Abstract
Context:
After an intense bout of exercise, foam rolling is thought to alleviate muscle fatigue and soreness (ie, delayed-onset muscle soreness [DOMS]) and improve muscular performance. Potentially, foam rolling may be an effective therapeutic modality to reduce DOMS while enhancing the recovery of muscular performance.
Objective:
To examine the effects of foam rolling as a recovery tool after an intense exercise protocol through assessment of pressure-pain threshold, sprint time, change-of-direction speed, power, and dynamic strength-endurance.
Design:
Controlled laboratory study.
Setting:
University laboratory.
Patients or Other Participants:
A total of 8 healthy, physically active males (age = 22.1 ± 2.5 years, height = 177.0 ± 7.5 cm, mass = 88.4 ± 11.4 kg) participated.
Intervention(s):
Participants performed 2 conditions, separated by 4 weeks, involving 10 sets of 10 repetitions of back squats at 60% of their 1-repetition maximum, followed by either no foam rolling or 20 minutes of foam rolling immediately, 24, and 48 hours postexercise.
Main Outcome Measure(s):
Pressure-pain threshold, sprint speed (30-m sprint time), power (broad-jump distance), change-of-direction speed (T-test), and dynamic strength-endurance.
Results:
Foam rolling substantially improved quadriceps muscle tenderness by a moderate to large amount in the days after fatigue (Cohen d range, 0.59 to 0.84). Substantial effects ranged from small to large in sprint time (Cohen d range, 0.68 to 0.77), power (Cohen d range, 0.48 to 0.87), and dynamic strength-endurance (Cohen d = 0.54).
Conclusions:
Foam rolling effectively reduced DOMS and associated decrements in most dynamic performance measures.
Key Words: pain, athletic performance, magnitude-based inference, massage
Key Points
The delayed-onset muscle soreness (DOMS) protocol effectively induced DOMS and substantially decreased performance measures.
After the DOMS protocol, foam rolling enhanced recovery and reduced physical performance decrements.
A 20-minute bout of foam rolling on a high-density roller immediately postexercise and every 24 hours thereafter may reduce muscle tenderness and decrements in multijointed dynamic movements due to DOMS.
Self-massage through foam rolling could benefit athletes seeking a recovery modality that is relatively affordable, easy to perform, and time efficient and that enhances muscle recovery.
Exercise often can induce various degrees of fatigue in the musculoskeletal, nervous, and metabolic systems. Various amounts of discomfort or pain and inflammation can be associated with exercise, depending on the frequency, intensity, duration, and type of exercise performed. After intense exercise, this discomfort and pain commonly are associated with disruption of the intracellular muscle structure, sarcolemma, and extracellular matrix, which leads to prolonged impairment of muscle function and delayed-onset muscle soreness (DOMS).1 Several physiologic theories, including excitation-contraction coupling impairment, damage to various muscle fibers, metabolic impairments, and fatigue, have been proposed to explain how DOMS impairs muscle function.
Delayed-onset muscle soreness is classified as a type 1 muscle strain,2 produces tenderness or stiffness to palpation or movement,2 and predominantly is seen in or amplified by unaccustomed exercise.3 Sensations associated with DOMS are highly variable and range from slight muscle stiffness that subsides with regular daily activity to severely debilitating pain that restricts any movement.3 Typically, the intensity of DOMS increases within the first 24 hours postexercise, peaks between 24 and 72 hours, and subsides and eventually disappears in 5 to 7 days.4
In terms of athletic performance, DOMS can have negative consequences. Muscle soreness and structural damage to muscles and connective tissue may result in altered muscle function and joint mechanics.5 These alterations may substantially reduce performance or optimal training intensity for athletes.3 In a recent review, Byrne et al1 reported the negative effects of DOMS on sprint, power, jump height, and drop-jump performance, all of which are important during many athletic events. Other DOMS-induced impairments that may reduce athletic performance include decreased joint proprioception,6 overestimation of force production,6 decreased joint range of motion,7 decreased strength and power measures,8 alterations in agonist and antagonist strength ratios,9 changes in recruitment patterns,3 and increased risk of injury.10 With any combination of these, joint mechanics and muscle function are compromised; thus, individuals adapt compensatory movement patterns,6 possibly leading to reduced athletic performance. Although the aforementioned literature demonstrates the negative consequences of DOMS, research on DOMS and athletic performance (ie, sprinting, jumping, agility) has received little attention.1
Massage is an intervention technique commonly proposed to prevent DOMS. Researchers11,12 have shown decreases in pain associated with DOMS after postexercise massage. However, it is unclear whether postexercise massage is beneficial for muscular function. Various combinations of therapist-provided massage had no effect on isokinetic11,12 and isometric muscle force.12 Viitasalo et al13 showed that warm, underwater jet massage was beneficial for continuous jumping power during a week of intense training. Thus, massage may be beneficial for multijoint dynamic measures but not for isometric and single-joint exercises.
Another form of massage that therapists use to aid recovery (ie, to alleviate DOMS) is foam rolling, which has become a common practice for treating or preventing soft tissue restrictions. During foam rolling, individuals use their own body mass on a foam roller to exert pressure on the soft tissue. The motions place both direct and sweeping pressure on the soft tissue, stretching it and generating friction between it and the foam roller. Foam rolling can be considered a form of self-induced massage because the pressure that the roller exerts on the muscles resembles the pressure exerted on the muscles through manual manipulation by a massage therapist. To our knowledge, there is only one study14 in the literature that has examined the effects of foam rolling on neuromuscular performance for up to 72 hours post-DOMS. Compared with the control group, the foam-rolling group had reduced muscle soreness and increased voluntary muscle activation, vertical jump height, and flexibility. In a non-DOMS study,15 it was also found that an acute bout of foam rolling increased range of motion without subsequently decreasing neuromuscular (isometric) function. Similar to massage, foam rolling may benefit recovery of dynamic (multijoint, sport-specific movements) measures for the duration of the DOMS. Hence, it is plausible that foam rolling will aid in recovery from DOMS and help to maintain physical performance. Therefore, the purpose of our study was to determine the effects of self-induced massage via foam rolling as a recovery tool from an intense exercise protocol (10 × 10 barbell back squat) on the pressure-pain threshold, sprint speed (30-m sprint time), power (broad-jump distance), change-of-direction speed, and dynamic strength-endurance (maximal number of squat repetitions at 70% of the 1-repetition maximum [1RM]).
METHODS
Participants
Eight healthy men (age = 22.1 ± 2.5 years, height = 177.0 ± 7.5 cm, mass = 88.4 ± 11.4 kg) from a university population volunteered for the study. All participants were recreational resistance trainers and were classified by the Canadian Society for Exercise Physiology as moderately to very physically active. They completed a Physical Activity Readiness Questionnaire16 before participation. All participants provided written informed consent, and The Memorial University of Newfoundland Human Investigation Committee approved the study.
Experimental Design
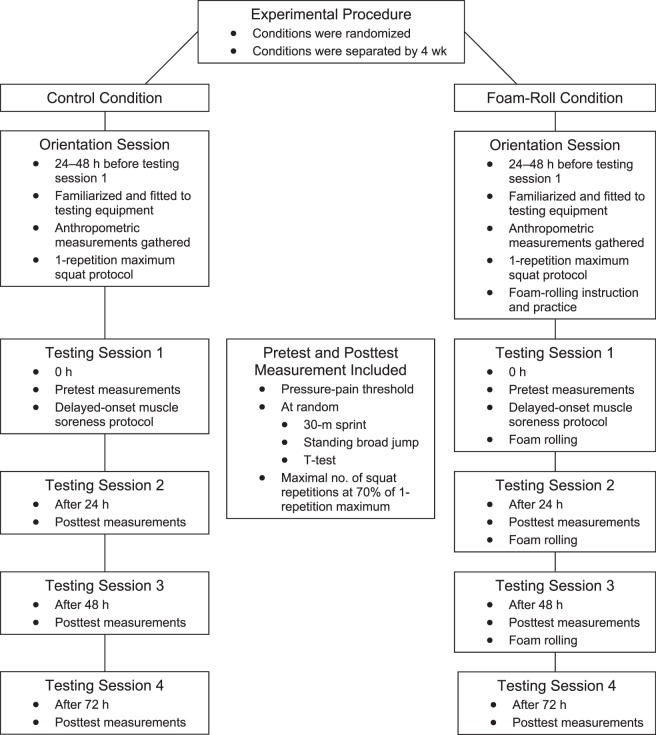
We used a repeated-measures design to examine the effects of foam rolling the quadriceps, adductors, hamstrings, iliotibial (IT) band, and gluteal muscles on the following dependent variables: (1) pressure-pain threshold of the quadriceps, (2) sprint speed (30-m sprint time), (3) power (broad-jump distance), (4) change-of-direction speed (T-test), and (5) dynamic strength-endurance (maximal back 15-squat repetitions at 70% of 1RM) after a 10 × 10 squat protocol, which hereafter is referred to as the DOMS protocol. All participants performed the 2 experimental conditions: control and foam rolling. The conditions were separated by 4 weeks so the DOMS protocol would be unaccustomed and, therefore, more likely to induce DOMS.3 The order of condition was assigned randomly. The conditions consisted of an orientation session and 4 testing sessions and were separated by 4 weeks. During the control condition, the orientation session consisted of a 1-RM squat17 and practice of each test. During the foam-rolling condition, the orientation session was similar to that of the control condition, but the participants were also introduced to the foam-rolling techniques.
At the beginning of each testing session, we measured the pressure-pain threshold of the quadriceps. Next, participants performed a warm-up on a cycle ergometer (model Ergomedic 839E; Monark Exercise AB, Vansbro, Sweden) for 5 minutes at an intensity of 70 revolutions per minute and 9.8 N (1 kp). Testing session 1 (consisting of baseline measurements and the DOMS protocol) commenced 24 to 48 hours after the control or foam-rolling orientation session. Testing sessions 2, 3, and 4 were conducted at 24, 48, and 72 hours, respectively, after the DOMS exercise protocol. During all testing sessions, 3 of 4 dependent variables (sprint speed, T-test, and power) were measured 2 consecutive times with a 4-minute rest between trials in a randomized order at baseline (preexercise) and 24, 48, and 72 hours post-DOMS protocol. Dynamic strength-endurance was performed last in each of the 4 testing sessions because the fatigue induced by this test would adversely affect performance of the other tests. All participants performed 10 repetitions at 35% of 1RM as a squat warm-up. After 4 minutes of rest, they executed maximal-repetition barbell back squats at 70% 1RM. In the foam-rolling condition, participants foam rolled after testing sessions 1, 2, and 3 were completed. Given that all participants had experience performing the dependent variable tests and had practiced each test in the orientation session, no learning effects for the dependent variables were present. Details of the experimental design are provided in Figure 1.
Figure 1.
The experimental procedure. Measurements before and after the delayed-onset muscle soreness protocol consisted of pressure-pain threshold, sprint speed (30-m sprint time), power (broad-jump distance), change-of-direction speed, and dynamic strength-endurance (maximal squat repetitions with a 70% of 1-repetition maximum load).
Participants were instructed to refrain from heavy exercise 24 hours before and throughout the experiment and to follow the Canadian Society for Exercise Physiology preliminary instructions (no eating, drinking caffeine, smoking, or drinking alcohol for 2, 2, 2, or 6 hours, respectively) before each test session.
Exercise
The exercise protocol consisted of participants performing 10 sets of 10 repetitions of barbell back squats at 60% of their 1RMs to a predetermined depth (Figure 2). Squat technique was adopted from the National Strength and Conditioning Association.17 The tempo for each repetition was a 4-second eccentric contraction, no pause, and a 1-second concentric contraction. Participants rested for 2 minutes between sets. Total squat time was 8 minutes, 20 seconds, and rest time was 18 minutes. We emphasized eccentric contractions because repetitive eccentric exercise has been shown to result in more DOMS than conventional weight training that emphasizes concentric contractions.3
Figure 2.
A participant demonstrates the depth achieved during all back squats. We stacked 5-cm spacers so that when the participants were at the end phase of the eccentric portion of the squat repetition, their femurs were parallel to the floor. Participants were required to touch the top of the stack during each squat repetition.
Foam Rolling
Participants used a custom-made foam roller that was constructed of a hollow polyvinylchloride pipe that had a 10.16-cm outer diameter and 0.5-cm thickness and was surrounded by neoprene foam with a 1-cm thickness. They were instructed to begin with the foam roller at the most distal portion of the muscle. We instructed them to place as much body mass as tolerable on the foam roller at all times and to roll their body mass back and forth along the roller as smoothly as possible at a cadence of 50 beats per minute (ie, 1 rolling motion per 1.2 seconds). Foam rolling was performed for 45 seconds and followed by a 15-second rest. This was accomplished for each muscle group in each lower extremity and repeated once. Total foam-rolling time, including rest, was 20 minutes.
Foam rolling was performed directly after we recorded the test measurements in testing sessions 1 (DOMS protocol), 2 (24 hours post-DOMS protocol), and 3 (48 hours post-DOMS protocol). Whereas DOMS was not immediately evident after testing session 1, we chose this time to foam roll because massage has been shown to enhance blood lactate removal and tissue healing.18 Furthermore, participants foam rolled after testing sessions 2 and 3 because the intensity of DOMS increases within the first 24 hours and peaks around 48 hours postexercise.4 We chose these time points because no empirical evidence recommending the most optimal duration and timing of postexercise foam rolling was available.
The foam-rolling technique for each muscle and the order in which each muscle was foam rolled follows.
Quadriceps
Starting in a prone position with the roller approximately 3 in (7.62 cm) inferior to the anterior-superior iliac spine, participants crossed 1 leg over the other (Figure 3A). They rolled down to a position superior to the patellar tendon and back using their elbows to guide movement.
Figure 3.
A participant demonstrates the 5 muscle groups foam rolled and the technique used for each muscle group. Foam rolling consisted of 45 seconds of rolling for each muscle in the left lower extremity, 15 seconds of rest, 45 seconds on the right lower extremity, and 15 seconds of rest for all muscles in the following order: A, quadriceps, B, adductors, C, hamstrings, D, iliotibial band, and E, gluteals. Total foam-rolling time was 20 minutes (15 minutes of rolling and 5 minutes of rest).
Adductors
Starting in a prone position with the hip flexed and externally rotated, participants positioned themselves on the roller with the proximal portion of the adductor group just inferior to the inguinal area (Figure 3B). They rolled down to a position superior to the medial condyle and back by shifting their body mass from side to side.
Hamstrings
Starting just inferior to the gluteal fold with the hips unsupported, participants crossed 1 foot over the other (Figure 3C). Their body mass was supported and maneuvered by the hands, which were posterior to the body. They rolled from the starting position down to the superior portion of the popliteal fossa and back.
Iliotibial Band
Starting in a side-lying position just inferior to the greater trochanter, participants placed the free lower extremity anterior to the supported extremity (Figure 3D). They rolled down to just superior to the lateral condyle and back with the free foot guiding the movement.
Gluteals
Starting just inferior to the posterior portion of the iliac crest on the lateral portion of the gluteal region, participants crossed 1 foot over the opposite knee in a figure-4 formation while supporting the body on 1 hand (Figure 3E). Using the support hand, they rolled down to a position superior to the gluteal fold and back.
Criterion Variables
Pressure-Pain Threshold
The pressure-pain threshold is used to assess muscle tenderness and is defined as the minimal amount of pressure that causes pain.19 A higher pressure-pain threshold indicates a smaller amount of muscle tenderness. We measured the pressure-pain threshold for each participant's right quadriceps at the beginning of each exercise session, before any other testing. Participants were instructed to say “yes” at the instant they felt pain rather than pressure.
With the participant in a relaxed standing position, the probe of an algometer (model 01163; Layfayette Instrument Company, Lafayette, IN) with a 1.0-cm2 stimulation area was placed into the midline of the right quadriceps midway between the iliac crest and the superior border of the patella. Force was gradually applied at a constant rate of 50 to 60 kPa/s until the participant indicated pain was present. Participants completed 3 trials with a 30-second interval between measurements, and data were recorded in kilograms per centimeters squared and converted to kilopascals (1 kg/cm2 = 98.1 kPa). The mean of the 3 trials was used for analysis.
Sprint Speed
Participants performed a 30-m sprint on an indoor synthetic track. They completed 2 submaximal sprint trials of increasing intensity with a 4-minute break between trials. Next, 2 maximal sprint trials were completed with a 4-minute break between trials. During the break, participants pursued a semiactive recovery as they walked slowly back to the starting line. Light gates (Kinematic Measurement System; Fitness Technology, Skye, South Australia) were used to record the time of the sprint. Participants started 0.5 m behind the first gate in a controlled 3-point stance with the dominant foot slightly behind the nondominant foot. Lights were set at a height of 0.4 m above the ground. Participants were instructed to start and were encouraged to give maximal effort when they were ready. Time started when they crossed the first gate, eliminating cognitive reaction-time factors, and ended when they crossed the second gate, which was 30 m away from the first. The faster of the 2 trials was used for analysis.
Power
A standing broad jump was used to measure dynamic power. Participants were instructed to stand with their feet 1 shoulder-width apart, jump out as far as they could, and land in a controlled manner on 2 feet without taking a step to maintain balance. We measured the jump from the toes of the starting position to the closest heel of the landing position. Each participant completed 2 trials that were separated by 4 minutes of rest. The farthest of the 2 jump trials was used for analysis.
Change-of-Direction Speed
We used the standard T-test to assess change-of-direction speed. The T-test was adopted from the National Strength and Conditioning Association.17 Light gates also were used to record the T-test time. Participants performed 1 submaximal trial of the T-test followed by a 4-minute break. Next, they performed 2 maximal trials with 4-minute rest periods between trials. Participants started 0.5 m behind the first gate in a 3-point stance, with the dominant foot slightly behind the nondominant foot. Lights were set at a height of 0.4 m above the ground. Participants were instructed to start and encouraged to give maximal effort when they were ready. Time started when they crossed the first gate and ended when they crossed the same gate at the end of the test. The faster of the 2 trials was used for analysis.
Dynamic Strength-Endurance
Dynamic strength-endurance was measured with barbell back squats at 70% of the participant's 1RM for a maximal number of repetitions. Participants were instructed to perform 1 set of back squats at 35% of their 1RM for 10 repetitions to a predetermined depth. They then performed back-squat repetitions with a 1-second eccentric phase, with no pause at the bottom, and a 1-second concentric phase. Participants performed repetitions to concentric failure or until squat technical criteria17 were no longer maintained. Only 1 trial was completed due to fatiguing effects.
Data Analysis
For data analysis, we used magnitude-based inferences and precision of estimation.20 Performance measures were log transformed before analysis to reduce the nonuniformity of error. Magnitude-based inferences on the interaction effects in the mean changes between the intervention trials (control and foam rolling) were determined. The interaction effect of time and foam rolling was calculated from the mean difference between preexercise and each time point (preexercise to 24, 48, and 72 hours postexercise) for the control and foam-rolling trials. The 2 differences then were subtracted to estimate the effect of foam rolling at each time point.
Qualitative descriptors of standardized effects were assessed using the following criteria: trivial (<0.2), small (0.2–0.5), moderate (0.5–0.8), and large (>0.8).21 Effects with 95% confidence limits (CLs) overlapping the thresholds for small positive and negative effects (exceeding 0.2 of the standard deviation on both sides of the null) were defined as unclear. Clear small or larger effect sizes were defined as substantial.22 Precision of estimates is indicated with mean difference ± 95% CLs, which defines the range representing the uncertainty in the true value of the unknown population mean. All magnitude-based inference calculations were performed in Excel (version 2007; Microsoft Corporation, Redmond, WA).
RESULTS
All raw data (mean ± SD) collected throughout the experimental conditions are presented in the Table.
Table.
Raw Data for All Dependent Variables Throughout the Experimental Conditions
| Test |
Time Point, h (Mean ± SD) |
|||
| Before Delayed-Onset Muscle Soreness |
24 |
48 |
72 |
|
| Foam roll | ||||
| 1-Repetition maximum squat, kg | 145.41 ± 31.50 | NA | NA | NA |
| Pressure-pain threshold, kPa | 940.78 ± 215.82 | 767.14 ± 168.73 | 758.31 ± 240.35 | 832.87 ± 205.03 |
| 30-m Sprint time, s | 4.39 ± 0.18 | 4.49 ± 0.20 | 4.53 ± 0.22 | 4.44 ± 0.17 |
| Broad-jump distance, cm | 226.75 ± 28.36 | 217.75 ± 22.11 | 219.00 ± 21.97 | 222.13 ± 20.79 |
| Change-of-direction speed, s | 10.28 ± 0.60 | 10.62 ± 0.62 | 10.44 ± 0.55 | 10.41 ± 0.62 |
| Squat repetitions, No. | 17.00 ± 6.59 | 13.88 ± 6.90 | 17.75 ± 6.69 | 17.38 ± 8.07 |
| Control | ||||
| 1-Repetition maximum squat, kg | 142.58 ± 33.73 | NA | NA | NA |
| Pressure-pain threshold, kPa | 934.90 ± 247.21 | 691.61 ± 190.31 | 650.4 ± 214.8 | 821.10 ± 253.10 |
| 30-m Sprint time, s | 4.38 ± 0.14 | 4.54 ± 0.17 | 4.51 ± 0.26 | 4.51 ± 0.22 |
| Broad-jump distance, cm | 233.88 ± 26.91 | 218.50 ± 26.76 | 215.00 ± 32.02 | 216.50 ± 29.25 |
| Change-of-direction speed, s | 10.40 ± 0.61 | 10.63 ± 0.43 | 10.60 ± 0.62 | 10.58 ± 0.52 |
| Squat repetitions, No. | 16.88 ± 5.64 | 13.50 ± 7.05 | 15.25 ± 6.48 | 16.63 ± 7.87 |
Abbreviation: NA, not applicable.
Effect of DOMS on Pain
Magnitude-based inferences demonstrated that DOMS reduced the pressure-pain threshold. It was almost certain (≥99% likely) that a large decrease in quadriceps pressure-pain threshold occurred 24 hours postexercise (245.25 kPa; 95% CL = 76.5, 410.1) and 48 hours postexercise (284.5 kPa; 95% CL = 87.3, 480.7), but only a small decrease occurred 72 hours postexercise (117.7 kPa; 95% CL = −77.5, 305.1).
Effect of DOMS on Performance
Magnitude-based inferences demonstrated that DOMS negatively affected all subsequent performance measurements when no foam rolling was performed. The increased 30-m sprint time was almost certain to be substantial at all 3 time points (≥97% likely), increasing by a large amount at 24 hours (0.17 seconds; 95% CL = 0.11, 0.22), 48 hours (0.16 seconds; 95% CL = 0.00, 0.33), and 72 hours (0.13 seconds, 95% CL = 0.04, 0.23) postexercise.
The reduction in broad-jump distance was almost certain (≥99% likely), decreasing by a moderate amount at 24 hours (15 cm; 95% CL = 8, 22), 48 hours (19 cm; 95% CL = 8, 30), and 72 hours (17 cm; 95% CL = 10, 25) postexercise.
The increase in change-of-direction speed time was also likely to be substantial (>75% likely), increasing a small amount at 24 hours (0.23 seconds; 95% CL = −0.14, 0.60), a moderate amount at 48 hours (0.31 seconds; 95% CL = −0.03, 0.65), and a small amount at 72 hours (0.19 seconds; 95% CL = 0.04, 0.42) postexercise.
A reduction in squat repetitions occurred at 24 hours postexercise (95% likely, 3.4 repetitions, moderate effect; 95% CL = 0.45, 6.3) but was unclear by 48 hours (69% likely, 1.6 repetitions, small effect; 95% CL = −0.69, 3.94) and 72 hours (61% likely, 0.25 repetitions, trivial effect; 95% CL = −0.26, 3.1) postexercise.
Effect of Foam Rolling on DOMS
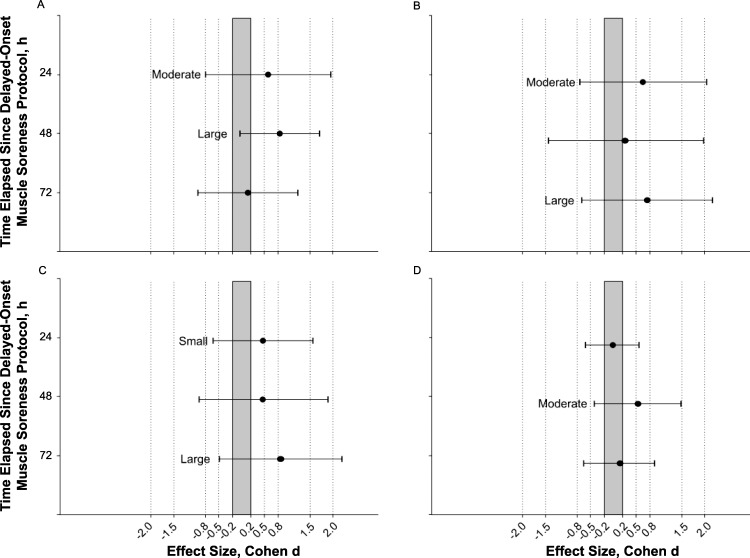
Foam rolling had a moderate effect on the decline in the pressure-pain threshold of the quadriceps at 24 hours postexercise (74% likely, 88.29 kPa; 95% CL = −120.66, 297.24) and a large effect at 48 hours postexercise (94% likely, 140.28; 95% CL = −7.95, 288.31), but it was unlikely (44% likely) to have a substantial effect at 72 hours postexercise (Figure 4A). That is, the quadriceps felt substantially better 24 and 48 hours after exercise with foam rolling.
Figure 4.
Magnitude-based inferences demonstrating the effect of foam rolling on A, muscle tenderness, B, sprint speed (30-m sprint time), C, power (broad-jump distance), and D, dynamic strength-endurance (maximal squat repetitions with a 70% of 1-repetition maximum load) after the delayed-onset muscle soreness (DOMS) protocol. Points represent the effect size (Cohen d) describing the interaction effect of foam rolling to control between each time point and pre-DOMS protocol. Error bars represent 95% confidence limits for the mean effect. A point in the shaded region represents a clinically trivial effect.
Effect of Foam Rolling on DOMS Protocol-Induced Decrements in Performance
We found that foam rolling had a moderate effect on the increase in sprint time at 24 hours (77% likely, 0.06 seconds; 95% CL = −0.07, 0.19) and at 72 hours (81% likely, 0.08 seconds; 95% CL = −0.08, 0.24) postexercise (Figure 4B). That is, sprint time was substantially less affected at the 24- and 72-hour postexercise time points with foam rolling.
Foam rolling had a small effect on the decline in broad-jump performance after exercise at 24 hours postexercise (72% likely, 5 cm; 95% CL = −6.5, 16.5) but a large effect at 72 hours postexercise (86% likely, 11 cm; 95% CL = −6.5, 28; Figure 4C). That is, the broad jump demonstrated less of a decline after foam rolling.
We found that the decrement in change-of-direction speed was unlikely to be affected by foam rolling by an amount greater than the smallest worthwhile change postexercise (all likelihood <65%).
Foam rolling had a moderate effect on squat repetitions at 48 hours postexercise, with participants squatting 1.9 repetitions more (79% likely; 95% CL = −1.5, 5.3), but any effect of foam rolling on squat repetitions 24 hours and 72 hours postexercise was unlikely (all likelihoods <55%; Figure 4D).
DISCUSSION
After intense bouts of exercise, many individuals use foam rolling to aid in recovery from muscular fatigue and soreness. We examined the use of foam rolling after a DOMS-inducing 10 × 10 squat protocol. Our most important findings were that (1) the protocol was a very effective way to induce DOMS and substantially decreased performance measures and (2) foam rolling enhanced recovery from DOMS and reduced physical performance decrements after the DOMS protocol. More specifically, foam rolling resulted in increased pressure-pain threshold score, sprint speed, power, and dynamic strength-endurance at various time points after exercise compared with the control condition. Our results provide strong evidence that foam rolling can reduce DOMS and the associated decrements in performance.
Delayed-onset muscle soreness is characterized by variable amounts of muscle tenderness, stiffness, and pain that can fluctuate from slight muscle stiffness on palpation to severe debilitation of athletic performance.2,5 The 10 × 10 squat protocol, which included 1-second concentric and 3-second eccentric contractions, promoted the development of severe DOMS and substantially decreased physical performance. Studies2,3,6,8,10–12,14,18,23,24 emphasizing eccentric exercise have resulted in muscular pain along with decrements in performance. In our study, pressure-pain thresholds were decreased at all time points postexercise, indicating an increase in muscle tenderness. We also observed decrements in performance measures for all dependent variables throughout the 72 hours postexercise: sprint time increased 3.02% to 3.77%, power decreased 6.57% to 8.07%, change-of-direction speed increased 1.8% to 2.2%, and squat repetition number decreased 1.48% to 20%. The reduction in performance may be due to a combination of several factors, including (1) physiologic damage to sarcomeres during intense exercise, such as tearing of Z-lines25; (2) a reduction in strength due to acute muscular fatigue26; (3) decreased range of motion; (4) increased inflammation; and (5) trepidation resulting from the pain of movement.27 The exact mechanism responsible for the reduction in performance remains unclear.
The reductions in performance differed between the control and foam-rolling groups. Similar to the effect of postexercise massage, foam rolling appears to aid in the recovery of muscle tenderness associated with DOMS. Similar to other research,14 we found that a 20-minute foam-rolling session caused participants to experience substantially less muscle tenderness. Researchers have shown that massage may reduce the pain associated with DOMS. However, it is unclear whether massage is an effective treatment for improving muscular function after DOMS. A combination of effleurage, tapotement, and petrissage had no effect on isokinetic11,12 and isometric muscle force.12 On the other hand, Viitasalo et al13 showed that warm underwater jet massage caused less of a decrease in continuous jumping power and less of an increase in ground contact time during a week of intense physical training. Similarly, in our study, foam rolling was beneficial for the recovery of physical performance involving dynamic movements after exercise that induced DOMS. Perhaps isometric and isokinetic measures of single-joint muscle performance are unaffected by massage and foam rolling, whereas dynamic movements involving multiple joints, such as jumping, squatting, and sprinting, may benefit.14
Among the different dynamic performance measures in our study, a discrepancy seems to exist as to how foam rolling affected each variable. Foam rolling had a trivial effect on change-of-direction speed, likely because performing a T-test requires a large amount of motor control and coordination due to the complex interaction of multiple muscles producing multiple actions (eg, acceleration; deceleration; lateral, forward, and backward movement).28 However, in contrast, foam rolling positively affected both sprint speed and power performances at 24 and 72 hours postexercise. These movements involve acceleration of the body in a single direction. Furthermore, these results are not surprising given that researchers have demonstrated very small correlations between T-test and jumping or sprinting performance,29 whereas strong correlations have been reported between sprinting and various measures of jumping performance.30 Regardless of whether the participants foam rolled, their total number of squat repetitions decreased similarly at 24 hours post-DOMS protocol. Squat repetitions returned to preexercise values by 48 hours postexercise during the foam-roll condition but not until 72 hours postexercise for the control condition. Whereas some substantial effects may have been missed due to our small number of participants, we observed a sufficient number of substantial effects to indicate that foam rolling is likely to be an effective DOMS recovery modality. Although some of the observed benefits we declare as likely have a possibility of clinically trivial or even harmful effects based on the magnitude of the 95% CLs, estimating clinical likelihoods requires a sufficiently substantial portion of the 95% CL to be greater than a trivial effect size. The likely accelerated recovery of physical performance measures could be critical for athletes training or competing with short durations of rest.
Various postulated mechanisms may explain why foam rolling enhanced the recovery from muscle tenderness and associated dynamic performance measures throughout the 72 hours postexercise. The most common mechanisms are decreased edema, enhanced blood lactate removal, and enhanced tissue healing,17 which are mainly due to the increase in muscular blood flow.31,32 Increased blood flow hinders the margination of neutrophils and reduces prostaglandin production, subsequently decreasing inflammation.24 Massage-induced muscular blood flow also increases oxygen delivery, which encourages mitochondrial resynthesis of adenosine triphosphate and the active transport of calcium back into the sarcoplasmic reticulum.4 However, considering that the roles of lactate and adenosine triphosphate depletion in fatigue are widely disputed, these explanations seem unlikely. Regardless, the action of foam rolling, similar to massage, could increase muscular blood flow and result in an enhanced recovery from DOMS in other ways. For example, foam rolling may have a systemic biochemical effect.24,33 Massage-related biochemical changes include (1) increased circulating neutrophil levels24; (2) smaller increases in postexercise plasma creatine kinase24; (3) activated mechanosensory sensors that signal transcription of COX7B and ND1, indicating that new mitochondria are being formed and presumably accelerating the healing of the muscle33; and (4) less active heat-shock proteins and immune cytokines, reflecting less cellular stress and inflammation.33 These biochemical changes were due to massage that applied constant pressure to the muscle. Perhaps the constant pressure on the muscle from foam rolling resulted in biochemical changes similar to those reported earlier.
Foam rolling substantially reduced the negative effect of DOMS on dynamic movements, which incorporate power, strength, and endurance. The foam-rolling–induced enhancement of recovery after the exercise protocol may have been due to a reduction in reduced pain, increased voluntary activation,14 and various other aforementioned mechanisms that were characterized by massage research. Given that only 2 studies support foam rolling as a recovery technique, it is unclear whether the foam-rolling prescription in our study optimized the duration, muscles, intensity, and number of sessions to achieve the best recovery. The time chosen for participants to foam roll was based on current clinical recommendations that 20 minutes of foam rolling is a substantial amount of time. Researchers should examine the frequency, intensity (amount of pressure placed on the foam roller), time (immediately postexercise versus other time points), and type (sweeping pressure versus undulations) of foam rolling that optimizes recovery after intense physical performance events.
CONCLUSIONS
Athletes commonly must train or compete during consecutive days despite discomfort and pain they may have sustained from previous exercise. At times of severe DOMS, athletes can experience decrements in physical performance up to and beyond 72 hours postexercise. To combat the adverse effects of DOMS, a 20-minute bout of foam rolling on a high-density roller immediately postexercise and every 24 hours thereafter may reduce the likelihood of muscle tenderness and decrements in multijointed dynamic movements. Just three 20-minute bouts (60 minutes total) of foam rolling can substantially enhance recovery after DOMS and alleviate muscle tenderness. This form of self-induced massage could benefit athletes seeking a recovery modality that is relatively affordable, easy to perform, and time efficient and that enhances muscular recovery.
REFERENCES
- 1.Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34(1):49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Gulick DT. Delayed onset muscle soreness: what is it and how do we treat it? J Sport Rehabil. 1996;5(3):234–243. [Google Scholar]
- 3.Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med. 2003;33(2):145–164. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16(6):529–538. [PubMed] [Google Scholar]
- 5.Rowlands AV, Eston RG, Tilzey C. Effect of stride length manipulation on symptoms of exercise-induced muscle damage and the repeated bout effect. J Sports Sci. 2001;19(5):333–340. doi: 10.1080/02640410152006108. [DOI] [PubMed] [Google Scholar]
- 6.Saxton JM, Clarkson PM, James R, et al. Neuromuscular dysfunction following eccentric exercise. Med Sci Sports Exerc. 1995;27(8):1185–1193. [PubMed] [Google Scholar]
- 7.Behm DG, Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol. 2011;111(11):2633–2651. doi: 10.1007/s00421-011-1879-2. [DOI] [PubMed] [Google Scholar]
- 8.Brown SJ, Child RB, Day SH, Donnelly AE. Indices of skeletal muscle damage and connective tissue breakdown following eccentric muscle contractions. Eur J Appl Physiol Occup Physiol. 1997;75(4):369–374. doi: 10.1007/s004210050174. [DOI] [PubMed] [Google Scholar]
- 9.Orchard J, Marsden J, Lord S, Garlick D. Preseason hamstring muscle weakness associated with hamstring muscle injury in Australian footballers. Am J Sports Med. 1997;25(1):81–85. doi: 10.1177/036354659702500116. [DOI] [PubMed] [Google Scholar]
- 10.Smith LL. Causes of delayed onset muscle soreness and the impact on athletic performance: a review. J Appl Sport Sci Res. 1992;6(3):135–141. [Google Scholar]
- 11.Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sports Med. 2003;37(1):72–75. doi: 10.1136/bjsm.37.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zainuddin Z, Newton M, Sacco P, Nosaka K. Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J Athl Train. 2005;40(3):174–180. [PMC free article] [PubMed] [Google Scholar]
- 13.Viitasalo JT, Niemela K, Kaappola R, et al. Warm underwater water-jet massage improves recovery from intense physical exercise. Eur J Appl Physiol Occup Physiol. 1995;71(5):431–438. doi: 10.1007/BF00635877. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald G, Button DC, Drinkwater E, et al. Foam rolling as a recovery tool after an intense bout of physical activity. Med Sci Sports Exerc. 2014;46(1):131–142. doi: 10.1249/MSS.0b013e3182a123db. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald G, Penney MD, Mullaley ME, et al. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res. 2013;27(3):812–821. doi: 10.1519/JSC.0b013e31825c2bc1. [DOI] [PubMed] [Google Scholar]
- 16.Canadian Society for Exercise Physiology (CSEP) The Canadian Physical Activity, Fitness & Lifestyle Approach (CPAFLA): CSEP-Health & Fitness Program's Health-Related Appraisal and Counselling Strategy. 3rd ed. Ottawa, ON: Canadian Society for Exercise Physiology and Health Canada;; 2003. p. 8. [Google Scholar]
- 17.Baechle TR, Earle RW. Essentials of Strength Training and Conditioning: National Strength and Conditioning Association. 3rd ed. Champaign, IL: Human Kinetics; 2008;264:350–351. [Google Scholar]
- 18.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–256. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fischer AA. Pressure algometry over normal muscles: standard values, validity and reproducibility of pressure threshold. Pain. 1987;30(1):115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates;; 1988. p. 99. [Google Scholar]
- 22.Liow DK, Hopkins WG. Velocity specificity of weight training for kayak sprint performance. Med Sci Sports Exerc. 2003;35(7):1232–1237. doi: 10.1249/01.MSS.0000074450.97188.CF. [DOI] [PubMed] [Google Scholar]
- 23.Francis KT, Hoobler T. Effects of aspirin on delayed muscle soreness. J Sports Med Phys Fitness. 1987;27(3):333–337. [PubMed] [Google Scholar]
- 24.Smith LL, Keating MN, Holbert D, et al. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: a preliminary report. J Orthop Sports Phys Ther. 1994;19(2):93–99. doi: 10.2519/jospt.1994.19.2.93. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen D, Brown LE, Coburn JW, et al. Effect of delayed-onset muscle soreness on elbow flexion strength and rate of velocity development. J Strength Cond Res. 2009;23(4):1282–1286. doi: 10.1519/JSC.0b013e3181970017. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto A, Maruyama T, Naito H, Sinclair PJ. Acute effects of high-intensity dumbbell exercise after isokinetic eccentric damage: interaction between altered pain perception and fatigue on static and dynamic muscle performance. J Strength Cond Res. 2010;24(8):2042–2049. doi: 10.1519/JSC.0b013e3181d8e881. [DOI] [PubMed] [Google Scholar]
- 27.Bottas R, Nicol C, Komi PV, Linnamo V. Adaptive changes in motor control of rhythmic movement after maximal eccentric actions. J Electromyogr Kinesiol. 2009;19(2):347–356. doi: 10.1016/j.jelekin.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Young W, Hawken M, McDonald L. Relationship between speed, agility and strength qualities in Australian Rules football. Strength Cond Coach. 1996;4(4):3–6. [Google Scholar]
- 29.Sassi RH, Dardouri W, Yahmed MH, Gmada N, Mahfoudhi ME, Gharbi Z. Relative and absolute reliability of a modified agility T-test and its relationship with vertical jump and straight sprint. J Strength Cond Res. 2009;23(6):1644–1651. doi: 10.1519/JSC.0b013e3181b425d2. [DOI] [PubMed] [Google Scholar]
- 30.Shalfawi SA, Sabbah A, Kailani G, Tonnessen E, Enoksen E. The relationship between running speed and measures of vertical jump in professional basketball players: a field-test approach. J Strength Cond Res. 2011;25(11):3088–3092. doi: 10.1519/JSC.0b013e318212db0e. [DOI] [PubMed] [Google Scholar]
- 31.Cafarelli E, Flint F. The role of massage in preparation for and recovery from exercise: an overview. Sports Med. 1992;14(1):1–9. doi: 10.2165/00007256-199214010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Hovind H, Nielsen SL. Effect of massage on blood flow in skeletal muscle. Scand J Rehabil Med. 1974;6(2):74–77. [PubMed] [Google Scholar]
- 33.Crane JD, Ogborn DI, Cupido C, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4(119) doi: 10.1126/scitranslmed.3002882. ra113. [DOI] [PubMed] [Google Scholar]