all levels of the motor pathway between the brain and the muscle.6–8 Furthermore, fatigue has been suggested as a contributing risk factor for noncontact ACL injury9–14 because the risk of noncontact knee injuries appears to increase later in games.15,16 Specifically, prolonged exercise, which contributes to the delayed activation of muscles agonistic to the ACL,13,17 has been suggested to increase risk of knee injury.13
The quadriceps and hamstrings play a critical role in providing dynamic stability of the knee joint during sports activities,18 so various lower extremity fatigue protocols have been used to decrease the force-producing capabilities of these muscles.10,19,20 Commonly, fatigue has been induced using isokinetic exercise protocols.12,14,21,22 However, the true nature of muscle function and its effect on functional knee-joint biomechanics during sporting activity is likely difficult to assess from isolated forms of isometric, concentric, or eccentric contractions. Exercise that results in complete volitional exhaustion of a single muscle or muscle group rarely occurs during functional activity. Therefore, fatigue protocols that involve total lower extremity actions incorporating submaximal stretch-shortening cycles23,24 may better mimic the type of muscular fatigue associated with prolonged weight-bearing activity.
A number of authors23,25,26 have examined the effect of lower extremity muscle fatigue on knee-joint biomechanics during jumping and landing activities. These results suggest that, depending on the fatigue protocol and task used, knee-flexion excursion (KFEXC) may be either decreased or increased postexercise, thus modulating joint stiffness.25,27 These changes in KFEXC appear to primarily depend on the peak knee flexion obtained,11,27 given that little to no change in the initial knee-flexion landing angle has been reported at ground contact in response to fatiguing exercise.9,20 Moran et al28 examined the effect of an incremental treadmill protocol and reported that exercise-induced alterations in tibial peak-impact acceleration were not attributed to changes in the knee angles at foot contact during a drop jump. This suggests that fatiguing exercise does not alter the initial knee-position angle at ground contact, but it may have a profound effect on knee-joint biomechanics during the weight-acceptance phase of landing. Because ACL injuries typically occur near the time of foot strike1,4 with the knee in shallow flexion (average, 23° of initial knee flexion),29 understanding the effect of fatiguing exercise on knee-joint biomechanics during this early weight-acceptance phase may lend further insight into the role of fatigue in ACL injury mechanisms.
As the knee transitions from non–weight bearing (NWB) to weight bearing (WB), the natural anterior translation of the tibia (ATT) relative to the femur at low knee-flexion angles (eg, 15°–30°)30,31 is restrained by the ACL.31 Greater axial loads30,32,33 and slowing of the quadriceps and hamstrings onset times in response to an anterior tibial load may contribute to increased ATT14 at shallow knee-flexion angles; hence, fatigue may compromise the biomechanics of the tibiofemoral joint during weight acceptance, thereby modifying the strain placed upon the ACL with continued loading and subsequent maneuvers (eg, plant and cut). This may be particularly problematic in landing situations where KFEXC decreases in response to fatiguing exercise.9,25,34 Although decreased KFEXC may represent a compensatory strategy to prevent collapse of the body due to fatigue of the quadriceps muscles,10,34 the reduced KFEXC may increase axial loads at the knee joint, and these greater axial loads may increase the amount of ATT.35
The purpose of our study was to investigate the effects of a lower extremity exercise protocol on tibiofemoral-joint biomechanics as the knee transitioned from NWB to WB in vivo. Based on previous fatigue studies of submaximal total lower extremity actions,9,25 our expectation was that fatiguing exercise would decrease KFEXC, increase axial compressive force (ACF), and subsequently increase ATT during transition from NWB to WB.
METHODS
Participants
Ten participants (5 men and 5 women; age = 25.3 ± 4.0 years, height = 170.9 ± 6.7 cm, weight = 68.5 ± 9.8 kg) who reported no previous history of knee-ligament injury to the preferred stance leg for kicking a ball were recruited. Participants were screened by a certified athletic trainer to ensure they had no current lower extremity orthopaedic dysfunction of the tested limb. Before data collection, all participants reviewed and signed an informed consent form approved by the university's institutional review board, which also approved the study. Before testing, leg length and circumference were recorded and subsequently used for calculating the loads of the counterweight system. Participants then completed a 5-minute warm-up on a stationary bicycle by pedaling at a self-selected pace.
Procedures
The participant was then placed supine in the Vermont Knee Laxity Device (VKLD) (University of Vermont, Burlington, VT), which is designed to measure the amount of ATT when an axial load aligned to the mechanical axis of the shank is applied to the bottom of the foot to simulate WB (Figure 1).36 The dominant foot was strapped to a foot cradle equipped with a 6-degrees-of-freedom force transducer (model MC3A; Advanced Medical Technology, Inc, Watertown, MA) to record axial compressive forces, and the foot cradle was attached to a linear slide with a pulley system attached to adjustable weights to allow the application of a known axial compressive load through the bottom of the foot. The second metatarsal was aligned perpendicular to the horizontal plane and in line with the anterior-superior iliac spine. Counterweights were applied to the thigh and shank to offset gravitational loads acting on the lower extremity to create an initial zero shear and compressive load across the knee joint. As reported previously,36 the thigh and shank counterweight magnitude and positions were ascertained by using the limb length and circumference measures along with the anthropometric model of Zatsiorsky.37 Three electromagnetic position sensors (model miniBIRD; Ascension Technology Corp, Burlington, VT) with a resolution of 0.5 mm and 0.1° were attached to the midpoint of the lateral thigh, the center of the patella, and the midpoint of the tibial shaft. The centroid method was used to estimate the center of rotation of the ankle (medial and lateral malleoli digitized), knee (medial and lateral epicondyles digitized), and hip (anterior-superior iliac spine and greater trochanter digitized).36 After the joint centers were estimated, the ankle and knee were flexed to 90° (neutral) and 20°, respectively, and the participant was instructed to relax the leg muscles. Ankle angle was determined visually, whereas knee-flexion angle (20°) was manually confirmed with a goniometer. Once the participant was properly positioned, knee position and zero axial force were confirmed via the MotionMonitor system (Innovative Sports Training, Chicago, IL), and a controlled axial load equal to 40% body weight (BW) was applied at random time intervals (ie, without visual or verbal cue) through the ankle and hip axes using a weight-and-pulley system (Figure 1) to simulate weight bearing.36 The participant was instructed to try and maintain the initial knee position (20° knee flexion) upon weight acceptance without anticipating the release of the axial load (40% BW). This lack of voluntary anticipation and preactivation of the thigh musculature allowed us to better understand the role of the passive-restraint system in weight acceptance. Data-capture time for each trial was 2 seconds after weight acceptance to ensure capture of the peak ACF (time to peak ACF postfatigue: 318 ± 44 milliseconds). Trials were considered successful if there was no limb movement before load release (confirmed subjectively and through examination of kinematic angle data), the load was successfully controlled for 2 seconds as defined by peak knee flexion less than 30° (as ascertained through pilot testing), and there was only 1 peak ACF to stop the released load. Before data collection, each participant performed 3 to 5 practice trials to become familiar with the task. Then, 3 successful trials were obtained before and after the lower extremity exercise protocol while position data from electromagnetic position sensors and force data from the 6-degrees-of-freedom force transducer were collected at 100 and 500 Hz, respectively. Each condition (prefatigue, postfatigue) consisted of 3 to 5 trials, depending upon the participant's efforts to meet the criteria for success.
Figure 1.
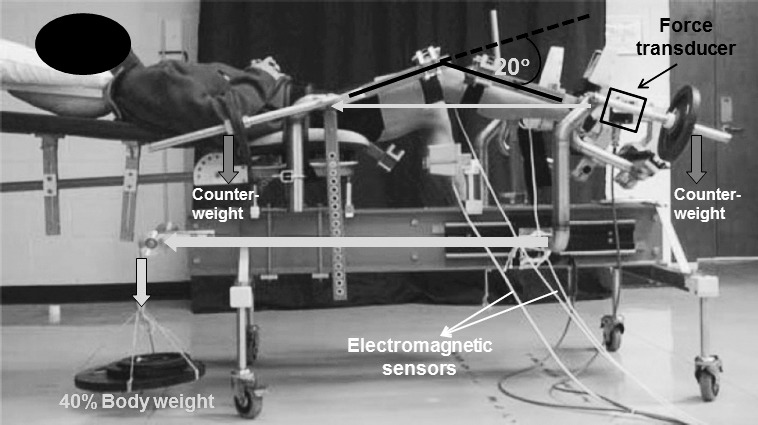
The Vermont Knee Laxity Device.
Lower extremity fatiguing exercise was elicited by having the participant remain in the same position in the VKLD while performing repeated leg presses. An additional 20% BW load was added to the existing 40% BW load (Figure 1), for a total resistance of 60% BW. Using a metronome, the participant was then instructed to flex the knee for 3 seconds and to extend the knee for 3 seconds from 10° to 40° of knee flexion. We defined 1 cycle of the leg-press exercise as 3 seconds of knee flexion and 3 seconds of knee extension without stopping the exercise at the end of the motion. This limited range of motion was necessary due to the mechanical design of the VKLD and the need to fatigue participants while positioned in the device to ensure postfatigue testing immediately upon exercise completion. The participant completed continuous cycles of 15 repetitions with 10 seconds of rest until he or she was unable to complete a full set of 15 repetitions at the prescribed pace of the metronome, at which point the participant was defined as fatigued. Oral encouragement was provided throughout the fatiguing exercise for maximal effort. Weight-acceptance trials were initiated within 20 seconds after the exercise protocol ended.
Data Reduction and Analysis
Raw position and force data were low-pass filtered at 10 and 60 Hz, respectively, using a fourth-order, zero-lag Butterworth filter.36 The ATT (mm) was defined as the change in anterior displacement of the tibia with respect to the patellar sensor from the initial NWB position to the peak ACF during WB (Figure 2). The KFEXC (°) was determined for the initial NWB position to the peak ACF during the WB interval by subtracting initial knee-flexion values obtained when NWB from the knee angle at peak ACF (N). The average ATT, KFEXC, and ACF values across the 3 trials for prefatigue and postfatigue conditions were used for analysis.
Figure 2.
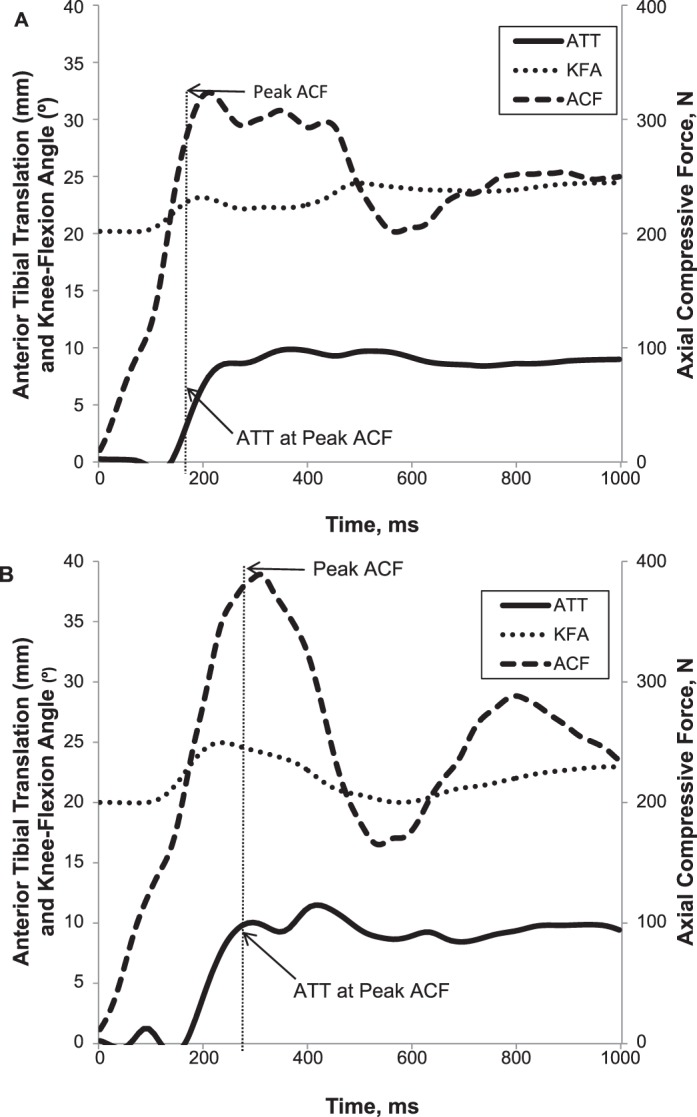
Representative graph showing anterior tibial translation (ATT), knee-flexion angle (KFA), and axial compressive force (ACF) A, before, and B, after fatigue.
Statistical Analysis
We calculated the mean, standard deviation, 95% confidence interval (CI), and effect size (η2) for each variable and 1-way repeated-measures analysis of variance to compare ACF, KFEXC, and ATT before and after the lower extremity fatigue protocol. Bivariate correlations were then used to examine whether changes in KFEXC and ACF from prefatigue to postfatigue were correlated with the change in ATT during that time. The α level was set at .05 for all comparisons. Statistical analyses were conducted in SPSS for Windows (version 15.0; SPSS Inc, Chicago, IL). A priori, we determined we had 80% power to detect an effect size of 0.4 (as determined by pilot testing) with a sample of 10 participants at P < .05.38
RESULTS
All data are reported as mean ± SD, 95% CI, and effect size (η2). From prefatigue to postfatigue, we observed a 6% increase in ACF (prefatigue = 351.8 ± 44.3 N, 95% CI = 320.0, 383.5 N and postfatigue = 374.0 ± 47.9 N, 95% CI = 340.0, 408.3 N; P = .018, η2 = 0.480), a 28% increase in KFEXC (prefatigue = 8.0° ± 4.0°, 95% CI = 5.1°, 10.9° and postfatigue = 10.2° ± 3.7°, 95% CI = 7.6°, 12.9°; P = .046, η2 = 0.366), and a 22% increase in ATT (prefatigue = 6.7 ± 1.7 mm, 95% CI = 5.5, 7.9 mm and postfatigue = 8.2 ± 1.9 mm, 95% CI = 6.9, 9.5 mm; P < .001, η2 = 0.845). However, we noted no significant correlations between the changes in KFEXC (Pearson r = 0.302, P > .397) or the change in ACF (Pearson r = −.130, P > .720) with the change in ATT from prefatigue to postfatigue.
DISCUSSION
Although many authors have reported lower extremity neuromuscular fatigue effects on knee-joint neuromechanics during functional activities such as landing, cutting, and jumping,11,14,25,39–42 the implications of muscular fatigue on tibiofemoral-joint biomechanics during the transition from early NWB to WB are less known. The current investigation revealed greater ACF and KFEXC after fatiguing exercise, which was accompanied by an approximate 1.5-mm increase in ATT. This increase represents approximately 22% of the total ATT motion preexercise. Reports from an in vivo study indicate that the ACL comes under strain during the transition from NWB to WB31 and that a 150-N anterior shear load at 30° of knee flexion in an NWB position is a strong predictor of ACL strain (R2 = 0.59).43 Collectively this suggests that the observed increase in ATT in response to fatiguing exercise may result in substantially greater loads on the ACL. Although increases in both ACF and KFEXC may seem counterintuitive relative to our hypothesis, visual analysis of knee-flexion curves after the fatigue protocol revealed a typical initial increase in knee flexion, followed by an abrupt halting and reversal of the knee-flexion angle into extension just before peak ACF. Exemplar data showing knee-flexion patterns during prefatigue and postfatigue weight-acceptance trials are graphically demonstrated in Figure 2. In the prefatigue condition, a more gradual and linear increase in knee flexion was observed beyond the occurrence of peak ACF. However, during the postfatigue condition, a more rapid increase in knee flexion was observed early in the weight-acceptance phase (average, 2°), but then knee flexion either peaked or the knee began to extend before peak ACF was attained.
To better understand the mechanics of weight acceptance beyond our initial hypotheses, we performed a secondary analysis of peak knee-flexion angular acceleration (calculated via the second derivative of the knee-flexion position data), which revealed an increase in peak angular-flexion acceleration, occurring immediately after weight acceptance from prefatigue to postfatigue (946.2° ± 255.7° ⋅ s−2 < 1108.0° ± 271.9° ⋅ s−2, P < .05). It has been suggested that greater peak ground reaction force is correlated with peak knee-flexion acceleration during running and vertical jumping.44,45 Thus, it is plausible that increased peak angular-flexion acceleration may lead to greater knee-extensor internal moments right before peak ACF in order to counteract knee-flexion motions. Furthermore, greater increases in knee-extensor moment have been associated with stiffer landings,46 resulting in greater ground reaction force.47 In turn, greater axial loads have been associated with greater ATT.35 Although direct measures of joint stiffness were unavailable for this study due to instrumentation limitations, a delayed, but more abrupt, knee-extensor moment may have occurred after fatiguing exercise that contributed to greater peak ACF and thus, greater ATT.35
Previous authors have reported both soft48,49 and stiff9,25 landing strategies after neuromuscular fatigue. Soft and stiff landings are associated with greater or lesser knee-flexion angles, respectively, affecting peak ground reaction force.41,50,51 Some investigators49,52 reported that participants demonstrated softer landing strategies (increased knee-flexion angles) after fatigue protocols, resulting in decreased ground reaction force. These may be compensatory responses to neuromuscular fatigue due to reduction in muscular strength of the lower extremity. In contrast, other researchers reported stiffer landing strategies after fatigue protocols; they attributed the small knee-flexion angle to a reduction in eccentric muscular strength, which is required to control the collapse of the lower extremities and avoid valgus of the knee joint.9,25 However, given the instructions to the participants to maintain knee-flexion angle upon weight acceptance in the current study, detailed comparisons to the landing literature are difficult.
In the present study, contrary to our expectation (decreased KFEXC after fatiguing exercise), KFEXC was initially increased about 2° immediately after fatiguing exercise, even with the instruction to maintain the initial knee position (20° of knee flexion). Studies11–13,53 of neuromuscular fatigue often demonstrate increased knee-flexion angle at foot contact during functional activities after fatiguing exercise. This may be explained by alterations in neuromuscular characteristics after fatigue, such as muscle-activation pattern, level, and onset time and knee proprioceptive function.13,14,40,49 Nyland et al40 revealed that activation of the quadriceps and hamstrings muscles was delayed during a task involving a run and rapid stop after a fatiguing protocol. Neuromuscular fatigue also impairs knee proprioception,54 resulting in slowing of the reflexive-muscle response.40,55,56 For these reasons, alterations in knee neuromuscular and proprioceptive functions may explain the increased KFEXC of about 2° after fatiguing exercise in spite of the instruction to maintain the initial knee position. In turn, an increase in ground reaction force during functional activities after neuromuscular fatigue might be explained by increased preparatory muscle actions and increased initial knee-extension angle.57 However, our experimental protocol study did not require preparatory muscle activation of the thigh muscles because participants were instructed to relax before the loading. Although participants were aware that the perturbation would occur at some point, the 40% BW load was released at a random time interval without warning. Our previous study35 demonstrated that muscle onsets were not detected until after weight release using the same methods in nonfatigued participants. Therefore, observed increases in knee extension before peak ACF may have occurred as a result of reactive or reflexive knee stiffening for the control of knee-joint motions.
Additionally, previous authors58,59 have identified that fatigue-inducing running exercises altered knee proprioception, such as joint position sense measured by absolute angular error. It has been suggested that neuromuscular fatigue deteriorated either mechanoreceptors or proprioceptive pathways, resulting in decreased joint position sense.58 As such, the intermittent leg-press exercise in the present study may reduce joint position sense. Therefore, the observed initial increase in KFEXC, followed by abrupt stiffening after the fatiguing exercise, may be due to a delay in the reflexive response to the WB load and result in rapid extension motion before peak ACF. The initial increase in knee flexion, followed immediately by increases in ACF to control the load, suggests that this strategy may increase the risk of ACL injury secondary to an increase in anterior shear forces induced by an increased ground reaction force during landing.
In this study, a prolonged function-specific fatigue protocol involving intermittent stretch-shortening cycle exercise was used to better mimic reductions in muscle function after prolonged submaximal efforts. The average duration of fatiguing exercise to volitional exhaustion was 25.9 ± 8.2 minutes. Due to the length of the exercise protocol, we cannot rule out the possibility that increases in joint laxity may also have contributed to the increase in ATT. Several investigators60–62 have reported an increase in anterior knee laxity after prolonged exercise designed to induce muscle fatigue in both male and female participants. Because greater anterior knee laxity has been associated with greater ATT during the transition from NWB to WB,36 the observed effects of fatiguing exercise on ATT in this study may be explained in part by exercise-induced increases in joint laxity. Although direct comparisons in the literature with our observed increase in ATT postfatigue are not available from axially direct loads, this value was consistent with a 1.2-mm increase in ATT observed in response to a 30-lb (14-kg) anteriorly directed load to the posterior leg after fatiguing exercise of the quadriceps and hamstrings.14 In this later study, the change in ATT was attributed to viscoelastic changes in the collagenous tissues of the knee,63 as well as reduced function of the dynamic stabilizers.14 We are unable to separate the potential influences of ACL extensibility and neuromuscular fatigue on ACL-strain biomechanics. Additionally, our study lacks an objective measure of fatigue; thus, it is difficult to fully assess whether the fatigue level generated in the current study reflects that found in actual physical activity. Further work is needed to fully understand the specific contributions of the level of muscular fatigue versus increased joint laxity to increased ATT after prolonged exercise and their collective effect on ACL-strain biomechanics.
A limitation of the current study was the lack of preparatory muscle activity in the VKLD model, which resulted in generally longer time to peak forces in VKLD compared with the landing task.64 Although this was purposeful so that we could understand the role of the passive restraint system in weight acceptance, we acknowledge that preactivation is often present in anticipation of landing. Thus, the current VKLD model may represent unanticipated landing situations during sport activity. Further study is needed to examine the effect of neuromuscular fatigue on knee biomechanics with and without preparatory muscle activity. The constrained weight-acceptance task we used differed from one involving upright WB postures, such as landing, but the VKLD model had the advantage of specifically controlling the direction and magnitude of the external load applied to the limb. We chose 40% BW to simulate a WB condition because each leg would experience about 40% BW during a double-legged stance.36 With 40% BW, ATT was increased about 1.5 mm at foot contact after fatiguing exercise. Accordingly, greater magnitudes of ATT may occur during actual sports activities (ie, those associated with higher axial loads),35 resulting in greater tension in the ACL. Finally, due to instrumentation limitations, the fatiguing exercise had to be restricted to a leg-press activity within the VKLD. Although we purposely chose a multi-joint action to induce fatigue, the limited uniplanar motion of our protocol may not mimic the extended physiologic and biomechanical demands encountered during common sports activities when injury may occur. Also, we did not ask participants to rate their perceived exertion, which limits our ability to compare across protocols. Moreover, the relative fatigue levels of the quadriceps and hamstrings muscle groups were unknown. Thus, the currently reported fatigue-related biomechanical changes may not wholly characterize conditions occurring during sports activity.
CONCLUSIONS
Our results suggest that after lower extremity neuromuscular fatigue, individuals experienced greater ATT, likely the result of higher ACF due to altered control of knee-joint motion characterized by small increases in knee flexion and then a rapid stiffening response of the knee joint. This initial rapid stiffening response likely increased ACF and may, in turn, have increased ATT, a finding that has previously been observed at shallow knee-flexion angles.30,35 This information may help us to further develop training programs that minimize such potentially injurious lower extremity biomechanics. Although the observed alterations in knee biomechanics with fatigue may suggest increased ACL strain during the latter part of the competition, more work is needed to understand potential confounding effects of exercise-induced increases in joint laxity.
REFERENCES
- 1.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 2.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Huston LJ, Greenfield ML, Wojtys EM. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop Relat Res. 2000;372:50–63. doi: 10.1097/00003086-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ireland ML. The female ACL: why is it more prone to injury? Orthop Clin North Am. 2002;33(4):637–651. doi: 10.1016/s0030-5898(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 5.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 6.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 7.Hunter SK, Duchateau J, Enoka RM. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev. 2004;32(2):44–49. doi: 10.1097/00003677-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kent-Braun JA. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol. 1999;80(1):57–63. doi: 10.1007/s004210050558. [DOI] [PubMed] [Google Scholar]
- 9.Benjaminse A, Habu A, Sell TC, et al. Fatigue alters lower extremity kinematics during a single-leg stop-jump task. Knee Surg Sports Traumatol Arthrosc. 2008;16(4):400–407. doi: 10.1007/s00167-007-0432-7. [DOI] [PubMed] [Google Scholar]
- 10.Nyland JA, Shapiro R, Caborn DN, Nitz AJ, Malone TR. The effect of quadriceps femoris, hamstring, and placebo eccentric fatigue on knee and ankle dynamics during crossover cutting. J Orthop Sports Phys Ther. 1997;25(3):171–184. doi: 10.2519/jospt.1997.25.3.171. [DOI] [PubMed] [Google Scholar]
- 11.Orishimo KF, Kremenic IJ. Effect of fatigue on single-leg hop landing biomechanics. J Appl Biomech. 2006;22(4):245–254. doi: 10.1123/jab.22.4.245. [DOI] [PubMed] [Google Scholar]
- 12.Pincivero DM, Gandhi V, Timmons MK, Coelho AJ. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J Biomech. 2006;39(2):246–254. doi: 10.1016/j.jbiomech.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Rozzi SL, Lephart SM, Fu FH. Effects of muscular fatigue on knee joint laxity and neuromuscular characteristics of male and female athletes. J Athl Train. 1999;34(2):106–114. [PMC free article] [PubMed] [Google Scholar]
- 14.Wojtys EM, Wylie BB, Huston LJ. The effects of muscle fatigue on neuromuscular function and anterior tibial translation in healthy knees. Am J Sports Med. 1996;24(5):615–621. doi: 10.1177/036354659602400509. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins RD, Fuller CW. A prospective epidemiological study of injuries in four English professional football clubs. Br J Sports Med. 1999;33(3):196–203. doi: 10.1136/bjsm.33.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahnama N, Reilly T, Lees A. Injury risk associated with playing actions during competitive soccer. Br J Sports Med. 2002;36(5):354–359. doi: 10.1136/bjsm.36.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehring D, Melnyk M, Gollhofer A. Gender and fatigue have influence on knee joint control strategies during landing. Clin Biomech (Bristol, Avon) 2009;24(1):82–87. doi: 10.1016/j.clinbiomech.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32(4):395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 19.James C, Sacco P, Jones DA. Loss of power during fatigue of human leg muscles. J Physiol. 1995;484(pt 1):237–246. doi: 10.1113/jphysiol.1995.sp020661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernozek TW, Torry MR, Iwasaki M. Gender differences in lower extremity landing mechanics caused by neuromuscular fatigue. Am J Sports Med. 2008;36(3):554–565. doi: 10.1177/0363546507308934. [DOI] [PubMed] [Google Scholar]
- 21.Kellis E, Kellis S. Effects of agonist and antagonist muscle fatigue on muscle coactivation around the knee in pubertal boys. J Electromyogr Kinesiol. 2001;11(5):307–318. doi: 10.1016/s1050-6411(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 22.Weir JP, Keefe DA, Eaton JF, Augustine RT, Tobin DM. Effect of fatigue on hamstring coactivation during isokinetic knee extensions. Eur J Appl Physiol Occup Physiol. 1998;78(6):555–559. doi: 10.1007/s004210050460. [DOI] [PubMed] [Google Scholar]
- 23.Horita T, Komi PV, Hamalainen I, Avela J. Exhausting stretch-shortening cycle (SSC) exercise causes greater impairment in SSC performance than in pure concentric performance. Eur J Appl Physiol. 2003;88(6):527–534. doi: 10.1007/s00421-002-0716-z. [DOI] [PubMed] [Google Scholar]
- 24.Komi PV. Stretch-shortening cycle: a powerful model to study normal and fatigued muscle. J Biomech. 2000;33(10):1197–1206. doi: 10.1016/s0021-9290(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 25.Chappell JD, Herman DC, Knight BS, Kirkendall DT, Garrett WE, Yu B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am J Sports Med. 2005;33(7):1022–1029. doi: 10.1177/0363546504273047. [DOI] [PubMed] [Google Scholar]
- 26.Hassani A, Patikas D, Bassa E, Hatzikotoulas K, Kellis E, Kotzamanidis C. Agonist and antagonist muscle activation during maximal and submaximal isokinetic fatigue tests of the knee extensors. J Electromyogr Kinesiol. 2006;16(6):661–668. doi: 10.1016/j.jelekin.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Kellis E, Kouvelioti V. Agonist versus antagonist muscle fatigue effects on thigh muscle activity and vertical ground reaction during drop landing. J Electromyogr Kinesiol. 2009;19(1):55–64. doi: 10.1016/j.jelekin.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Moran KA, Clarke M, Reilly F, Wallace ES, Brabazon D, Marshall B. Does endurance fatigue increase the risk of injury when performing drop jumps? J Strength Cond Res. 2009;23(5):1448–1455. doi: 10.1519/JSC.0b013e3181a4e9fa. [DOI] [PubMed] [Google Scholar]
- 29.Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 30.Torzilli PA, Deng X, Warren RF. The effect of joint-compressive load and quadriceps muscle force on knee motion in the intact and anterior cruciate ligament-sectioned knee. Am J Sports Med. 1994;22(1):105–112. doi: 10.1177/036354659402200117. [DOI] [PubMed] [Google Scholar]
- 31.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Rudy TW, Allen C, Sakane M, Woo SL. Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: a porcine study. J Orthop Res. 1998;16(1):122–127. doi: 10.1002/jor.1100160121. [DOI] [PubMed] [Google Scholar]
- 33.Meyer EG, Haut RC. Excessive compression of the human tibio-femoral joint causes ACL rupture. J Biomech. 2005;38(11):2311–2316. doi: 10.1016/j.jbiomech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Rodacki AL, Fowler NE, Bennett SJ. Multi-segment coordination: fatigue effects. Med Sci Sports Exerc. 2001;33(7):1157–1167. doi: 10.1097/00005768-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz RJ, Kim H, Shultz SJ. Effect of axial load on anterior tibial translation when transitioning from non-weight bearing to weight bearing. Clin Biomech (Bristol, Avon) 2010;25(1):77–82. doi: 10.1016/j.clinbiomech.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shultz SJ, Shimokochi Y, Nguyen AD, et al. Nonweight-bearing anterior knee laxity is related to anterior tibial translation during transition from nonweight bearing to weight bearing. J Orthop Res. 2006;24(3):516–523. doi: 10.1002/jor.20040. [DOI] [PubMed] [Google Scholar]
- 37.Chernyi GG, Regirer SA. Contemporary Problems of Biomechanics. Boca Raton, FL: CRC Press;; 1990. [Google Scholar]
- 38.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 39.Kellis E. The effects of fatigue on the resultant joint moment, agonist and antagonist electromyographic activity at different angles during dynamic knee extension efforts. J Electromyogr Kinesiol. 1999;9(3):191–199. doi: 10.1016/s1050-6411(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 40.Nyland JA, Caborn DN, Shapiro R, Johnson DL. Fatigue after eccentric quadriceps femoris work produces earlier gastrocnemius and delayed quadriceps femoris activation during crossover cutting among normal athletic women. Knee Surg Sports Traumatol Arthrosc. 1997;5(3):162–167. doi: 10.1007/s001670050045. [DOI] [PubMed] [Google Scholar]
- 41.Nyland JA, Shapiro R, Stine RL, Horn TS, Ireland ML. Relationship of fatigued run and rapid stop to ground reaction forces, lower extremity kinematics, and muscle activation. J Orthop Sports Phys Ther. 1994;20(3):132–137. doi: 10.2519/jospt.1994.20.3.132. [DOI] [PubMed] [Google Scholar]
- 42.Pincivero DM, Coelho AJ, Campy RM, Salfetnikov Y, Bright A. The effects of voluntary contraction effort on quadriceps femoris electromyogram median frequency in humans: a muscle and sex comparison. Eur J Appl Physiol. 2002;87(4–5):448–455. doi: 10.1007/s00421-002-0658-5. [DOI] [PubMed] [Google Scholar]
- 43.Fleming BC, Beynnon BD, Nichols CE, Johnson RJ, Pope MH. An in vivo comparison of anterior tibial translation and strain in the anteromedial band of the anterior cruciate ligament. J Biomech. 1993;26(1):51–58. doi: 10.1016/0021-9290(93)90612-i. [DOI] [PubMed] [Google Scholar]
- 44.Derrick TR. The effects of knee contact angle on impact forces and accelerations. Med Sci Sports Exerc. 2004;36(5):832–837. doi: 10.1249/01.mss.0000126779.65353.cb. [DOI] [PubMed] [Google Scholar]
- 45.Elvin NG, Elvin AA, Arnoczky SP, Torry MR. The correlation of segment accelerations and impact forces with knee angle in jump landing. J Appl Biomech. 2007;23(3):203–212. doi: 10.1123/jab.23.3.203. [DOI] [PubMed] [Google Scholar]
- 46.Zhang SN, Bates BT, Dufek JS. Contributions of lower extremity joints to energy dissipation during landings. Med Sci Sports Exerc. 2000;32(4):812–819. doi: 10.1097/00005768-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz RJ, Kulas AS, Perrin DH, Riemann BL, Shultz SJ. Sex differences in lower extremity biomechanics during single leg landings. Clin Biomech (Bristol, Avon) 2007;22(6):681–688. doi: 10.1016/j.clinbiomech.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Fagenbaum R, Darling WG. Jump landing strategies in male and female college athletes and the implications of such strategies for anterior cruciate ligament injury. Am J Sports Med. 2003;31(2):233–240. doi: 10.1177/03635465030310021301. [DOI] [PubMed] [Google Scholar]
- 49.Madigan ML, Pidcoe PE. Changes in landing biomechanics during a fatiguing landing activity. J Electromyogr Kinesiol. 2003;13(5):491–498. doi: 10.1016/s1050-6411(03)00037-3. [DOI] [PubMed] [Google Scholar]
- 50.Oliver J, Armstrong N, Williams C. Changes in jump performance and muscle activity following soccer-specific exercise. J Sports Sci. 2008;26(2):141–148. doi: 10.1080/02640410701352018. [DOI] [PubMed] [Google Scholar]
- 51.Wikstrom EA, Powers ME, Tillman MD. Dynamic stabilization time after isokinetic and functional fatigue. J Athl Train. 2004;39(3):247–253. [PMC free article] [PubMed] [Google Scholar]
- 52.James CR, Scheuermann BW, Smith MP. Effects of two neuromuscular fatigue protocols on landing performance. J Electromyogr Kinesiol. 2010;20(4):667–675. doi: 10.1016/j.jelekin.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 53.McLean SG, Fellin RE, Suedekum N, Calabrese G, Passerallo A, Joy S. Impact of fatigue on gender-based high-risk landing strategies. Med Sci Sports Exerc. 2007;39(3):502–514. doi: 10.1249/mss.0b013e3180d47f0. [DOI] [PubMed] [Google Scholar]
- 54.Givoni NJ, Pham T, Allen TJ, Proske U. The effect of quadriceps muscle fatigue on position matching at the knee. J Physiol. 2007;584(pt 1):111–119. doi: 10.1113/jphysiol.2007.134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melnyk M, Gollhofer A. Submaximal fatigue of the hamstrings impairs specific reflex components and knee stability. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):525–532. doi: 10.1007/s00167-006-0226-3. [DOI] [PubMed] [Google Scholar]
- 56.Horita T, Komi PV, Nicol C, Kyrolainen H. Stretch shortening cycle fatigue: interactions among joint stiffness, reflex, and muscle mechanical performance in the drop jump [corrected] Eur J Appl Physiol Occup Physiol. 1996;73(5):393–403. doi: 10.1007/BF00334415. [DOI] [PubMed] [Google Scholar]
- 57.James CR, Scheuermann BW, Smith MP. Effects of two neuromuscular fatigue protocols on landing performance. J Electromyogr Kinesiol. 2010;20(4):667–675. doi: 10.1016/j.jelekin.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Miura K, Ishibashi Y, Tsuda E, Okamura Y, Otsuka H, Toh S. The effect of local and general fatigue on knee proprioception. Arthroscopy. 2004;20(4):414–418. doi: 10.1016/j.arthro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Skinner HB, Wyatt MP, Hodgdon JA, Conard DW, Barrack RL. Effect of fatigue on joint position sense of the knee. J Orthop Res. 1986;4(1):112–118. doi: 10.1002/jor.1100040115. [DOI] [PubMed] [Google Scholar]
- 60.Sakai H, Tanaka S, Kurosawa H, Masujima A. The effect of exercise on anterior knee laxity in female basketball players. Int J Sports Med. 1992;13(7):552–554. doi: 10.1055/s-2007-1024562. [DOI] [PubMed] [Google Scholar]
- 61.Skinner HB, Wyatt MP, Stone ML, Hodgdon JA, Barrack RL. Exercise-related knee joint laxity. Am J Sports Med. 1986;14(1):30–34. doi: 10.1177/036354658601400106. [DOI] [PubMed] [Google Scholar]
- 62.Steiner ME, Grana WA, Chillag K, Schelberg-Karnes E. The effect of exercise on anterior-posterior knee laxity. Am J Sports Med. 1986;14(1):24–29. doi: 10.1177/036354658601400105. [DOI] [PubMed] [Google Scholar]
- 63.Lam TC, Thomas CG, Shrive NG, Frank CB, Sabiston CP. The effects of temperature on the viscoelastic properties of the rabbit medial collateral ligament. J Biomech Eng. 1990;112(2):147–152. doi: 10.1115/1.2891165. [DOI] [PubMed] [Google Scholar]
- 64.Thomas AC, McLean SG, Palmieri-Smith RM. Quadriceps and hamstrings fatigue alters hip and knee mechanics. J Appl Biomech. 2010;26(2):159–170. doi: 10.1123/jab.26.2.159. [DOI] [PubMed] [Google Scholar]


