Abstract
To evaluate 18F-labeled-fluorodeoxyglucose (18F-FDG-) and 18F-labeled-sodium fluoride (18F-NaF-) positron emission tomography/computed tomography (PET/CT) as biomarkers in metastatic castrate-resistant prostate cancer (mCRPC). Nine men (53-75 years) in a phase 1 trial of abiraterone and cabozantinib had 18F-FDG-PET/CT, 18F-NaF-PET/CT and standard imaging (99mTc-labeled-methylene-diphosphonate (99mTc-MDP) bone scan and abdominal/pelvic CT) at baseline and after 8 weeks of therapy. Baseline disease was classified as widespread 18F-FDG-avid, oligometastatic 18F-FDG-avid (1 site), or non-18F-FDG-avid. Metabolic response was classified using European Organisation for Research and Treatment of Cancer (EORTC) criteria. Treatment response using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, Prostate Cancer Working Group 2 (PCWG2) guidelines and days on trial (DOT) were recorded. All men were followed for 1 year or until progression. Four men had 18F-FDG-avid disease: two with widespread (DOT 53 and 76) and two with oligometastatic disease (DOT 231 and still on trial after 742+ days). Five men had non-18F-FDG-avid disease; three remained stable or improved (2 still on trial while one discontinued for non-oncologic reasons; DOT 225-563+), and 2 progressed (DOT 285 and 532). Despite the small sample size, Kaplan-Meier analysis showed a significant difference in progression free survival (PFS) between men with widespread 18F-FDG-avid, oligometastatic 18F-FDG-avid and non-18F-FDG-avid disease (p < 0.01). All men had 18F-NaF-avid disease. Neither 18F-NaF-avid disease extent nor intensity was predictive of treatment response. 18F-FDG-PET/CT may be superior to 18F-NaF-PET/CT and standard imaging in men with mCRPC on abiraterone and cabozantinib. 18F-FDG-PET/CT may have potential to stratify men into 3 groups (widespread vs. oligometastatic 18F-FDG-avid vs. non-18F-FDG-avid mCRPC) to tailor therapy. Further evaluation is warranted.
Keywords: PET/CT, FDG, NaF, prostate cancer, bone scan
Introduction
It is estimated that 233,000 new cases of prostate cancer will be diagnosed and 29,480 men will die of the disease in the United States in 2014 (http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf). Although most men initially respond to androgen deprivation therapy (ADT), progression to castration-resistant prostate cancer (CRPC) occurs in almost all cases and leads to death. Approximately 85% of men with metastatic CRPC (mCRPC) have bone metastases, and less than half have soft tissue disease [1].
Technetium-99m-methylene diphosphonate (99mTc-MDP) skeletal scintigraphy and diagnostic abdominal/pelvic CT are the standard imaging modalities used to evaluate metastases and play an important role in patient management (http://www.nccn.org/patients/guidelines/prostate/index.html#24). Positron emission tomography/ computed tomography with fluorine-18-sodium fluoride (18F-NaF-PET/CT) has higher sensitivity and specificity for the detection of osseous metastases than standard imaging [2-5], however, it does not assess soft tissue disease and is not currently considered standard of care. 18F-fluorodeoxyglucose (18F-FDG) PET/CT can assess both soft tissue and osseous disease extent. Since mCRPC is often non-18F-FDG-avid, the utility of 18F-FDG-PET/CT in this disease has been questioned [6-9]. Recently, a growing number of studies are showing that 18F-FDG uptake is an independent prognostic factor in men with mCRPC and suggest 18F-FDG-PET/CT may detect a subgroup of biologically more aggressive disease [10-14].
Over the last few years there has been a proliferation of therapies for men with mCRPC, although survival remains poor and determining the optimal approach to therapy is challenging [15-21]. Furthermore, certain medications such as cabozantinib, modulate osteoblastic turnover and can have a profound effect on 99mTc-MDP bone scans, limiting the ability of 99mTc-MDP bone scan to accurately reflect disease response to therapy [21-24]. 18F-FDG- and 18F-NaF-PET/CT have the potential to provide a quantitative evaluation of metastatic disease that may be complementary to standard imaging. Our aim was to evaluate 18F-FDG- and 18F-NaF-PET/CT as biomarkers in men with metastatic castrate-resistant prostate cancer (mCRPC) enrolled in a phase 1 trial of abiraterone and cabozantinib. Namely we sought to evaluate the metabolic appearance and disease response of mCRPC in men on abiraterone and cabozantinib using 18F-FDG- and 18F-NaF-PET/CT and to compare this with standard imaging and progression-free survival (PFS).
Materials and methods
Experimental details & clinical trial conduct
The first nine men in a phase I multicenter trial of cabozantinib (Exelixis, San Francisco, CA) and abiraterone (Janssen Pharmaceuticals, Titusville, NJ) had whole-body 18F-FDG- and 18F-NaF-PET/CT at baseline and after 8 weeks of therapy as well as standard of care imaging (99mTc-MDP bone scan and diagnostic abdominal/pelvic CT) at baseline and every 8 weeks on therapy. Institutional review board approval was obtained, each patient included in the study gave informed consent and the study was Health Insurance Portability and Accountability Act (HIPAA) compliant. All patients had progressive mCRPC and no prior treatment with cytochrome 17A1 (CYP17A1), mesenchymal-epithelial transition factor (MET) or vascular endothelial growth factor receptor (VEGFR) inhibitors. A history of radiation to a bone metastasis was allowed if this was more than 14 days prior to enrolling on the trial. Treatment cycles were 28 days with cycles repeated until disease progression or unacceptable toxicity. Efficacy of therapy was determined using standard criteria - a combination of symptomatic responses and PSA levels every 28 days as well as evaluation of standard of care imaging. Treatment response using Prostate Cancer Working Group 2 (PWCG2) guidelines [25] and days on trial (DOT) were recorded. All men were followed prospectively until removed from the trial.
Image acquisition and analysis
Whole-body 99mTc-MDP bone scans were acquired in the anterior and posterior projections approximately 3 hours after IV administration of 20 mCi 99mTc-MDP. The diagnostic IV contrast-enhanced CT scans of the abdomen and pelvis were performed using collimation less than or equal to 3 mm and 5 mm reconstructed axial images. Each 18F-FDG-PET/CT was acquired in three-dimensional mode, 60 ± 10 minutes after the intravenous administration of 10-15 mCi 18F-FDG. Imaging was performed from the skull vertex through the mid thighs using a hybrid PET/CT scanner with images corrected for detector efficiency, attenuation, scatter, decay and random coincidences. Patients fasted for 6 hours prior to the 18F-FDG-PET/CT. The 18F-NaF-PET/CT scan acquisition in three-dimensional mode from skull vertex to mid thighs was obtained 30-60 minutes after the IV injection of approximately 10 mCi 18F-NaF. There was no specific patient preparation required, however, patients were encouraged to drink 500-1000 ml of water shortly before and up to 500 ml water shortly after the radiopharmaceutical administration.
Consistency of image acquisition was maintained by adherence to a standard protocol and quality assurance program in accordance with National Cancer Institute (NCI) consensus guidelines [26]. Imaging was centrally reviewed for quality control and analysis without knowledge of patient status or outcome. Anatomic tumor response was classified where possible according to the best response achieved using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 applied to standard imaging obtained after 8 weeks of therapy and then at intervals of 2 months [27]. PET/CT studies were evaluated for disease extent and response. Baseline disease extent was classified as widespread 18F-FDG-avid disease, oligometastatic 18F-FDG-avid disease (1 site), and non-18F-FDG-avid disease (Figures 1 and 2). For each PET/CT scan, the maximum standardized uptake value (SUVmax) of the most intense site of disease and the average SUVmax of up to the 5 most intense sites of disease (chosen to include as many involved organ systems as possible) were assessed at baseline and follow-up. Metabolic tumor response after 8 weeks of therapy was classified using European Organization for Research and Treatment of Cancer (EORTC) criteria based on thresholds in percentage change for SUVmax and average SUVmax of target lesions relative to baseline where: partial metabolic response (PR) ≤ -25% < stable metabolic disease (SD) < +25% ≤ progressive metabolic disease (PD) [28]. A new site of metabolically active metastatic disease was classified as PD regardless of percentage change.
Figure 1.
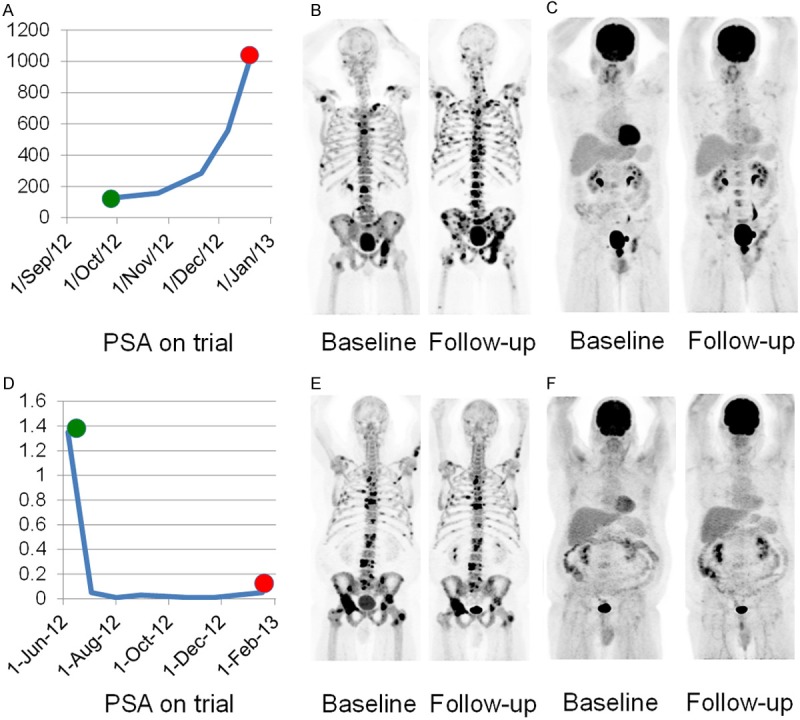
Change in PSA (A), 18F-NaF-PET/CT (B) and 18F-FDG-PET/CT (C) at baseline and 8 week follow-up in a man with mCRPC and widespread 18F-NaF-avid and 18F-FDG-avid disease who remained on trial for 53 days; Change in PSA (D), 18F-NaF-PET/CT (E) and 18F-FDG- PET/CT (F) at baseline and 8 week follow-up in a man with mCRPC, widespread 18F-NaF-avid and non-18F-FDG-avid disease who remained on trial for 225 days.
Figure 2.
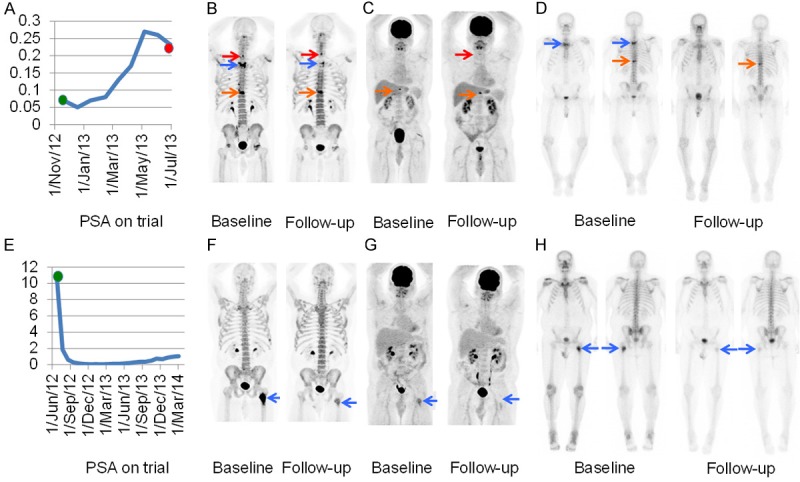
Change in PSA (A), 18F-NaF-PET/CT (B), 18F-FDG-PET/CT (C) and 99mTc-MDP bone scan (D) at baseline and 8 week follow-up in a man with baseline oligometastatic 18F-FDG-avid disease, metabolic disease progression on 18F-FDG-PET/CT, mixed change on 18F-NaF-PET/CT and response on 99mTc-MDP bone scan following radiation from T1 to T4 prior to enrolling on the trial. Of note, the 99mTc-MDP bone scan performed after 6 months of therapy (not shown) suggested worsening disease similar to the 18F-FDG-PET/CT performed at 8 weeks. The red, blue and orange arrows depict the C7, T2 and T10 lesions. Change in PSA (E), 18F-NaF-PET/CT (F), 18F-FDG-PET/CT (G) and 99mTc-MDP bone scan (H) at baseline and 8 week follow-up in a man with baseline oligometastatic 18F-FDG-avid disease and metabolic disease response on 18F-FDG-PET/CT, 18F-NaF-PET/CT and 99mTc-MDP bone scan following radiation to the left femoral lesion (blue arrow) prior to enrolling on the trial.
Results
Nine men (age 53-75 years) with mCRPC had 18F-FDG- and 18F-NaF-PET/CT scans at baseline and after 8 weeks of therapy (Table 1). Four (44%) had 18F-FDG-avid disease and 5 (56%) had non-18F-FDG-avid baseline disease. All men with 18F-FDG-avid baseline disease had 18F-FDG-avid disease after 8 weeks of therapy while all men without 18F-FDG-avid baseline disease had non-18F-FDG-avid disease after 8 weeks of therapy. All 9 men had 18F-NaF-avid disease (widespread in 8 men) at baseline and follow-up.
Table 1.
Summary of response in 9 men with mCRPC
| # | DOT | FDG category | Metabolic Response 18F-NaF- PET/CT SUVmax | Metabolic Response 18F-NaF- PET/CT SUVavg | Metabolic Response 18F-FDG- PET/CT SUVmax | Metabolic Response 18F-FDG- PET/CT SUVavg | Anatomic Response RECIST 1.1 after 8 weeks therapy | PCWG2 at time of last visit | TTP Based on clinical & radiologic findings |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 285 | Non-avid | PR | SD | Non-avid | Non-avid | Non-measurable | SD | Clinical PD after 285 DOT |
| 2 | 225 | Non-avid | PR | PR | Non-avid | Non-avid | Non-measurable | SD | Suspected PD after 225 DOT determined to be non-oncologic on follow-up |
| 3 | 742+ | Oligometastatic disease | PR | PR | PR | N/A | Non-measurable | SD | Still on trial (DOT 742+) |
| 4 | 53 | Widespread disease | PD | PD | PD | PD | PD | SD | Clinical & imaging PD after 53 DOT |
| 5 | 532 | Non-avid | SD | SD | Non-avid | Non-avid | Non-measurable | SD | Clinical PD after 532 DOT |
| 6 | 231 | Oligometastatic disease | SD | SD | PD | N/A | Non-measurable | SD | Clinical PD after 231 DOT |
| 7 | 563+ | Non-avid | PR | PR | Non-avid | Non-avid | Non-measurable | SD | Still on trial (DOT 563+) |
| 8 | 76 | Widespread disease | PR | PR | PD | PD | SD | SD | Clinical PD after 76 DOT |
| 9 | 516+ | Non-avid | PR | PR | Non-avid | Non-avid | PR | PR | Still on trial (DOT 516+) |
DOT = days on trial, TTP = time to progression, PR = partial response, SD = stable disease, PD = progressive disease.
Two of 4 men with 18F-FDG-avid baseline disease had widespread disease and the shortest time to progression (DOT 53 and 76); of the two patients with oligometastatic 18F-FDG-avid disease, one had progressive disease (DOT 231) and one is without progression (still on trial after 742+ days). Overall, the SUVmax and average SUVmax were higher in men with widespread versus oligometastatic 18F-FDG-avid disease (Table 2). Five men had non-18F-FDG-avid baseline disease and three had stable or improved disease. Of these three, two are still on trial (DOT 516+ and 563+, respectively) and one discontinued for non-oncologic reasons (DOT 225). The remaining 2 men with non-18F-FDG-avid baseline disease had progressive disease but with longer DOT (285 and 532) than the 3 men with 18F-FDG-avid progressive disease. Despite our small sample size, univariate analysis (Figure 3) showed a statistically significant difference in response among the 3 groups of men (p < 0.01); those with widespread 18F-FDG-avid disease progressed more rapidly than men with oligometastatic or non-18F-FDG-avid disease. Of the 2 men with oligometastatic disease, one had a single site of 18F-FDG-avid disease at T10 and a history of radiation from T1 to T4 prior to enrolling on the trial. Follow-up 18F-FDG-PET/CT showed a new site of 18F-FDG-avid disease at C7 and progression of 18F-FDG-avid disease at T10. This man was removed from the trial due to cord compression from mCRPC disease at T10, the only site of baseline 18F-FDG-avid disease. The other patient had a single site of baseline 18F-FDG-avid disease in the left femur, which was radiated prior to enrolling on the trial. Follow-up imaging showed disease improvement at this site (Figure 2).
Table 2.
Semiquantitative PET/CT analysis in 9 men with mCRPC
| Man # | SUV | 18F-NaF- PET/CT Baseline | 18F-NaF- PET/CT Follow-up | 18F-FDG- PET/CT Baseline | 18F-FDG- PET/CT Follow-up |
|---|---|---|---|---|---|
| 1 | SUVmax | 33.1 | 23.5 | Non-avid | Non-avid |
| Average SUVmax | 19.3 | 16.1 | |||
| 2 | SUVmax | 68.8 | 47.1 | Non-avid | Non-avid |
| Average SUVmax | 58.0 | 35.5 | |||
| 3 | SUVmax | 51.2 | 14.3 | 4.9 | 2.9 |
| Average SUVmax | 19.9 | 10.2 | (1 lesion) | (1 lesion) | |
| 4 | SUVmax | 74.0 | 55.8 | 15.6 | 15.8 |
| Average SUVmax | 45.7 | 38.7 | 7.0 | 5.7 | |
| 5 | SUVmax | 33.7 | 32.1 | Non-avid | Non-avid |
| Average SUVmax | 25.8 | 25.5 | |||
| 6 | SUVmax | 61.8 | 59.8 | 7.0 | 9.1 |
| Average SUVmax | 39.4 | 37.6 | (1 lesion) | 6.7 (2 lesions) | |
| 7 | SUVmax | 77.2 | 39.6 | Non-avid | Non-avid |
| Average SUVmax | 40.2 | 16.7 | |||
| 8 | SUVmax | 43.9 | 18.3 | 21.7 | 35.3 |
| Average SUVmax | 37.4 | 14.5 | 15.1 | 13.8 | |
| 9 | SUVmax | 19.1 | 11.6 | Non-avid | Non-avid |
| Average SUVmax | 12.0 | 8.5 |
Figure 3.
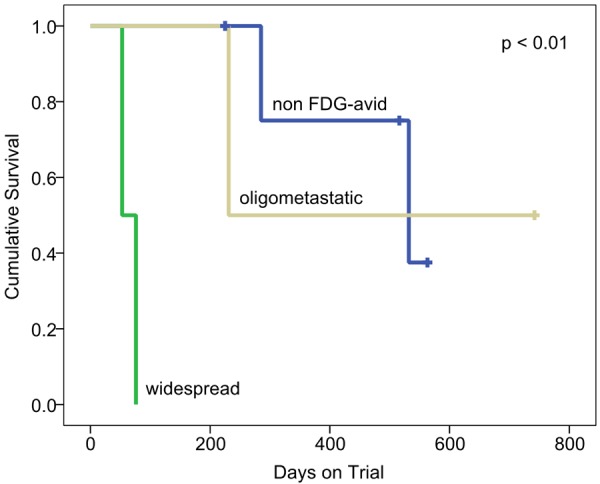
Progression-free survival of the 9 men with mCRPC included in the study according to baseline disease classification on 18F-FDG-PET/CT.
All men had 18F-NaF-avid disease, which was widespread in 8 men. Only one had relatively limited 18F-NaF-avid disease (3 sites). Subjectively 18F-NaF-PET/CT detected more sites of skeletal metastatic disease than 99mTc-MDP bone scan regardless of whether the disease was sclerotic, lytic or 18F-FDG-avid.
Subjectively 18F-NaF-PET/CT and 18F-FDG-PET/CT could identify therapy response at an earlier time-point than standard imaging. Indeed, 6 of 9 men (67%) had metastatic disease only to bone and the disease response using RECIST 1.1 could not be measured. With PCWG2 criteria, 8 of 9 men (89%) had SD at the time of their last scheduled clinic trial visit and scanning; this included 2 men with rapid interval clinical progression on therapy (DOT 53 and 76) and 2 men who are still on trial (DOT 563+ and 742+) at the time of reporting. Further, we suggest that 18F-FDG appeared to be better than 18F-NaF for the evaluation of therapeutic response (Table 1, Figures 1, 2 and 3). Indeed, 18F-NaF-avid baseline disease extent and intensity did not appear to be associated with clinical treatment response, while 18F-FDG-avid baseline disease extent and intensity did appear to be associated with treatment response.
Discussion
Recent advances have led to the approval of several new therapies for men with mCRPC [15-19]. Another agent that is currently under investigation is cabozantinib. Results from a phase II randomized discontinuation trial have shown that cabozantinib is associated with pain response, decreased markers of bone turnover, improvement on bone scans, and longer PFS. In this trial, 171 men with mCRPC were given 100 mg cabozantinib daily, 149 men had evidence of bone metastases at baseline, and 116 men (78%) had at least one follow-up bone scan evaluable for response. Bone scans were improved in 79 men (68%). Baseline pain was reported by 92 men with bone metastases, with 71 men taking narcotics to control the pain. Among men with at least one available follow-up assessment of pain or narcotic use, 67% (56 of 83 men) reported an improvement in pain control and 56% (31 of 55 patients) reported a decrease or discontinuation of narcotic use. Thirty-one men with stable disease after 12 weeks on trial were randomly assigned to receive cabozantinib or placebo and the median PFS was 23.9 weeks (95% CI, 10.7 to 62.4 weeks) with cabozantinib versus 5.9 weeks (95% CI, 5.4 to 6.6 weeks) with placebo (hazard ratio, 0.12; p < 0.001) [21]. Preliminary results in the literature suggest there is an effect of cabozantinib at doses ranging from 20 mg to 100 mg on 99mTc-MDP bone scans [22]. However, this may be due to a combination of osteoblast modulation and anti-tumor effect [23-24]. Since 99mTc-MDP uptake is linked with osteoblastic activity and cabozantinib appears to block MET and VEGFR-2 phosphorylation in prostate cancer and osteoblast cells, bone scans may not be an accurate measure of anti-tumor therapy response. Therefore, in men on cabozantinib it is possible that standard imaging is limited and there is a need to pursue different imaging modalities such as PET/CT in this context.
18F-NaF- and 18F-FDG are the two most ubiquitous PET tracers available. Several studies have shown 18F-NaF-PET/CT has superior sensitivity and specificity compared with 99mTc-MDP bone scans [2-5]. Single photon emission computed tomography (SPECT) alone or in combination with CT (SPECT/CT) can improve the sensitivity and specificity of 99mTc-MDP bone scans [3-4] but still remains inferior to 18F-NaF-PET/CT. Tateishi et al. [5] published a meta-analysis on the topic in 2010 in which they concluded that the pooled sensitivity and specificity of 18F-NaF PET/CT across 10 studies was 96% and 98% respectively compared with the pooled sensitivity and specificity of 99mTc-MDP bone scans (with and without SPECT) across 8 studies, which was 57% and 98% respectively. In an effort to assess 18F-NaF-PET/CT for the detection of skeletal metastases, the National Oncologic PET Registry (NOPR), a collaboration of the American College of Radiology Imaging Network, the American College of Radiology, and the Academy of Molecular Imaging, enrolled 20,238 patients in a registry between February 2010 and April 2013 [29]. In total, 3,531 scans were performed in 3,396 men with prostate cancer. A preliminary data analysis published in 2014 showed 18F-NaF-PET/CT significantly affected patient management in terms of initial staging, suspected first skeletal metastasis and progression of known skeletal disease. Although 18F-NaF-PET/CT likely provides a better estimate of disease burden than 99mTc-MDP bone scans, 18F-NaF uptake is related to osteoblastic turnover and not to the presence of malignant cells themselves. Thus, 18F-NaF-PET/CT provides an indirect measure of osseous metastases similar to 99mTc-MDP bone scans, and does not accurately measure the burden of malignant cells. 18F-FDG-PET/CT has also been investigated in mCRPC. However, the benefit in men with prostate cancer is often questioned, largely because 18F-FDG uptake by prostate cancer cells is variable and often low [30]. Minamimoto et al. and Watanabe et al. found that 18F-FDG-PET/CT had a sensitivity < 40% for the detection of prostate cancer [6-7]. This may be due to a combination of factors including, low glucose metabolism related to decreased expression of glucose transporters (such as Glut-1), or to the use of fructose rather than glucose [31]. Jadvar et al. also found low sensitivity for 18F-FDG-PET/CT in men with prostate cancer [8]. Indeed, the results of 18F-NaF- and 18F-FDG-PET/CT in 37 men with biochemical relapse after definitive therapy for localized prostate cancer suggested 18F-NaF-PET/CT was useful to detect occult osseous metastases while 18F-FDG-PET/CT was limited. However, these men were at an earlier stage in the continuum of prostate cancer disease than men in our study (biochemical recurrence versus mCRPC, respectively) and had limited long-term follow-up (median 24 weeks, range 1-49 weeks). Thus, it might be expected that the burden of aggressive disease would be lower in the cohort of men studied by Jadvar et al. Further, based on the reported results, it is difficult to determine if sites of 18F-FDG-avid disease was more aggressive in the long-term. Recently, several studies have shown 18F-FDG uptake reflects prognosis. When Jadvar and colleagues evaluated 18F-FDG-PET/CT in 87 men with mCRPC they found the sum of lesions’ SUVmax contributed independent prognostic information on overall survival (OS) [12]. Vargas et al. studied 18F-FDG-PET/CT in 38 men with mCRPC and suggested that the number of lesions was associated with OS [32].
In our study, we assessed 9 men with mCRPC treated with abiraterone and cabozantinib. The majority of men (89%) had extensive baseline osseous metastatic disease, which is consistent with the skeleton being the most common site of prostate cancer metastases [1] and the men in our study had advanced disease. 18F-NaF-PET/CT showed more skeletal lesions than conventional imaging. Results in the literature suggest the number of osseous lesions is an independent prognostic factor in prostate cancer [32-34], however the large number of men with mCRPC and extensive 18F-NaF-avid disease makes it difficult to adopt a practical approach using 18F-NaF-PET/CT to stratify patients in a way that provides a priori insight into prognosis. Also, the intensity of baseline 18F-NaF-avid disease did not provide information on therapy response and changes on 18F-NaF-PET/CT following therapy did not consistently reflect DOT. Since cabozantinib may impact both osteoblastic and cancer cells, 18F-NaF-PET/CT may be limited in its ability to measure anti-cancer therapy response in this population.
18F-FDG-PET/CT provided complementary information to 18F-NaF-PET/CT and conventional imaging. Five of nine men (56%) had non-18F-FDG-avid disease at baseline. We found that 18F-FDG-PET/CT identified patients likely to respond to abiraterone and cabozantinib therapy a priori. Of the 4 men with 18F-FDG-avid disease at baseline, DOT was significantly shorter than for the 5 men with non-18F-FDG-avid disease. Our results suggest 18F-FDG-PET/CT may be a practical tool for classifying baseline disease into one of 3 categories: widespread 18F-FDG-avid disease, oligometastatic 18F-FDG-avid disease (1 lesion) and non-18F-FDG-avid disease. The main limitation of this study was the small sample size as this was conducted to generate pilot data in this patient population. However, despite our small sample size, univariate analysis revealed a statistically significant difference (p < 0.01) among these 3 groups. The two men with widespread disease did worse than the two men with oligometastatic disease, while the five men with non-18F-FDG-avid disease did better.
The concept of an oligometastatic disease state has previously been defined as a state where the number of metastatic deposits is limited [35]. The literature suggests that eradication of oligometastatic tumors either with surgery or radiation may increase survival [36-39]. Although not standard of care, it has been suggested that it may be worth considering aggressive local treatment to all sites of metastases in men with prostate cancer and ≤ 5 metastatic lesions [40]. There were two men with a single site of 18F-FDG-avid oligometastatic disease included in our preliminary data. One man, with a solitary site of baseline 18F-FDG-avid disease and no history of radiation prior to enrolling on the trial, developed cord compression from progression at this site of baseline 18F-FDG-avid disease while on the trial and was radiated to this site of disease following removal from the trial (DOT 231). The other man, with a solitary site of baseline 18F-FDG-avid disease, had a history of radiation to the only site of 18F-FDG-avid prior to enrolling on the trial. Although this site of disease demonstrated baseline 18F-FDG avidity, it improved on follow-up imaging and this man had significantly longer PFS (DOT 742+). Our preliminary data suggest that a lower burden of 18F-FDG-avid disease is associated with longer PFS. Also, radiation targeted to the site of 18F-FDG-avid oligometastatic disease may provide benefit despite the presence of more extensive 18F-NaF-avid disease elsewhere (Figure 2).
Finally, we found that, overall, the SUVmax and average SUVmax was higher in men with widespread versus oligometastatic 18F-FDG-avid disease. Although both men with widespread intensely 18F-FDG-avid osseous disease had 18F-FDG-avid soft tissue disease as well, and did poorly (DOT 53 and 76), there was one man who had non-18F-FDG-avid soft tissue and skeletal disease, who remained on the trial for a considerably longer period of time (DOT 516+). This suggests that 18F-FDG-avid bone disease rather than the presence of soft tissue disease may be a more important factor in terms of survival.
Conclusion
Our data suggest that baseline 18F-FDG-PET/CT may identify men with mCRPC who are likely to benefit from cabozantinib and abiraterone therapy more reliably than standard imaging. Further, 18F-FDG may be superior to 18F-NaF in this patient population. Finally, 18F-FDG-PET/CT has the potential to stratify men with mCRPC into 3 groups (widespread 18F-FDG-avid disease, oligometastaic 18F-FDG-avid disease and non-18F-FDG-avid disease) to tailor therapy. A major unmet clinical need in men with mCRPC is the development of biomarkers to identify those who will benefit from therapy while avoiding futile expensive and toxic treatment. Ideally, a non-invasive biomarker could be used to personalize therapy. Given the relatively easy access to 18F-FDG compared with novel PET tracers, 18F-FDG-PET/CT may prove to be an attractive, practical tool to triage men with mCRPC to receive appropriate therapy that could easily be adopted into clinical practice. Further evaluation is warranted.
Acknowledgements
We acknowledge funding from the Radiological Society of North America Research Scholar Grant #RSCH 1221, the Prostate Cancer Foundation, Exelixis, the Dana-Farber/Harvard Cancer Center Prostate SPORE # P50CA090-381 and the Brigham and Women’s Hospital Radiology Department.
Disclosure of conflict of interest
Dr. Sweeney and Dr. Kantoff served as a consultant for from Exelixis and Janssen during the conduct of the study; the remaining co-authors have nothing to disclose.
References
- 1.de Bono J, Logothetis C, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI COU-AA-Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;264:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 3.Schirrmeister H, Glatting G, Hetzel J, Nüssle K, Arslandemir C, Buck AK, Dziuk K, Gabelmann A, Reske SN, Hetzel M. Prospective evaluation of the clinical value of planar bone scans, SPECT, and 18F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42:1800–1804. [PubMed] [Google Scholar]
- 4.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy. Single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 5.Tateishi U, Morita S, Taguri M, Shizukuishi K, Minamimoto R, Kawaguchi M, Murano T, Terauchi T, Inoue T, Kim EE. A meta-analysis of 18F-fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med. 2010;24:523–531. doi: 10.1007/s12149-010-0393-7. [DOI] [PubMed] [Google Scholar]
- 6.Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Murano T, Fukuda H, Iinuma T, Uno K, Nishizawa S, Tsukamoto E, Iwata H, Inoue T, Oguchi K, Nakashima R, Inoue T. The current status of an FDG-PET cancer screening program in Japan based on a 4-year (2006-2009) nationwide survey. Ann Nucl Med. 2013;27:46–57. doi: 10.1007/s12149-012-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe H, Kanematsu M, Kondo H, Kako N, Yamamoto N, Yamada T, Goshima S, Hoshi H, Bae KT. Preoperative detection of prostate cancer: a comparison with 11C-choline PET, 18F-fluorodeoxyglucose PET, and MR imaging. J Magn Reson Imaging. 2010;31:1151–1156. doi: 10.1002/jmri.22157. [DOI] [PubMed] [Google Scholar]
- 8.Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, Gross ME, Pinski JK, Quinn DI. Prospective evaluation of 18FNaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick J, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, Alcaraz A, Bahl A, Bracarda S, Di Lorenzo G, Efstathiou E, Finn SP, Fosså S, Gillessen S, Kellokumpu-Lehtinen PL, Lecouvet FE, Oudard S, de Reijke TM, Robson CN, De Santis M, Seruga B, de Wit R. Optimal management of metastatic castration-resistant prostate cancer: Highlights from a European Expert Consensus Panel. Eur J Cancer. 2014;50:1617–1627. doi: 10.1016/j.ejca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, Shizukuishi K, Tateishi U, Kubota Y, Inoue T. The potential of FDG PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–27. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 11.Oyama N, Akino H, Suzuki Y, Kanamaru H, Miwa Y, Tsuka H, Sadato N, Yonekura Y, Okada K. Prognostic value of 2-deoxy-2-[F-18] fluoro-Dglucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 12.Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, Pinski JK, Quinn DI. Baseline 18F-FDG parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris MJ, Akhurst T, Larson SM, Ditullio M, Chu E, Siedlecki K, Verbel D, Heller G, Kelly WK, Slovin S, Schwartz L, Scher HI. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res. 2005;11:3210–3216. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meirelles GS, Schoder H, Ravizzini GC, Gönen M, Fox JJ, Humm J, Morris MJ, Scher HI, Larson SM. Prognostic value of baseline [18F] fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin Cancer Res. 2010;16:6093–6099. doi: 10.1158/1078-0432.CCR-10-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA TAX Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg CN, de Bono JS, Chi KN, Fizazi K, Mulders P, Cerbone L, Hirmand M, Forer D, Scher HI. Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol. 2014;25:429–434. doi: 10.1093/annonc/mdt571. [DOI] [PubMed] [Google Scholar]
- 18.Parker C, Nilsson S, Heinrich , Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzén L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O ALSYMPCA Investigators. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 19.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 20.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 21.Smith D, Smith M, Sweeney C, Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PN Jr, Lin CC, Srinivas S, Sella A, Schöffski P, Scheffold C, Weitzman AL, Hussain M. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J. Clin. Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RJ, Saylor PJ, Michaelson MD, Rothenberg SM, Smas ME, Miyamoto DT, Gurski CA, Xie W, Maheswaran S, Haber DA, Goldin JG, Smith MR. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res. 2013;19:3088–3094. doi: 10.1158/1078-0432.CCR-13-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham TJ, Box G, Tunariu N, Crespo M, Spinks TJ, Miranda S, Attard G, de Bono J, Eccles SA, Davies FE, Robinson SP. Preclinical evaluation of imaging biomarkers for prostate cancer bone metastasis and response to cabozantinib. J Natl Cancer Inst. 2014;106:dju033. doi: 10.1093/jnci/dju033. [DOI] [PubMed] [Google Scholar]
- 24.Dai J, Zhang H, Karatsinides A, Keller JM, Kozloff KM, Aftab DT, Schimmoller F, Keller ET. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin Cancer Res. 2014;20:617–630. doi: 10.1158/1078-0432.CCR-13-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scher H, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M Prostate Cancer Clinical Trials Working Group. Design and end points of a clinical trial for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer trials working group. J. Clin. Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D National Cancer Institute. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F] -fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1182. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 29.Hillner BE, Siegel BA, Hanna L, Duan F, Shields AF, Coleman RE. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the National Oncologic PET Registry. J Nucl Med. 2014;55:574–581. doi: 10.2967/jnumed.113.130005. [DOI] [PubMed] [Google Scholar]
- 30.Kukuk D, Reischl G, Raguin O, Wiehr S, Judenhofer MS, Calaminus C, Honndorf VS, Quintanilla-Martinez L, Schönberger T, Duchamp O, Machulla HJ, Pichler BJ. Assessment of PET tracer uptake in hormone-independent and hormone-dependent xenograft prostate cancer mouse models. J Nucl Med. 2011;52:1654–1663. doi: 10.2967/jnumed.110.086702. [DOI] [PubMed] [Google Scholar]
- 31.Reinicke K, Sotomayor P, Cisterna P, Delgado C, Nualart F, Godoy A. Cellular distribution of Glut-1 and Glut-5 in benign and malignant human prostate tissue. J Cell Biochem. 2012;113:553–562. doi: 10.1002/jcb.23379. [DOI] [PubMed] [Google Scholar]
- 32.Vargas HA, Wassberg C, Fox JJ, Wibmer A, Goldman DA, Kuk D, Gonen M, Larson SM, Morris MJ, Scher HI, Hricak H. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology. 2014;271:220–229. doi: 10.1148/radiol.13130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbatini P, Larson SM, Kremer A, Zhang ZF, Sun M, Yeung H, Imbriaco M, Horak I, Conolly M, Ding C, Ouyang P, Kelly WK, Scher HI. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J. Clin. Oncol. 1999;17:948–957. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]
- 34.Rigaud J, Tiguert R, Le Normand L, Karam G, Glemain P, Buzelin JM, Bouchot O. Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol. 2002;168:1423–1426. doi: 10.1016/S0022-5347(05)64465-5. [DOI] [PubMed] [Google Scholar]
- 35.Hellman S, Weichselbaum RR. Oligometastases. J. Clin. Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 36.The International Registry of Lung Metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 37.Lo SS, Moffatt-Bruce SD, Dawson LA, Schwarz RE, Teh BS, Mayr NA, Lu JJ, Grecula JC, Olencki TE, Timmerman RD. The role of local therapy in the management of lung and liver oligometastases. Nat Rev Clin Oncol. 2011;8:405–416. doi: 10.1038/nrclinonc.2011.75. [DOI] [PubMed] [Google Scholar]
- 38.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, Gospodarowicz M, Sanders K, Kostashuk E, Swanson G, Barber J, Hiltz A, Parmar MK, Sathya J, Anderson J, Hayter C, Hetherington J, Sydes MR, Parulekar W; NCIC CTG PR.3/MRC UK PR07 investigators. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, Lund JA, Tasdemir I, Hoyer M, Wiklund F, Fosså SD Scandinavian Prostate Cancer Group Study 7; Swedish Association for Urological Oncology 3. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 40.Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP, Messing E, Okunieff P. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004;58:3–10. doi: 10.1016/s0360-3016(03)01442-1. [DOI] [PubMed] [Google Scholar]


