Abstract
Context:
Near-infrared (NIR) light therapy is purported to act as an ergogenic aid by enhancing the contractile function of skeletal muscle. Improving muscle function is a new avenue for research in the area of laser therapy; however, very few researchers have examined the ergogenic effects of NIR light therapy and the influence it may have on the recovery process during rehabilitation.
Objective:
To evaluate the ergogenic effect of NIR light therapy on skeletal muscle function.
Design:
Crossover study.
Setting:
Controlled laboratory.
Patients or Other Participants:
Thirty-nine healthy men (n = 21) and women (n = 18; age = 20.0 ± 0.2 years, height = 169 ± 2 cm, mass = 68.4 ± 1.8 kg, body mass index = 23.8 ± 0.4 kg/m2).
Intervention(s):
Each participant received active and sham treatments on the biceps brachii muscle on 2 separate days. The order of treatment was randomized. A class 4 laser with a cumulative dose of 360 J was used for the active treatment. After receiving the treatment on each day, participants completed an elbow-flexion resistance-exercise protocol.
Main Outcome Measure(s):
The dependent variables were elbow range of motion, muscle point tenderness, and strength (peak torque). Analysis of variance with repeated measures was used to assess changes in these measures between treatments at baseline and at follow-up, 48 hours postexercise. Additionally, immediate strength loss postexercise was compared between treatments using a paired t test.
Results:
Preexercise to postexercise strength loss for the active laser treatment, although small, was less than with the sham treatment (P = .05).
Conclusions:
Applied to skeletal muscle before resistance exercise, NIR light therapy effectively attenuated strength loss. Therefore, NIR light therapy may be a beneficial, noninvasive modality for improving muscle function during rehabilitation after musculoskeletal injury. However, future studies using higher treatment doses are warranted.
Key Words: lasers, photobiomodulation, ergogenic aids, contractile function, biceps brachii muscle
Key Points
Near-infrared light therapy administered before resistance exercise enhanced the contractile function of skeletal muscle by attenuating strength loss.
Disuse atrophy and pain can be significant impediments to recovery after musculoskeletal injury. Therefore, the ability to enhance muscle function during rehabilitation is of great benefit to patients.
Various forms of light therapies have been used over the past 4 decades. Light therapy involves the application of light to a tissue to promote healing and functional recovery.1 Near-infrared (NIR) light has shown promise as a therapeutic modality for treating acute and chronic musculoskeletal injuries.1,2 Near-infrared light modalities emit photons in a narrow bandwidth with wavelengths ranging from 700 to 1000 nm. Examples of NIR light modalities include class 3 and class 4 lasers as well as light-emitting diodes. Light-emitting diodes emit light in the red to infrared range at intensities that fall within the range of class 3 lasers. Positive therapeutic effects of light therapy are attributed to its ability to transmit photons through the skin to penetrate deeper into soft tissues, where the photons are absorbed by cellular and blood-bound proteins known as chromophores.1 Photon absorption by endogenous chromophores stimulates biochemical reactions in tissues that translate to improvements in cellular growth, repair, and function.1 This process has been referred to as photobiomodulation.1,3
The majority of research conducted on NIR light therapy has focused on the ability of photostimulation to improve soft tissue healing. Investigators4–7 have begun to explore the ergogenic effects of NIR light therapy in reducing skeletal muscle fatigue and attenuating strength loss during and after resistance exercise. Previous authors8–10 have provided evidence for the ability of light therapy to enhance blood circulation, resulting in an increase in tissue oxygenation. Increasing circulation in the irradiated tissue can lead to enhanced oxygen and substrate delivery, thereby improving the overall ability of the muscle to perform work. An increase in oxygen delivery to the working muscle will also lead to increased adenosine triphosphate (ATP) production via aerobic respiration. With increased ATP bioavailability, the local musculature is able to perform more work and resist fatigue more readily as it limits the accumulation of metabolites and the impairment of oxygen delivery. Enhancement of blood flow, oxygen delivery, and ATP synthesis within working muscle provides a physiologic basis of support for light therapy as an ergogenic aid. Any complementary therapy administered to skeletal muscle before exercise that can produce an ergogenic effect would be beneficial in rehabilitation by enhancing the target muscle's capacity to perform work.
Research has also implicated light therapy as an effective tool for enhancing skeletal muscle contractile function and for increasing the number of repetitions and the time it takes to fatigue a muscle.4–6,11,12 Leal Junior et al12 showed a significant delay in the fatigue response to repeated electronically evoked tetanic contractions in the tibialis anterior muscles of rats exposed to light therapy. In human studies,4–7,11 muscles exposed to laser therapy demonstrated enhanced performance by maintaining contractile force output and delaying the onset of fatigue when exposed to resistance exercise. Empirical evidence also demonstrated that light therapy can limit exercise-induced muscle damage, thereby improving biochemical and functional recovery by reducing inflammation and oxidative stress.4,6,7,11 Therefore, the primary objective of our investigation was to evaluate the ergogenic effect of NIR light therapy on skeletal muscle function during resistance exercise for the biceps brachii muscle. We hypothesized that NIR light therapy would attenuate strength loss in the biceps brachii muscle and enhance recovery after resistance exercise by protecting the muscle from exercise-induced damage.
METHODS
Participants
Thirty-nine participants were enrolled in the study. Participants consisted of healthy men (n = 21) and women (n = 18) of any racial or ethnic background who were active in upper extremity resistance training more than 2 days per week (age = 20.0 ± 0.2 years, height = 169 ± 2.0 cm, weight = 68.4 ± 1.8 kg, body mass index = 23.8 ± 0.4 kg/m2). We obtained institutional review board approval before recruiting participants. Written informed consent was obtained from all study participants.
We excluded volunteers who (1) had a recent (in the previous 6 months) injury to their upper extremity; (2) had participated in upper extremity resistance training less than once per week for the past 8 weeks; (3) took pain or prescription medication or nutritional supplements on a daily basis; (4) had any tattoos on or around the area of treatment; or (5) had a history of any of the following conditions: cancerous tumors or blood clots in the upper extremity area, skin allergies, severe muscle weakness, or serious neurologic injury. Participants were instructed to continue their regular exercise schedule during the course of the study.
Experimental Design
A double-blind, repeated-measures, sham-controlled crossover design was used in this study. Each participant received both active and sham treatments. The order of treatment was randomized. For each treatment, participant's elbow-flexion range of motion (ROM), muscle-point tenderness, and isometric elbow-flexor strength were measured before and 48 hours after receiving treatment and performing elbow-flexor resistance exercise. Muscle strength was also measured immediately after the exercise to assess the strength loss due to exercise. All participants attended 4 laboratory visits (2 visits for each treatment; Table 1).
Table 1.
Experimental Design
| Treatment-Order Group |
Visit 1 (Day 0) |
Visit 2 (Day 2) |
Visit 3 (Day 8) |
Visit 4 (Day 10) |
| Active-sham | 1. Preintervention measurementsa | Follow-up measurementsa | 1. Preintervention measurementsa | Follow-up measurementsa |
| 2. Active light therapy intervention | 2. Sham light therapy intervention | |||
| 3. Exercise | 3. Exercise | |||
| 4. Assessment of MVIC | 4. Assessment of MVIC | |||
| Sham-active | 1. Preintervention measurementsa | Follow-up measurementsa | 1. Preintervention measurementsa | Follow-up measurementsa |
| 2. Sham light therapy intervention | 2. Active light therapy intervention | |||
| 3. Exercise | 3. Exercise | |||
| 4. Assessment of MVIC | 4. Assessment of MVIC |
Abbreviation: MVIC, maximal voluntary isometric contraction.
Included assessment of range of motion, muscle-point tenderness, and MVIC.
Procedure
During the first visit, participants read and signed the informed consent agreement, completed a brief medical health questionnaire, and became familiar with the exercise protocol by completing a mock trial. The mock trial consisted of a set of 10 low-load isokinetic elbow-flexor exercises. A low load was used for this trial so as not to affect the subsequent measurements. The participants then had preintervention (baseline) measurements taken, which included assessments of elbow ROM, muscle-point tenderness, and isometric elbow-flexor strength. After preexercise measures, participants received either the active or sham laser treatment and completed the exercise protocol for the elbow flexors.
Measurements
Range of Motion
Both pain-free and forced active elbow-flexion and -extension ROM were evaluated with a standard plastic goniometer. The goniometer approximated the axis of rotation for the humeroulnar joint, and the goniometer arms were aligned with the humerus and forearm. Forced ROM was assessed by asking the participant to flex or extend the elbow as far as possible. Pain-free ROM was assessed by asking the participant to flex or extend the elbow only through the ROM that caused no pain or discomfort. A relaxed arm angle was measured with a goniometer by having the participant stand with the arm relaxed in a supinated position at the side. All ROM measures were performed by the same research assistant to avoid intertester differences. The research assistant was a certified athletic trainer and well experienced in taking all clinical measurements.
Muscle-Point Tenderness
Muscle-point tenderness was measured by applying focal pressure to a targeted area of the biceps muscle using an instrumented algometer (Force Ten FDX; Wagner Instruments, Greenwich, CT). Pressure was applied to the muscle at a rate of 2.2 kg/s. The participant was instructed to indicate at what point the pressure turned to pain. The measurement was performed at 3 sites on the biceps (proximal musculotendinous junction, midbelly, and distal musculotendinous junction). The test was performed 3 times at each site and the average of the 3 scores was recorded in kilograms of force.
Muscle Strength
The Kin-Com (model 125 AP; Isokinetic International, Chattanooga, TN) is a computerized testing and training device used for neuromuscular performance evaluation and therapeutic exercise and rehabilitation. The device is capable of measuring isokinetic (fixed-speed) and isometric (fixed-position) muscle actions using a dynamometer that is interfaced with a computer.
Elbow-flexion muscle strength was assessed as the peak torque produced during the maximal voluntary isometric contraction (MVIC). Participants were seated with the dominant arm placed at their side in 90° of elbow flexion. Each participant performed 3 MVICs of the dominant arm. Each contraction was held for 5 seconds, and a 30-second recovery period was provided between contractions. The most forceful contraction of the 3 values was recorded as the peak torque in newton meters. The MVIC was measured before and immediately after the exercise protocol, and the scores were used to calculate the strength-loss index (SLI).
The NIR Light Therapy
A commercially available, FDA-approved laser device (model K-Laser kl6d; Laser Therapy Products, LLC, Franklin, TN) was used for all phototherapy applications. The K-Laser is a class 4 laser that emits NIR light using a double wavelength of 800 and 970 nm. Each participant received a 4-minute treatment with the output power of the laser set at 3 W (50% duty cycle, 1.5 W average power output) and an overall treatment dose of 360 J delivered to the biceps brachii muscle in a typical grid pattern. Before each laser application, the surface area to be treated was cleaned with isopropyl alcohol. A dose of 360 J was selected based on previous research from our laboratory.13 The NIR light treatments were performed using a direct-contact technique following a set grid pattern covering 15 points oriented along the biceps brachii muscle group. The technician administered light to each point for 3 to 4 seconds. The grid pattern followed a proximal-to-distal approach, starting at the proximal end of the biceps and moving distally down the muscle belly to the distal myotendinous region in a lateral-to-medial fashion to ensure that the entire muscle was treated. The 15 grid points did not cover the entire muscle; however, the beam of light diverges as it penetrates the tissue layers, thus spreading light energy evenly throughout the entire muscle. All participants, along with the laser technician, wore safety eyewear to protect themselves from potential eye injury. The safety eyewear was able to filter laser light up to 1070 nm. All laser treatments were performed in a confined room.
Resistance Exercise
Force attenuation was induced using sustained maximal concentric and eccentric actions of the biceps brachii muscle group. An isokinetic testing and exercise device (model Kin-Com 125 AP) provided resistance during the arm exercise. The exercise protocol required participants to perform repeated maximal concentric and eccentric actions for the elbow-flexor muscle group. This exercise protocol has been used previously and mimics the cellular and functional processes observed with acute bouts of exercise.14 Such processes include loss of ROM and strength and clinical pain. The angular velocity was set at 45°/s for concentric actions and 60°/s for eccentric actions. Each participant completed 3 sets of 20 repetitions. Each participant was instructed to perform repetitions “as hard as you can.” The investigator provided oral encouragement during the exercise protocol.
Postexercise Procedures
Immediately after the exercise protocol, each participant had his or her strength reassessed to determine the SLI (visit 1). The SLI is the percentage decline in MVIC from the beginning to the end of the exercise protocol and was calculated for each participant as ([postexercise − preexercise] / preexercise) · 100. Participants were asked to return 48 hours postexercise for follow-up measurements to assess recovery (visit 2). Participants were asked not to stretch, massage, or ice the area and to refrain from taking any medications with anti-inflammatory or analgesic properties. During visit 2, the measurements of elbow-flexion ROM, muscle-point tenderness, and isometric elbow-flexor strength were repeated following the same procedure. Participants returned for their third visit 1 week after the original testing and exercise session. The third visit consisted of procedures identical to those of visit 1. Participants then received the remaining NIR light therapy intervention (sham or active) as indicated by their treatment-order group. Participants again returned 48 hours postexercise, on day 10, for follow-up measurements.
Statistical Analysis
All data analyses were performed using SPSS for Windows (version 16.0; SPSS, Inc, Chicago, IL). Data are presented as means and standard deviations. To determine if a carryover effect occurred because of the order of treatment between groups, we conducted independent t tests to compare baseline muscle strength between the groups. An additional 2-way analysis of variance was calculated to assess changes in MVIC at the 3 time points (preexercise, immediately postexercise, and 48 hours postexercise). Paired comparisons were used to determine differences between the active laser and sham treatments when a significant interaction effect was found.
We used a dependent paired t test to analyze the SLI between the active and sham laser treatments. An α level of .05 was the criterion for statistical significance.
RESULTS
A total of 39 participants consented and completed the described experimental protocol. One female participant dropped out during the study because of arm discomfort and her data were subsequently withdrawn.
Crossover Effects
Muscle strength did not differ between the group that received the active laser first and the group that received it second (t37 = 0.900, P = .374) or between the group that received the sham treatment first and the group that received it second (t37 = 1.01, P = .320).
Strength Loss and Recovery
No significant time-by-treatment interaction was observed for strength (F1,38 = 2.451, P = .094). All means and standard deviations for the strength data are presented in Table 2. The SLI showed less of a decline in MVIC after the active laser treatment than after the sham laser treatment (t1,38 = 2.024, P = .05; Figure).
Table 2.
Experimental Results for Muscle-Point Tenderness and Strength
| Measurement |
Time |
Treatment (Mean ± SD) |
F or t Value |
P Value |
|
| Active |
Sham |
||||
| Muscle-point tenderness, kg | Preexercise | 4.72 ± 1.5 | 4.39 ± 1.4 | F = 2.559 | .118 |
| Postexercise | 3.86 ± 1.6 | 4.05 ± 1.6 | |||
| Strength (MVIC) | Preexercise | 32.57 ± 11.2 | 30.71 ± 11.7 | F = 2.451 | .094 |
| Postexercise | 14.24 ± 6.8 | 12.06 ± 6.6 | |||
| At 48 h | 30.23 ± 13.7 | 31.78 ± 14.1 | |||
| Change in MVIC, % (strength-loss index) | 56.48 ± 12.7 | 60.75 ± 12.3 | t = 2.024 | .05a | |
Abbreviation: MVIC, maximal voluntary isometric contraction.
Indicates statistical significance (P ≤ .05).
Figure.
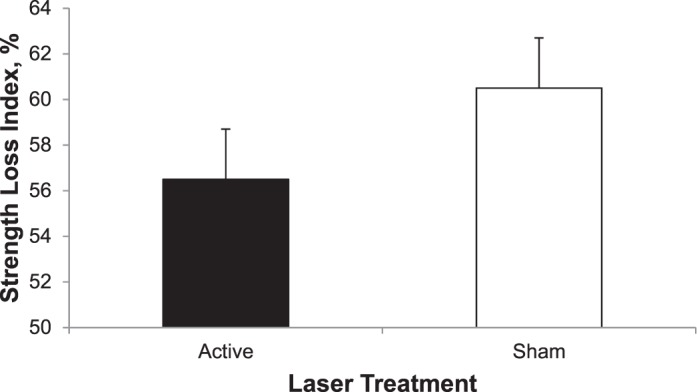
Percentage change in torque between preexercise and postexercise maximal voluntary isometric contraction for the active laser and sham laser treatments. Difference between treatments (P = .05).
Muscle-Point Tenderness and ROM
We found no differences between the active laser and sham laser treatments at either time point (F1,38 = 2.559, P = .118; Table 2). No differences were noted between the active laser and sham treatments for any of the ROM measures at either time point: full flexion (F1,38 = 0.153, P = .698), pain-free flexion (F1,38 = 0.360, P = .552), full extension (F1,38 = 1.608, P = .212), pain-free extension (F1,38 = 2.234, P = .143), and resting arm angle (F1,38 = 2.734, P = .106; Table 3).
Table 3.
Range-of-Motion Measures Preexercise and Postexercise, °
| Measurement |
Elbow Motion |
Mean ± SD |
|||
| Preexercise Treatment |
Postexercise Treatment |
||||
| Active Laser |
Sham Laser |
Active Laser |
Sham Laser |
||
| Full range of motion | Flexion | 143.23 ± 5.45 | 143.98 ± 5.9 | 142.76 ± 5.7 | 143.18 ± 6.3 |
| Extension | 178.32 ± 8.3 | 178.31 ± 7.8 | 176.10 ± 9.1 | 177.50 ± 7.8 | |
| Pain-free range of motion | Flexion | 143.25 ± 5.5 | 144.24 ± 5.7 | 138.25 ± 9.4 | 140.48 ± 7.8 |
| Extension | 178.32 ± 8.3 | 178.31 ± 7.8 | 168.57 ± 16.8 | 173.56 ± 11.9 | |
| Resting arm angle | 159.15 ± 7.6 | 158.15 ± 7.5 | 157.03 ± 9.1 | 158.87 ± 8.6 | |
DISCUSSION
A crossover study design was used to limit the amount of variability among participants and to reduce the influence of confounding covariates. We observed no differences in strength at the beginning of the 2 treatment sessions. Therefore, we can eliminate the possibility of a crossover or unwanted learning effect from the first and second treatment sessions. The SLI was different between treatment conditions, with MVIC losses after the exercise protocol of 56.5% for the active laser treatment and 60.8% for the sham treatment. This difference demonstrates the ability of the laser to preserve maximal capacity for torque production and attenuate strength losses that are typically associated with fatiguing muscle exercises. However, no difference was observed for the MVIC at the 48-hour postexercise follow-up between the active and sham laser treatments.
Our results are in partial agreement with those of previous studies using light-emitting diode therapy as an ergogenic aid.4–6,11 Muscle torque has also been evaluated in these studies by measuring MVIC. Laser therapy was performed on the quadriceps femoris muscle groups of 36 healthy male participants.4 Maximal voluntary isometric contractions were measured immediately after exercise and again at 24 and 48 hours postexercise. At each time point, a difference was evident between the laser therapy treatment and the sham control treatment. Strength loss was attenuated with laser treatment after exercise by 11% immediately postexercise and 13% and 15% at 24 and 48 hours postexercise, respectively. In a similar study,11 light-emitting diode therapy attenuated strength loss by a difference of 16% when compared with the sham treatment. These researchers observed greater conservation of strength with laser therapy than we did. It is possible that, although we reached the threshold to stimulate physiologic function, we did not exceed it sufficiently to produce the benefit seen in previous studies. Similarly, our MVIC results were not different at 48 hours postexercise, indicating that our measure of recovery was not affected by the laser treatment. However, we did not measure biomarkers of muscle damage or postexercise recovery, unlike previous investigators.4,7,12 Therefore, further research is needed to examine recovery measures more thoroughly at 48 hours postexercise and beyond. To adequately assess the effects of laser therapy on muscle recovery, future authors should focus on the analysis of biochemical markers of muscle and cell damage and impairment measures beyond 48 hours after exercise-induced muscle fatigue.
The exact mechanism by which light therapy modulates contractile function in exercising muscle has yet to be elucidated. In addition to the strength-related ergogenic effects of light therapy, exercise and the ability to perform work can also be greatly influenced by circulatory and cellular mechanisms.15 Because decrements in skeletal muscle contractile function may be associated with inadequate blood flow, it can be postulated that laser-mediated vasodilation is responsible for the enhanced skeletal muscle function and the attenuated strength loss.9,10,16,17 Maegawa et al9 investigated the effect of light therapy on rat mesenteric microcirculation in vivo and showed vastly improved vasomotor relaxation and, consequently, improved blood flow. Ihsan18 observed accelerated collateral circulation and enhanced microcirculation in rabbits exposed to light therapy after femoral artery occlusion. In human populations, 3 clinical trials evaluated the effects of light therapy on microcirculatory changes. Most recently, Larkin et al8 demonstrated a dose-response effect of laser therapy for increasing blood flow in soft tissue. When participants were exposed to a 4-minute laser treatment consisting of a 360-J dose, forearm blood flow increased, starting at minute 4 of treatment and continuing for 2 minutes after the treatment ended.
Increasing blood circulation in tissue exposed to light therapy would lead to an increase in the delivery of oxygen and other substrates to exercising muscles, thereby increasing the muscle's ability to perform work. An increase in oxygen delivery to the working muscle would also be beneficial to the cell by increasing ATP production via aerobic respiration, as observed in previous studies.19 In addition, the stimulatory effect on cytochrome c oxidase activity that has been observed in previous research lends additional physiologic support for an increase in ATP production.4,13,19–22 Increasing local circulation to exercising muscles also limits the accumulation of metabolites and the impairment of oxygen delivery, resulting in further enhancement in muscle function. Therefore, enhancement of the physiologic processes related to blood flow, oxygen delivery, and ATP synthesis observed in previous laser therapy research provides additional support for light therapy's ability to improve muscle function and protect the muscle from exercise-induced damage. However, the relationship between these physiologic enhancements and muscle performance require further investigation.
Clinical Applications
Research on NIR light therapy has implicated laser as an effective treatment modality for enhancing contractile function, as well as increasing the number of repetitions and the time it takes to fatigue a muscle.4–6,11,12 Such findings have provided substantive evidence for the use of light therapy as an effective ergogenic aid to exercising muscles. We evaluated the effectiveness of NIR light therapy in reducing strength loss and muscle impairment associated with strenuous resistance exercise.
During rehabilitative exercise programs, decrements in skeletal muscle contractile force production may significantly limit functional progression, produce symptoms such as pain and soreness, prolong recovery time, and negatively affect clinical outcomes. A therapeutic modality administered just before rehabilitative exercise that can produce an ergogenic effect on the muscle would be beneficial for the patient because it could improve a muscle's capacity to work and perhaps reduce the risk for exercise-induced muscle damage. Athletes in recovery from musculoskeletal injury or reconstructive surgery rely on therapeutic exercise to reconnect impaired neural networks and restore strength levels. Decrements in contractile function of skeletal muscle can be a limiting factor to functional progression. Near-infrared light treatments administered before therapeutic exercise may improve and sustain muscle performance, thereby restoring neuromuscular control and facilitating the return to full function.
Limitations and Future Studies
Our study focused on the ergogenic effects of NIR light therapy. The nonsignificant findings with respect to the impairment measures (ROM and muscle-point tenderness) may be attributed to inadequate dosing. It is possible that stronger dosing is necessary to produce full conservation of all impairment measures. Lack of adequate dosing at the biceps brachii may have resulted from low power output or an inadequate duration of laser application, or both. Also, it is unlikely that our research participants were using their biceps brachii muscles alone throughout all concentric and eccentric elbow-flexion contractions. Application of the laser to the surrounding musculature might be useful, as compensatory muscle actions will occur when performing an arm-exercise protocol.
Impairment measures may have also been affected by the participants' activities of daily living, as we did not place strict limitations on their activities between testing sessions. Participants were asked not to stretch, massage, or ice the area and to refrain from taking any medications with anti-inflammatory or analgesic properties. Although these instructions were delivered to all participants after the exercise protocol, it is unlikely that we could control for such extraneous factors. In addition, we imposed no dietary restrictions or other intake instructions. We appreciate the effect of diet on recovery from exercise; however, we did not control for this and, therefore, it could have limited the study outcomes.
CONCLUSIONS
In conclusion, NIR light therapy was effective in attenuating strength loss after resistance exercise. Therefore, NIR light therapy may be a beneficial, noninvasive modality for improving muscle function during rehabilitation after musculoskeletal injury. The ability to enhance muscle performance during rehabilitation is of great benefit to patients when disuse atrophy and musculoskeletal pain impede recovery. However, the effect of laser on strength loss was relatively small and was nonexistent 48 hours after the treatment. Thus, future studies using higher treatment doses are warranted.
REFERENCES
- 1.Huang YY, Chen ACH, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. PM R. 2010;2(12 suppl 2):S292–S305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 4.Baroni BM, Leal Junior EC, De Marchi T, Lopes AL, Salvador M, Vaz MA. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010;110(4):789–796. doi: 10.1007/s00421-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 5.Leal Junior EC, Lopes-Martins RA, Dalan F, et al. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008;26(5):419–424. doi: 10.1089/pho.2007.2160. [DOI] [PubMed] [Google Scholar]
- 6.Leal Junior EC, Lopes-Martins RA, Vanin AA, et al. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009;24(3):425–431. doi: 10.1007/s10103-008-0592-9. [DOI] [PubMed] [Google Scholar]
- 7.Leal Junior EC, Lopes-Martins RA, Frigo L, et al. Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports. 2010;40(8):524–532. doi: 10.2519/jospt.2010.3294. [DOI] [PubMed] [Google Scholar]
- 8.Larkin KA, Martin JS, Zeanah EH, True JM, Braith RW, Borsa PA. Limb blood flow after class 4 laser therapy. J Athl Train. 2012;47(2):178–183. doi: 10.4085/1062-6050-47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maegawa Y, Itoh T, Hosokawa T, Yaegashi K, Nishi M. Effects of near-infrared low-level laser irradiation on microcirculation. Lasers Surg Med. 2000;27(5):427–437. doi: 10.1002/1096-9101(2000)27:5<427::AID-LSM1004>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Samoilova KA, Zhevago NA, Menshutina MA, Grigorieva NB. Role of nitric oxide in the visible light-induced rapid increase of human skin microcirculation at the local and systemic level, I: diabetic patients. Photomed Laser Surg. 2008;26(5):433–442. doi: 10.1089/pho.2007.2197. [DOI] [PubMed] [Google Scholar]
- 11.Baroni BM, Leal Junior EC, Geremia JM, Diefenthaeler F, Vaz MA. Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed Laser Surg. 2010;28(5):653–658. doi: 10.1089/pho.2009.2688. [DOI] [PubMed] [Google Scholar]
- 12.Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol. 2010;108(6):1083–1088. doi: 10.1007/s00421-009-1321-1. [DOI] [PubMed] [Google Scholar]
- 13.Desmet KD, Paz DA, Corry JJ, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24(2):121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 14.Borsa PA, Sauers EL. The importance of gender on myokinetic deficits before and after microinjury. Med Sci Sports Exerc. 2000;32(5):891–896. doi: 10.1097/00005768-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586(pt 1):11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindl A, Heinze G, Schindl M, Pernerstorfer-Schön H, Schindl L. Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res. 2002;64(2):240–246. doi: 10.1006/mvre.2002.2429. [DOI] [PubMed] [Google Scholar]
- 17.Tullberg M, Alstergren PJ, Ernberg MM. Effects of low-power laser exposure on masseter muscle pain and microcirculation. Pain. 2003;105(1–2):89–96. doi: 10.1016/s0304-3959(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 18.Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005;23(3):289–294. doi: 10.1089/pho.2005.23.289. [DOI] [PubMed] [Google Scholar]
- 19.Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol. 2000;76(6):863–870. doi: 10.1080/09553000050029020. [DOI] [PubMed] [Google Scholar]
- 20.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504(1):46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 21.Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg. 2005;23(1):3–9. doi: 10.1089/pho.2005.23.3. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Xing D, Gao X, Chen WR. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol. 2009;218(3):603–611. doi: 10.1002/jcp.21636. [DOI] [PubMed] [Google Scholar]


