Abstract
We aimed to explore the effects of 90Y-DOTATOC and 90Y-DOTATOC plus 177Lu-DOTATOC on survival of patients with metastasized gastrinoma. Patients with progressive metastasized gastrinoma were treated with repeated cycles of 90Y-DOTATOC or with cycles alternating between 90Y-DOTATOC and 177Lu-DOTATOC until tumor progression or permanent toxicity. Multivariable Cox regression analyses were used to study predictors of survival. A total of 36 patients were enrolled; 30 patients received 90Y-DOTATOC (median activity per patient 11.8GBq; range: 6.1-62.2GBq) and 6 patients received 90Y-DOTATOC plus 177Lu-DOTATOC (median activity per patient: 14.8GBq; range: 7.4-14.8GBq). Response was found in 26 patients (72.2%), including morphological (n=12, 33.3%), biochemical (n=14, 38.9%) and/or clinical response (n=6, 16.2%). A total of 21 patients (58.3%) experienced hematotoxicity grade 1/2, while 1 patient (2.8%) experienced hematotoxicity grade 3; no grade 4 hematotoxicity occurred. Furthermore, 2 patients (5.6%) developed grade 4 renal toxicity; no grade 5 renal toxicity occurred. Responders had a significantly longer median survival from time of enrollment than non-responders (45.1 months, range: 37.1-53.1 months vs. 12.6 months, range: 11.0-14.2, hazard ratio: 0.12 (0.027-0.52), p=0.005). Additionally, there was a trend towards longer median survival with 90Y-DOTATOC plus 177Lu-DOTATOC as compared to 90Y-DOTATOC alone (60.2 months, range: 19.8-100.6 months vs. 27.0 months, range: 4.0-50.0, hazard ratio: 0.21 (0.01-3.98), p=0.16). Response to 90Y-DOTATOC and 90Y-DOTATOC plus 177Lu-DOTATOC therapy is associated with a longer survival in patients with metastasized gastrinoma. Both treatment regimens are promising tools for management of progressive gastrinoma.
Keywords: Yttrium, lutetium, somatostatin, survival, gastrinoma
Introduction
Gastrinomas are among the most common pancreatic neuroendocrine tumors and surgical resection is curative for localized disease. However, gastrinomas have approximately a 50% malignant potential and up to 33% of patients with gastrinomas have liver metastases on diagnosis [1]. Therapeutic options for patients with metastatic disease are limited, and distant metastases are associated with increased mortality and an expected 5-year survival of 60% [2,3]. The most common chemotherapeutics for treatment of metastasized gastrinomas are etoposide, cisplatin and doxorubicin, which are associated with significant toxicity [4-6], while neither liver transplantation, chemo-embolization, nor alpha-interferon therapy have demonstrated significant survival benefit [7,8].
High expression of somatostatin receptors in gastrinoma tissues prompted the use of somatostatin receptor-based radiopeptide imaging for detection of gastrinomas with 111In-DTPA-Octreotide, which demonstrated superior diagnostic value compared to morphologic imaging [9] and provided the rational to investigate somatostatin-based radiopeptide treatment in progressive gastrinoma.
Somatostatin-based radiopeptide therapy with tetraazacyclododecane-tetraacetic acid modified Tyr-octreotide (DOTATOC) is a valuable therapeutic tool for patients with metastasized neuroendocrine cancers [10]. The current practice in radiopeptide therapy is the use of a single radioisotope, typically 90yttrium (90Y) or 177lutetium (177Lu). 90Y has a long-range, high-energy β emission that deposits high radiation doses in large metastases while 177Lu has a short-range, lower-energy β emission that concentrates in small metastases. Given their complementary characteristics, there is interest in treatment regimens combining the two isotopes, and we recently found that DOTATOC therapy with both 90Y and 177Lu was more efficacious than DOTATOC therapy with 90Y alone in a large cohort of patients with neuroendocrine tumors [11].
A previous report in 11 gastrinoma patients showed that 90Y-DOTATOC or 177Lu-DOTATATE resulted in symptomatic improvement and some degree of tumor response (complete, partial or stabilization) [12]. Here, we report the response rate, long-term toxicity profile and survival after treatment with 90Y-DOTATOC or 90Y-DOTATOC plus 177Lu-DOTATOC in 36 patients with progressive metastasized gastrinoma.
Methods
Patients
At the University Hospital Basel, we included patients with histologically confirmed gastrinoma, metastasized disease, disease progression within 12 months before study entry and visible tumor uptake in the pre-therapeutic somatostatin receptor scintigraphy. Patients with concurrent anti-tumor treatment other than somatostatin treatment were excluded. Furthermore, patients with pregnancy, breast-feeding, urine incontinence, preexisting hematological toxicities grade 3/4, and severe concomitant illness including severe psychiatric disorders were also excluded. The study was designed and carried out in accordance with good clinical practice guidelines, Swiss drug laws and the Declaration of Helsinki were used to design and carry out the study. Approval from the local ethics committee for human studies (EKBB-Reference-No.: M120/97; www.ekbb.ch) was obtained and the study was registered (ClinicalTrials.gov number NCT00978211). All participants or their legal representatives gave written informed consent.
Treatment
A 5-step process was used to synthesize DOTA-TOC according to Good Laboratory Practice [13]. From October 1997 to February 2001, 90Y-DOTATOC was the standard treatment. After February 2001, combination therapy utilizing alternating cycles of 90Y-DOTATOC and 177Lu-DOTATOC was available [14]. Due to the uncertain benefit, 177Lu-DOTATOC treatment was performed only in patients living in close proximity to Basel.
Radiolabeling was performed with: 3.7GBq/m2 body surface of 90Y and 0.111GBq of 111Indium for intratherapeutic imaging, or with 7.4GBq of 177Lu. Lyophilized DOTA-TOC kits were incubated with the radioisotopes for 30 minutes at 95°C. Solid phase extraction and high performance liquid chromatography were performed for quality control with a minimum required labeling yield ≥99.5%.
Amino acid solutions containing lysine and arginine were administered before and after radiopeptide injection to inhibit tubular re-absorption [14-16]. Long-acting somatostatin analogues were withheld for at least 6 weeks and short-acting somatostatin analogues were withheld for at least 3 days prior to radiopeptide therapy. Following each cycle, patients were hospitalized for 3 days in accordance with Swiss regulations for radiation protection.
Based on the results of our pilot study [15], repeated treatment cycles were performed with a minimum interval of 6 weeks in case of: (i) stabilization or decrease in the sum of the longest diameters of all pre-therapeutically detected tumor lesions, (ii) improvement in at least one of the five key symptoms: flush, diarrhea, pain, fatigue and involuntary weight-loss or (iii) measurable post-therapeutic serum gastrin decrease after pre-therapeutic progression of serum gastrin. There was no a priori defined upper limit of the numbers of treatment cycles. Intratherapeutic imaging and image scoring were performed as previously described [14,17,18].
Follow-up
Clinical status and vital signs were monitored for 72 hours following each therapeutic application with continuous monitoring for evidence of toxicity. Following discharge, hematologic parameters were measured at biweekly intervals for 10 weeks after each cycle or until normalization of marker levels. Post-therapeutic morphological imaging was planned 6-8 weeks after each cycle. Measurement of serum gastrin levels was planned 2-8 weeks after treatment.
Further cycles were withheld in case of disease progression, permanent toxicity or loss of the ability to travel to the treatment center. At this point, follow-up was performed to obtain information on survival and long-term toxicities until the patient’s death. Data were obtained from the referring centers with a minimum frequency of 2 follow-up visits per year, adapted to the patient’s individual requirements. All follow-up data were centrally collected and each case was reviewed and approved for completeness at the study center. Primary physicians and/or the patients were directly contacted if additional results were needed.
Acute and long-term adverse events were graded according to the Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf) of the National Cancer Institute. The Modification of Diet in Renal Disease (MDRD) formula [19] was used to assess kidney function. The guidelines of the National Kidney Foundation (www.kidney.org) were used to classify renal toxicities, where grade 4 and 5 renal toxicity was defined as a glomerular filtration rate <30 and <15 mL/min/1.73 m2, respectively.
Outcomes and statistical analyses
Main outcomes of interest were response to treatment, toxicity and survival. Response was defined as previously described [14]: (i) Stabilization or decrease in the sum of the longest diameters of all pre-therapeutically detected tumor lesions, (ii) improvement in at least one of the five key symptoms: flush, diarrhea, pain, fatigue and involuntary weight-loss or (iii) measurable post-therapeutic serum gastrin decrease after pre-therapeutic progression of serum gastrin. Intention-to-treat principles were used to define the overall response rate, whereby loss of follow-up after treatment was regarded as treatment failure.
Pre- and post-therapeutic serum gastrin levels were compared using Related Samples Wilcoxon Signed Rank Test. Survival was assessed from time of first DOTA-TOC treatment to death from any cause. Multivariable Cox regression was used to study predictors of survival with the following pre-specified candidate variables: age, response vs. no response, and combined treatment with 90Y-DOTATOC plus 177Lu-DOTATOC vs. treatment with 90Y-DOTATOC alone. Effect estimates were expressed as hazard ratios (HR) with 95% confidence intervals (CI). The frequency of hematological toxicity in both treatment groups were compared using binary logistic regression with the pre-specified candidate variables used for all survival analyses.
Sensitivity analyses were performed to assess the influence of the cumulative 90Y-DOTATOC and 177Lu-DOTATOC activities and the year of treatment on all study endpoints. Two-sided p values of <0.05 indicate statistical significance.
Results
Patients
Between October 1996 and February 2010, 45 patients were screened for eligibility. Of these, 3 patients (6.6%) were not eligible because of absent pre-therapeutic tumor uptake or impaired kidney function. Furthermore, 5 eligible patients (11.1%) declined participation and 1 patient (2.2%) was referred for other treatment. The remaining 36 patients (80.0%) were recruited (Figure 1). The patients in our study came from Europe, North America and Asia.
Figure 1.
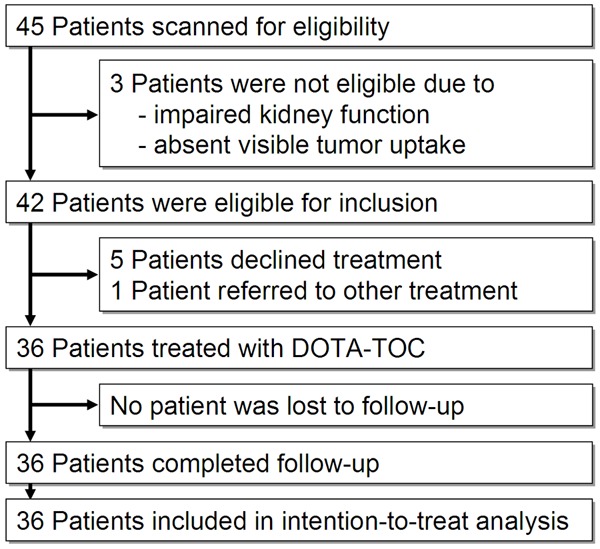
Patient Flow.
Treatment
A total of 92 treatment cycles (1-8 cycles per patient) were performed, including 81 cycles of 90Y-DOTATOC (median cumulative activity per patient: 11.8GBq; range: 6.1-62.2GBq) and 11 cycles of 177Lu-DOTATOC (median cumulative activity per patient: 14.8GBq; range: 7.4-14.8GBq). Intratherapeutic scintigraphy revealed visual tumor uptake grade 3 in all 36 patients (Table 1, Figure 2).
Table 1.
Baseline Characteristics (n=36)
| Characteristic | All Patients (n=36) | Responders (n=26) | Non-responders (n=10) | |
|---|---|---|---|---|
| Gender | Women | 19 (52.8%) | 12 (46.2%) | 7 (70.0%) |
| Men | 17 (47.2%) | 14 (53.8%) | 3 (30.0%) | |
| Age [y] | Median | 51.9 | 53.3 | 50.5 |
| Range | 16.2-77.4 | 16.2-77.4 | 31.4-75.3 | |
| Disease Duration [y] | Median | 1.9 | 2.2 | 1.4 |
| Range | 0.1-37.8 | 0.1-37.8 | 0.2-27.6 | |
| Pretreatment | Surgery | 20 (55.6%) | 15 (57.7%) | 5 (50.0%) |
| Chemotherapy | 9 (25.0%) | 6 (23.1%) | 3 (30.0%) | |
| Radiation | 1 (2.8%) | 0 (0%) | 1 (10.0%) | |
| Extent | Single Metastasis | 3 (8.3%) | 3 (11.5%) | 0 (0%) |
| Liver Metastases | 36 (100%) | 26 (100%) | 10 (100%) | |
| Bone Metastases | 5 (13.8%) | 1 (3.8%) | 4 (40.0) | |
| Creatinine [µmol/L] | Median | 68 | 69 | 59 |
| Range | 32-130 | 38-119 | 32-130 | |
| Tumor Uptake | Score 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| Score 2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Score 3 | 36 (100%) | 26 (100%) | 10 (100%) | |
| Kidney Uptake | Score 0 | 1 (2.8%) | 0 (0%) | 1 (10.0%) |
| Score 1 | 6 (16.7%) | 3 (11.1%) | 3 (30.0%) | |
| Score 2 | 15 (41.7%) | 13 (50.0%) | 2 (20.0%) | |
| Score 3 | 13 (36.1%) | 10 (38.5%) | 3 (30.0%) |
Figure 2.
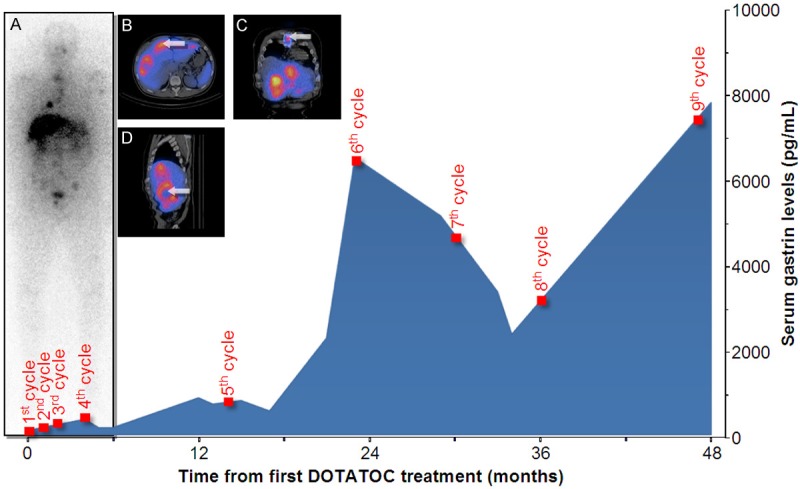
Example of a 51-year old man with metastasized gastrinoma. The intratherapeutic 111In-DOTATOC-scan displays focal grade 4 tracer uptake in the liver, right lung, left scapula, spine, ribs, pelvis and left humerus (A). Co-registered SPECT-CT confirms specific uptake in liver metastases (B). The thoracic lesion is confirmed as a sternal metastasis via SPECT-CT (arrow, C). High uptake in viable tumor and absent uptake in necrotic parts of metastases is demonstrated (arrow, D). The serum gastrin did not drop after the first four treatment cycles; however, at later cycles a significant drop in serum gastrin levels occurred.
Response was found in 26 patients (72.2%), including morphological (n=12, 33.3%), biochemical (n=14, 38.9%) and/or clinical response (n=6, 16.2%). Overall, the median pre-therapeutic serum gastrin was 1124 pg/mL (range: 23-66000 pg/mL), while the median post-therapeutic level was 750 pg/mL (range: 37-526000 pg/mL, n=36, p=0.26, Figure 3). In biochemical responders, the median pre-therapeutic serum gastrin was 1838 pg/mL (range: 132-66000 pg/mL), while the median post-therapeutic level was 750 pg/mL (range: 58-21167 pg/mL, n=14, p=0.001, Figure 3). In biochemical non-responders, the median pre-therapeutic serum gastrin was 399 pg/mL (range: 23-43500 pg/mL), while the median post-therapeutic level was 1000 pg/mL (range: 37-526000 pg/mL, n=14, p=0.012, Figure 3).
Figure 3.
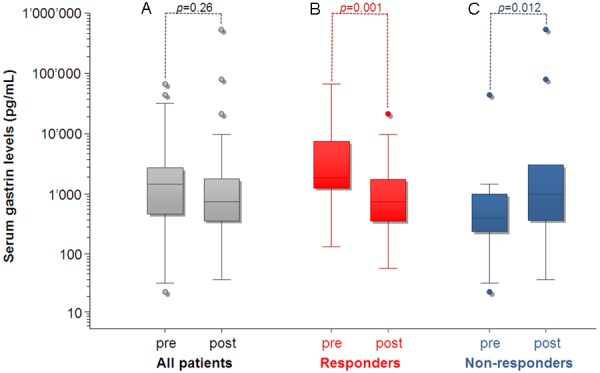
Pre- and post-therapeutic serum gastrin levels in all patients (A), biochemical responders (B) and biochemical non-responders (C).
Survival
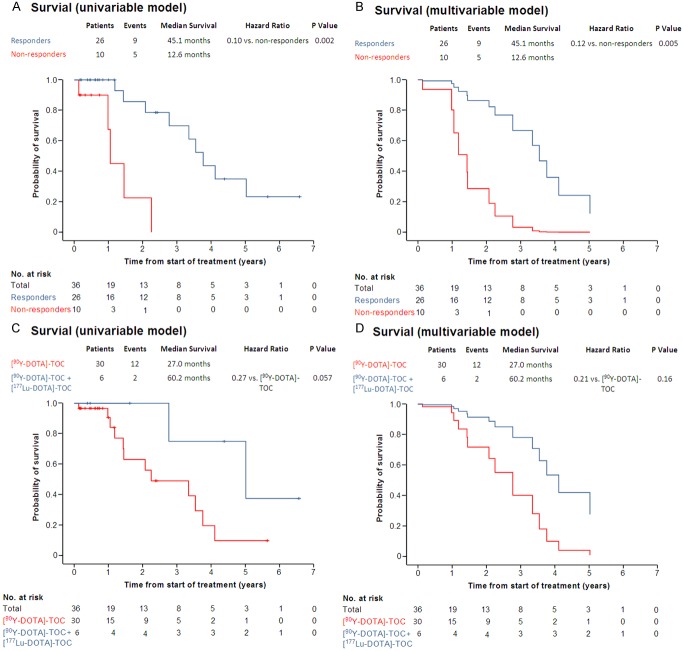
After a median follow-up period of 12.3 months (range: 1.6-78.8 months), 14 patients (38.9%) died and 23 (61.1%) survived. The median survival was 157.4 months from time of diagnosis and 40.1 months from time of enrollment.
Responders had a significantly longer survival from time of enrollment (45.1 months, range: 37.1-53.1 months vs. 12.6 months, range: 11.0-14.2), in the uni-variable (hazard ratio: 0.096 (2.4-45.2), p=0.002, Figure 4A) and in the multi-variable analysis (hazard ratio: 0.12 (0.027-0.52), p=0.005, Figure 4B and Table 3). Furthermore, there was a trend towards longer survival with 90Y-DOTATOC plus 177Lu-DOTATOC as compared to 90Y-DOTATOC alone (60.2 months, range: 19.8-100.6 months vs. 27.0 months, range: 4.0-50.0), in the uni-variable (hazard ratio: 0.27 (0.06-1.25), p=0.057, Figure 4C) and in the multi-variable analysis (hazard ratio: 0.21 (0.01-3.98), p=0.16, Figure 4D and Table 3).
Figure 4.
Survival of responders vs. non-responders in the uni-variable (A) and multivariable analysis (B). Survival of patients treated with 90Y-DOTATOC vs. patients treated with 90Y-DOTATOC plus 177Lu-DOTATOC in the uni-variable (C) and multivariable analysis (D).
Table 3.
Hazard Ratios for Overall Survival (n=36)
| Covariate | Hazard Ratio (95% CI)* | P Value | |
|---|---|---|---|
| Age | (per year) | 1.01 (0.97-1.06) | 0.54 |
| Response | (vs. no response) | 0.12 (0.027-0.52) | 0.005 |
| 90Y-DOTATOC plus 177Lu-DOTATOC | (vs. 90Y-DOTATOC) | 0.27 (0.043-1.70) | 0.16 |
Estimates for each co-variable have been adjusted for the cumulative DOTATOC activity as well as for all other co-variables listed.
Toxicities
In total, 21 patients (58.3%) experienced hematotoxicity grade 1/2, while 1 patient (2.8%) experienced hematotoxicity grade 3; no grade 4 hematotoxicity occurred (Table 2). There was no significant difference in the frequencies of grade 1/2 hematotoxicities (p=0.23) and no difference in the frequencies of grade 3/4 hematotoxicities (p=0.99) with 90Y-DOTATOC plus 177Lu-DOTATOC as compared to 90Y-DOTATOC alone. Two patients (5.6%) developed grade 4 renal toxicity and no grade 5 renal toxicity occurred.
Table 2.
Hematological Toxicities (n=36)
| Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| 90Y-DOTATOC (n=30) | |||||
| Leukopenia | 9 (30.0%) | 2 (6.7%) | 7 (23.3%) | 0 (0%) | 0 (0%) |
| Anemia | 20 (66.7%) | 16 (53.3%) | 3 (10%) | 1 (3.3%) | 0 (0%) |
| Thrombocytopenia | 7 (23.3%) | 5 (16.7%) | 2 (6.7%) | 0 (0%) | 0 (0%) |
| 90Y-DOTATOC plus 177Lu-DOTATOC (n=6) | |||||
| Leukopenia | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anemia | 1 (16.7%) | 0 (0%) | 1 (16.7%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 1 (16.7%) | 1 (16.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
All toxicities are scored according to the NCI criteria.
Discussion
Given the limited therapeutic options available to patients with metastasized gastrinoma, there is a need to establish well-tolerated agents that prolong survival. Traditional agents used in the treatment of metastasized gastrinoma are known to cause frequent serious side effects without proven prolonged survival [5-7,20]. More promising results have been seen with targeted therapies, which exploit aberrant molecular pathways known to be overexpressed or overactive in gastrinomas. The somatostatin analog octreotide LAR has been shown to induce disease control in about 50% of patients with metastasized gastrinoma [21], with similar results seen for the protein kinase inhibitors Everolimus and Sunitinib [22,23], however long term survival data is lacking for these agents. In our cohort, 72% of patients showed a response to DOTATOC therapy that was associated with longer overall survival, indicating that radiopeptide therapy with somatostatin based analogues may be an effective means of treatment for patients with progressive metastasized gastrinoma.
The clinical paradigm of gastrinoma, known as Zollinger-Ellison Syndrome, is secondary to gastric acid hypersecretion and causes serious peptic ulcers, gastro-esophageal reflux and diarrhea, significantly contributing to the morbidity of patients with gastrinoma [24]. About one third of patients in our cohort demonstrated a biochemical response to DOTATOC therapy with a significant reduction in post-therapeutic gastrin levels. As demonstrated by the patient example, significant reduction in gastrin levels may occur later in the course of therapy even with initial nonresponse. While clinical response can often be induced with radiopeptide treatment, protein kinase inhibitors rarely have this effect and instead can result in a multitude of other side effects that contribute to patient morbidity (e.g. rash, stomatitis, diarrhea, arthralgia).
DOTATOC therapy was generally well tolerated in our cohort of patient. Hematotoxicity is a well-known toxicity associated with radiopeptide therapy that is thought to occur due to irradiation from 90Y-DOTATOC circulating through the body or binding to somatostatin receptors on bone marrow cells [25], or due to the small fraction of free 90Y that is administered during treatment cycles that integrates into the bone matrix [26]. Low-grade hematotoxicity was relatively common in our cohort, with approximately 60% of patients experiencing grade 1 or 2 hematotoxicity. Overall, there was no significant difference in the frequencies of hematotoxicities with combination therapy as compared to 90Y-DOTATOC alone. Renal toxicity, the main adverse effect after radiopeptide therapy, occurred at rates similar to or lower than those reported for most radiopeptide trials using 90Y or 177Lu [16,27-29].
The trend toward longer survival with combined 90Y-DOTATOC plus 177Lu-DOTATOC therapy compared to 90Y-DOTATOC alone in patients with metastasized gastrinoma is consistent with findings we previously reported in patients with neuroendocrine tumors [11]. The improved survival associated with combination therapy with long-range, high-energy 90Y and short-range, lower-energy 177Lu is thought to be due to spatial differences in the deposition of energy [30]. To our knowledge, no randomized trials have been performed on the superiority of one of these radionuclides in radiopeptide therapy, or on identifying in which cases these radionuclides may be preferred (i.e. tumor location, tumor perfusion, large tumor size). Such work would be helpful in defining which patient cohorts would most benefit from 90Y- and 177Lu-based radiopeptide therapy.
Previously, we have shown that qualitative analysis of scintigraphic uptake of the radiopeptide is able to predict survival in patients with neuroendocrine tumors undergoing 90Y-DOTATOC treatment [14]. These findings suggest that a quantitative approach using 68Ga-DOTATOC-PET could be used in the future to even more precisely predict radiotherapeutic outcome. In the present study, however, all tumors demonstrated high tracer uptake, precluding any correlation analysis on uptake and survival.
The present study has strengths and limitations. To our knowledge, this is the largest study to date showing a treatment-related survival benefit in patients with metastasized gastrinoma. The relatively long patient follow-up was sufficient to obtain the significant results described above. The patients in our study came from Europe, North America and Asia, resulting in a diverse cohort that is more widely representative of a global population rather than that limited to a single country. Given that there is currently no accepted standard regimen for DOTATOC therapy in the Nuclear Medicine community, the administration of treatment at a single center ensured homogeneity of intervention. However, because of factors such as year of enrollment, proximity to the University Hospital Basal and baseline renal function, patients were not randomly stratified to the different treatment regimens. Additionally, our patients came from a variety of different referral centers at which initial staging and follow up was performed. This sometimes resulted in non-uniform ordering of imaging and laboratory tests. Finally, this study was conducted over a long time period given the relative rarity of metastasized gastrinoma. Our current findings warrant prospective evaluation in a randomized trial.
In conclusion, the longer survival associated with response to DOTATOC radiotherapy makes it a promising treatment option for patients with metastasized gastrinoma.
Acknowledgements
The authors are grateful to all patients and referring institutions for participation. Furthermore, we thank our nursing, laboratory and technical staff for their support.
Disclosure of conflict of interest
There are no financial disclosures, no funding sources, and potential conflicts of interest.
References
- 1.Lau WY, Ho S, Leung WT, Chan M, Lee WY, Johnson PJ. What determines survival duration in hepatocellular carcinoma treated with intraarterial Yttrium-90 microspheres? Hepatogastroenterology. 2001;48:338–340. [PubMed] [Google Scholar]
- 2.Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J. Clin. Oncol. 1999;17:615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 3.Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 4.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 5.Rivera E, Ajani JA. Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma. Am J Clin Oncol. 1998;21:36–38. doi: 10.1097/00000421-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Ruszniewski P, Hochlaf S, Rougier P, Mignon M. Intravenous chemotherapy with streptozotocin and 5 fluorouracil for hepatic metastases of Zollinger-Ellison syndrome. A prospective multicenter study in 21 patients. Gastroenterol Clin Biol. 1991;15:393–398. [PubMed] [Google Scholar]
- 7.Pisegna JR, Slimak GG, Doppman JL, Strader DB, Metz DC, Benya RV, Orbuch M, Fishbeyn VA, Fraker DL, Norton JA, et al. An evaluation of human recombinant alpha interferon in patients with metastatic gastrinoma. Gastroenterology. 1993;105:1179–1183. doi: 10.1016/0016-5085(93)90965-f. [DOI] [PubMed] [Google Scholar]
- 8.Pellicano R, De Angelis C, Resegotti A, Rizzetto M. Zollinger-Ellison syndrome in 2006: concepts from a clinical point of view. Panminerva medica. 2006;48:33–40. [PubMed] [Google Scholar]
- 9.Gibril F, Reynolds JC, Doppman JL, Chen CC, Venzon DJ, Termanini B, Weber HC, Stewart CA, Jensen RT. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas. A prospective study. Ann Intern Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Marincek N, Jorg AC, Brunner P, Schindler C, Koller MT, Rochlitz C, Muller-Brand J, Maecke HR, Briel M, Walter MA. Somatostatin-based radiotherapy with [90Y-DOTA] -TOC in neuroendocrine tumors: long-term outcome of a phase I dose escalation study. Journal of Translational Medicine. 2013;11:17. doi: 10.1186/1479-5876-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, Ng QK, Macke HR, Muller-Brand J, Rochlitz C, Briel M, Walter MA. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA] -TOC versus [(90)Y-DOTA] -TOC plus [(177)Lu-DOTA] -TOC in neuroendocrine cancers. Journal of Clinical Oncology: Official Journal of The American Society of Clinical Oncology. 2012;30:1100–1106. doi: 10.1200/JCO.2011.37.2151. [DOI] [PubMed] [Google Scholar]
- 12.Grozinsky-Glasberg S, Barak D, Fraenkel M, Walter MA, Mueller-Brand J, Eckstein J, Applebaum L, Shimon I, Gross DJ. Peptide receptor radioligand therapy is an effective treatment for the long-term stabilization of malignant gastrinomas. Cancer. 2011;117:1377–1385. doi: 10.1002/cncr.25646. [DOI] [PubMed] [Google Scholar]
- 13.Otte A, Jermann E, Behe M, Goetze M, Bucher HC, Roser HW, Heppeler A, Mueller-Brand J, Maecke HR. DOTATOC: a powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med. 1997;24:792–795. doi: 10.1007/BF00879669. [DOI] [PubMed] [Google Scholar]
- 14.Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, Macke HR, Rochlitz C, Muller-Brand J, Walter MA. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA] -TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 15.Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, Maecke HR, Muller J. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26:1439–1447. [PubMed] [Google Scholar]
- 16.Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA] -D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol. 2001;12:941–945. doi: 10.1023/a:1011160913619. [DOI] [PubMed] [Google Scholar]
- 17.Iten F, Muller B, Schindler C, Rochlitz C, Oertli D, Macke HR, Muller-Brand J, Walter MA. Response to [90Yttrium-DOTA] -TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res. 2007;13:6696–6702. doi: 10.1158/1078-0432.CCR-07-0935. [DOI] [PubMed] [Google Scholar]
- 18.Iten F, Muller B, Schindler C, Rasch H, Rochlitz C, Oertli D, Maecke HR, Muller-Brand J, Walter MA. [(90)Yttrium-DOTA] -TOC response is associated with survival benefit in iodine-refractory thyroid cancer: long-term results of a phase 2 clinical trial. Cancer. 2009;115:2052–2062. doi: 10.1002/cncr.24272. [DOI] [PubMed] [Google Scholar]
- 19.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 20.Ruszniewski P, Malka D. Hepatic arterial chemoembolization in the management of advanced digestive endocrine tumors. Digestion. 2000;62(Suppl 1):79–83. doi: 10.1159/000051860. [DOI] [PubMed] [Google Scholar]
- 21.Shojamanesh H, Gibril F, Louie A, Ojeaburu JV, Bashir S, Abou-Saif A, Jensen RT. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer. 2002;94:331–343. doi: 10.1002/cncr.10195. [DOI] [PubMed] [Google Scholar]
- 22.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Oberg K. Everolimus for advanced pancreatic neuroendocrine tumors. The New England Journal of Medicine. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Horsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. The New England Journal of Medicine. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 24.Jensen RT, Niederle B, Mitry E, Ramage JK, Steinmuller T, Lewington V, Scarpa A, Sundin A, Perren A, Gross D, O’Connor JM, Pauwels S, Kloppel G. Gastrinoma (duodenal and pancreatic) Neuroendocrinology. 2006;84:173–182. doi: 10.1159/000098009. [DOI] [PubMed] [Google Scholar]
- 25.Oomen SP, Hofland LJ, van Hagen PM, Lamberts SW, Touw IP. Somatostatin receptors in the haematopoietic system. Eur J Endocrinol. 2000;143(Suppl 1):S9–14. doi: 10.1530/eje.0.143s009. [DOI] [PubMed] [Google Scholar]
- 26.Chen PS, Terepka AR, Hodge HC. The Pharmacology and Toxicology of the Bone Seekers. Annual Review of Pharmacology. 1961;1:369–396. [Google Scholar]
- 27.Bodei L, Cremonesi M, Grana C, Rocca P, Bartolomei M, Chinol M, Paganelli G. Receptor radionuclide therapy with 90Y-[DOTA] 0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2004;31:1038–1046. doi: 10.1007/s00259-004-1571-4. [DOI] [PubMed] [Google Scholar]
- 28.Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, Kwekkeboom DJ, Bouterfa H, Krenning EP. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3] octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147–156. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, Haldemann A, Mueller-Brand J. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 30.de Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2005;46(Suppl 1):13S–17S. [PubMed] [Google Scholar]