Abstract
Multi-modal imaging approaches of tumor metabolism that provide improved specificity, physiological relevance and spatial resolution would improve diagnosing of tumors and evaluation of tumor progression. Currently, the molecular probe FDG, glucose fluorinated with 18F at the 2-carbon, is the primary metabolic approach for clinical diagnostics with PET imaging. However, PET lacks the resolution necessary to yield intratumoral distributions of deoxyglucose, on the cellular level. Multi-modal imaging could elucidate this problem, but requires the development of new glucose analogs that are better suited for other imaging modalities. Several such analogs have been created and are reviewed here. Also reviewed are several multi-modal imaging studies that have been performed that attempt to shed light on the cellular distribution of glucose analogs within tumors. Some of these studies are performed in vitro, while others are performed in vivo, in an animal model. The results from these studies introduce a visualization gap between the in vitro and in vivo studies that, if solved, could enable the early detection of tumors, the high resolution monitoring of tumors during treatment, and the greater accuracy in assessment of different imaging agents.
Keywords: Glucose, glucose uptake, FDG, deoxyglucose, FDG-PET, multi-modal imaging
Introduction
Glucose uptake is a main indication of cellular metabolism, a key hallmark of cancer invasion and progression [1]. Imaging the cellular uptake of glucose in tumor models and clinical samples is therefore an important biomedical research and diagnostic tool. In order to best do this, however, analogs of glucose need to be created that have properties conducive to the desired imaging modality. The most important of these in current clinical use has been 18F labeled 2-fluoro-2-deoxy-D-glucose (FDG) designed for positron emission tomography (PET). The synthesis of 18F labeled FDG was first described in 1969 by Pacak et al [2]. Its use as a tracer for PET imaging was investigated in the 1970’s and early 1980’s [3-7]. Since then, it has been used in hundreds of thousands of PET diagnostic imaging procedures and has been the subject of over 15,000 studies [8-11]. These studies have elucidated the glycolytic activity of tumors in animal models and humans in response to various cancer treatments.
Due to the clinical importance of FDG PET approaches, there has been recent effort to characterize the intratumoral distribution of the molecule in various tumor models [12,13]. However, this is not an easy task due to sensitivity and spatial resolution challenges. There have already been several studies that have yielded rough distributions of FDG, mainly through the use of PET imaging [14]. However, the low intrinsic resolution of PET makes the determination of the distribution of FDG on a cellular level impossible with traditional approaches.
The use of other imaging modalities may offer new ways to investigate the intratumoral distribution of FDG. There are other imaging modalities that do not have the resolution limits that PET has, such as magnetic resonance imaging (MRI) or optical imaging. While each of these modalities does have limitations, they can be used for many different applications to provide complementary information to the information that is already available through PET [15,16]. A multi-modal approach with FDG-PET and other imaging modalities could provide improvements in physiological sensitivity and spatial characterization.
Imaging glucose uptake with other imaging modalities introduces a new and major problem in detection. The method of contrast in imaging with FDG is positron emission through the decay of 18F. Other imaging modalities such as optical and MRI will not be able to take advantage of this decay to provide contrast. Therefore, several deoxyglucose analogs have been developed such as ones that are fluorescently labeled for optical imaging, and 19F-FDG, labeled for MRI and NMR, that will help researchers study molecules that are similar to FDG with new imaging modalities [17-19]. There is also at least one example of a deoxyglucose analog that was developed for PET imaging, but that goes through a slightly different metabolic pathway [20].
Many cancer treatment plans take FDG-PET information into account [21,22]. Due to this clinical importance an increased understanding of the distribution of FDG within a tumor could lead to better treatment planning for a more effective treatment overall. There are many factors that influence the distribution of FDG, biologically. The most immediate factor is a tumor’s proliferation levels. FDG is a deoxyglucose analog and is taken up at higher levels in cell’s that are highly proliferating [12,13]. It is this property of FDG that is currently exploited for tumor imaging and diagnosis.
However, on a cellular level, a particular cell’s uptake of FDG depends on many more factors than just its metabolism or proliferation levels. It depends on the expression levels of the protein(s) that transport FDG into the cell [14]. It also depends on the expression levels of the protein(s) that will act on FDG [14]. Finally, it depends on an individual cell’s ability to access FDG. This last factor is involved with vascularization and angiogenesis within a tumor and a molecule’s ability to penetrate into a tumor.
The aim of this review is to highlight several recent attempts to image metabolic changes in cancer via detecting the intratumoral distribution of deoxyglucose. These studies are both in vivo and in vitro. They will include novel imaging techniques as well as the development of deoxyglucose analogs similar to FDG, all in an attempt to provide better information about this distribution. This review will also provide background on the cellular uptake and metabolism of FDG. A final aim of this review is to comment on the clinical importance of this work, so that a context is provided for future related studies.
18F-FDG
Molecule and synthesis
FDG is a modified glucose molecule with the radionuclide fluorine-18 (18F) in place of the hydroxyl group on the 2 carbon. To produce FDG, 18F must first be made. This is typically done with the proton beam produced from a cyclotron, by using it to bombard 18O enriched water [23]. This creates no-carrier-added 18F ions dissolved in the water through a (p,n) reaction. These ions can be used to synthesize FDG. 18F has a half-life of about 1.8 hours, so FDG must be transported rapidly from its production site to its site of use. The structure of FDG is shown in Figure 1 alongside the structure of glucose. Note the fluorination on the 2-carbon of glucose.
Figure 1.
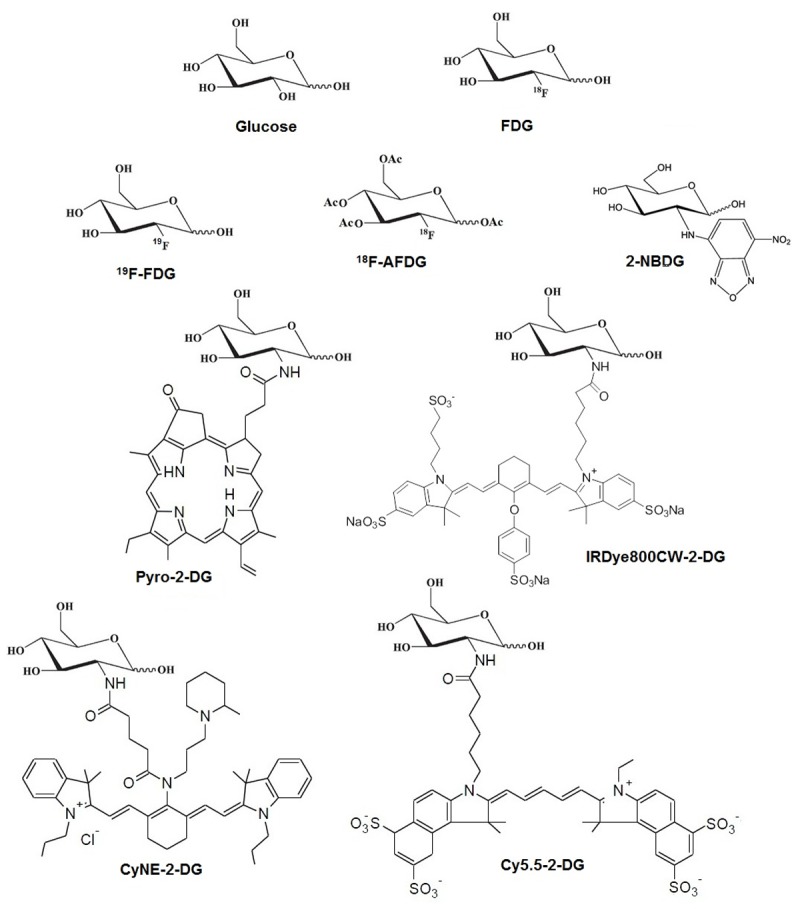
Chemical structures of all the molecules discussed in this review.
FDG uptake and metabolism
Several of the hallmarks of cancer introduced by Hanahan and Weinberg involve cell proliferation [1]. These include an insufficiency in anti-growth signals, a self-sufficiency in growth signals, and tissue invasion and metastatic behavior. In addition, an emerging hallmark, reprogramming energy metabolism, is particularly relevant to FDG uptake. When these factors combine, tumors exhibit uncontrolled growth. They multiply when normal cells are at rest, and they need less growth factors than normal cells to do so. They also need more anti-growth factors than normal cells to stop doing so [1]. In order to sustain this level of growth, tumor cells must have higher levels of cellular energy metabolism.
Glucose is the key fuel for cellular energy metabolism [24]. Warburg demonstrated that tumor cells have different glucose metabolism than normal cells in 1925 [25]. It was originally thought that this increased metabolism in glucose was a result of increased uptake. This increased uptake was demonstrated by Hatanaka in 1974 [26]. However, this was found to be too simplistic, as the rate of glucose metabolism was shown to not be directly related to glucose transport across the cellular membrane, and that other factors were involved.
Glucose is transported into the cell by cellular membrane proteins called glucose transporters [24]. These transport proteins are activated by insulin, a hormone created in the pancreas. Glucose transporters are among a family of membrane proteins called heterogeneously glycosylated integral membrane proteins [27-29]. Glucose in circulation binds the outer part of these transport proteins on the outside of the cell membrane. This induces the protein complex to change conformation to move the glucose molecule inside the cell. A model of this mechanism can be seen in Figure 2.
Figure 2.
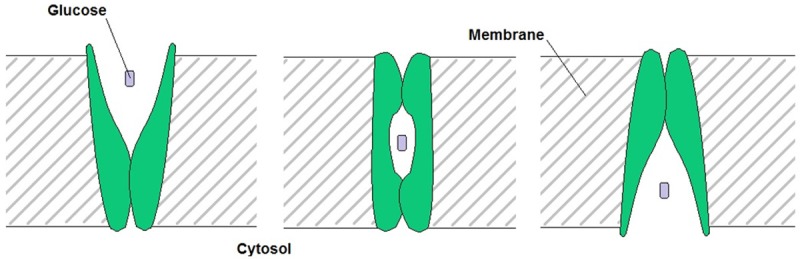
Schematic depicting the method of facilitated glucose transport via glucose transport proteins (GLUT), shown in green. Adapted from Pauwels et al. in 1998 [24].
These transport proteins move glucose both ways across the cellular membrane, depending on glucose concentrations inside and outside of the cell. They move glucose from high to low concentrations. So far, seven different glucose transport proteins have been discovered. They were named GLUT 1-7 and they all have a similar polypeptide chain of about 500 amino acids. This peptide chain has a similar folding pattern in all 7 of these transport proteins [24,30]. This is further evidence of their common purpose.
After glucose is taken up into a living cell, it undergoes phosphorylation by hexokinase. This results in a molecule of glucose-6-phosphate(P). Glucose-6-P is then free to enter further downstream metabolic pathways. FDG is transported into the cell via the same glucose transport proteins. It is also then phosphorylated by hexokinase [13]. However, in this case, the resulting molecule is 2-deoxy-2-F-glucose-6-P. The next step in the glycolytic pathway requires a hydroxyl group on the 2 carbon. FDG is missing this hydroxyl group and thus cannot proceed through glycolysis. This effectively traps the 18F signal inside the cell. [13]. The transport and metabolism of FDG is shown in Figure 3.
Figure 3.

Schematic showing the transport of FDG into the cell and the subsequent metabolism of it into FDG-6P. Like many chemical reactions within the body, this process is reversible, and the variables k1, k2, k3 and k4 are the kinetic constants that describe the transport of FDG into the cell, its phosphorylation to FDG-6-Phophate, its dephosphorylation back to FDG and its transport out of the cell, respectively.
The uptake and metabolism of glucose or FDG clearly depends on at least two variables, the expression and activity of glucose transport proteins and the expression and activity of hexokinase in an individual cell. However, this is only looking at the individual cell and not the tumor as a whole. As mentioned earlier, the direction of transport of glucose or FDG into a cell depends on the concentrations of glucose or FDG inside and outside of that cell. These concentrations depend on the overall tumor biology.
One of the main factors that dictates how much glucose or FDG will be present in an area of a tumor is that area’s angiogenesis, or the extent of its vasculature. Molecules like FDG will be able to penetrate into areas of a tumor that have high vasculature, but they will not be able to do so in areas with low vasculature. A complex picture of FDG uptake and metabolism is beginning to emerge. High-resolution imaging of FDG distributions within well-defined tumor models would help elucidate the interplay between FDG uptake and metabolism and the factors discussed here.
Clinical importance
FDG is one of the most commonly used radiotracers in nuclear medicine. It is typically used for cancer diagnosis through PET imaging. However, PET images lack the resolution necessary to identify distributions of FDG within a tumor other than large areas of increased or decreased uptake. This has a direct effect on treatment planning that utilizes PET imaging.
When PET information is available it is often used in the treatment planning of radiotherapy [21,22]. PET information helps define tumor volumes by adding information to images obtained from CT or MRI, in which tumor identification might be difficult. These tumor volumes define a target region during a radiotherapy operation. The target dose to that region is uniform in most cases.
However, the uniformity of the dose to the target volume is not consistent with the proliferation rates of the tumor itself. In an ideal case, the non-uniformity of FDG uptake and proliferation levels within a tumor would be mirrored in dose distributions. Developing ways to reliably image the distribution of FDG within a tumor will help to achieve this goal and could enable the use of more effective treatments of tumors.
Fluorescent deoxyglucose analogs
There is great interest in developing fluorescent glucose analogs that could take advantage of the improved spatial resolution and specificity of fluorescence optical imaging. The following represent examples of such molecules. From their structures (see Figure 1), one striking feature of some of these molecules is the size of the fluorescent group that was attached to the 2-carbon of glucose. It should be noted that the increased size of this group has the potential to affect a molecule’s transport into the cell.
2-NBDG
In 1985, a fluorescent glucose analog, 6-NBDG, was developed, but it was determined that while the molecule did enter the cell through GLUTs, it did so at a relatively slow rate, implying a low affinity for the transporters [31]. This molecule had a fluorescent group on the C-6 position of glucose. In the hopes of creating a fluorescent glucose analog that would enter the glycolytic pathway, Yoshioka et al. synthesized 2-NBDG in 1996 [32]. This molecule featured the same fluorescent group as 6-NBDG, but had it on the C-2 position rather than the C-6 position. The C-2 position is the same position that the 18F atom is bound to in FDG.
In order to determine if this new molecule behaved as compared to FDG, Yoshioka et al. began to describe the intracellular fate of 2-NBDG later in 1996 [17]. In order to do this, they used Escherichia coli cells and performed several studies to identify all of the metabolites of 2-NBDG as it proceeded through the glycolytic pathway. They found that 2-NBDG is almost immediately converted into another fluorescent derivative after uptake into the cell. They called this derivative the 2-NBDG metabolite [32]. After this, the fluorescent 2-NBDG metabolite was then decomposed into non-fluorescent forms.
This decomposition of the fluorescent 2-NBDG metabolite into a non-fluorescent form could prove useful in studying glucose uptake activity [32]. This is because the fluorescent intensity that is seen within a cell after 2-NBDG uptake will represent a dynamic equilibrium level between the generation and the decomposition of the 2-NBDG metabolite. This equilibrium should be sensitive to glucose uptake activity. There have been several studies, discussed later, that have evaluated 2-NBDG for use as a fluorescent glucose analog and as an indicator of glucose uptake activity.
IRDye800CW 2-DG
IRDye800CW 2-DG is a proprietary fluorescent probe made by Licor (Lincoln, Nebraska). It is very similar to 2-NBDG in that it also has a fluorescent group in the C-2 position of its ring. That fluorescent group in this case is an organic dye that emits in the near infrared (NIR) range. It was used by Garafalakis et al. in their in vivo study of FDG-PET imaging in parallel with fluorescence diffuse optical tomography (fDOT) in 2012 [18]. This study is presented in more detail later.
Cy5.5-2-DG
Cy5.5-D-glucosamine (Cy5.5-2-DG) was developed at Stanford University by Gambhir’s group in 2006 [33]. It is a fluorescent deoxyglucose analog that emits in the NIR range. It was developed for imaging tumor metabolism in living subjects. Its ability to target tumors was assessed and compared to other fluorescent deoxyglucose analogs [33].
During this study, tumor localization was investigated after tail vein delivery of Cy5.5-2-DG. In some cases, the contrast ratio in tumor compared to healthy tissue approached 3:1. This compares well to FDG. However, high extracellular glucose concentration did not inhibit Cy5.5-2-DG uptake. This suggests that the pathways that moderate glucose uptake, GLUT and hexokinase, do not mediate the uptake of this tracer. This is in contrast to 2-NBDG, although the stability of Cy5.5-2-DG is greater than that of 2-NBDG.
Pyro-2-DG
Pyropheophorbide 2-deoxyglucosamide (Pyro-2-DG) was developed at the University of Pennsylvania by Zheng’s group in 2003 [34]. Like IRDye800CW 2-DG and Cy5.5-2-DG, Pyro-2-DG is tagged with a fluorescent group at the 2 carbon of deoxyglucose that emits in the NIR range. They later assessed this molecule’s use as a tumor-targeting agent for fluorescence imaging [35]. During this study, mouse and rat models received tail vein injections of Pyro-2-DG. After surgical excision of tumors, the ratio of accumulation in tumors compared to normal tissue was found to be almost 10:1, significantly higher than FDG.
CyNE-2-DG
CyNE 2-DG was synthesized by Chang’s group at the National University of Singapore. It is another fluorescent analog to deoxyglucose that emits in the NIR range. This probe was characterized by comparing it to IRDye800CW 2-DG [36]. They found that CyNE 2-DG yielded significantly higher signal after incubation in cancer cells than IRDye 800CW 2-DG. Chang and his group speculated that the relative reduction in signal observed with IRDye800CW 2-DG was due to it having a more negative charge, which could decrease its cell membrane permeability.
Non-fluorescent deoxyglucose analogs
18F-AFDG
As discussed earlier, FDG accumulation in tumor cells is directly affected by two factors, the levels of glucose transporter protein (GLUT) and the levels of hexokinase present in a cell. This complicates the mechanism of higher uptake of FDG. In an attempt to un-complicate this picture, Waki et al. investigated another analog to FDG in 1998 [20]. 1,3,4,6-tetra-acetyl-2-[18F]-2-deoxy-D-glucose (18F-AFDG) is the analog that they chose.
18F-AFDG is a lipophilic FDG analog. This property makes its accumulation in cells independent of glucose transport proteins, because it can pass through the cellular membrane without a transport protein. To demonstrate that 18F-AFDG did in fact accumulate in cells without a glucose transport protein, Waki et al. used cytochalasin B, a compound that inhibits transport via GLUT proteins. They found that the presence of cytochalasin B did not affect 18F AFDG uptake [20].
In another study described in the same paper, Waki et al. studied the level of 18F-AFDG uptake in the presence of excess glucose [20]. In media that contained double the glucose level of the control, FDG uptake was decreased by about 50%. In the same media, 18F-AFDG uptake was unaffected. This further demonstrates the independence of 18F-AFDG uptake to GLUT proteins, as higher levels of glucose inhibit GLUT function.
A linear relationship was observed between 18F-AFDG uptake and 18F-FDG-6P production even after a 10-fold increase in 18F-AFDG concentration [20]. The study of analogs to FDG that are GLUT-independent, such as 18F-AFDG, may help to clarify the mechanism surrounding FDG uptake by simplifying the metabolic mechanism.
19F-FDG
19F-FDG, or “cold” FDG, is a glucose molecule that is fluorinated at the 2-carbon with 19F and not 18F. When 18F-FDG is synthesized, the vast majority of the resulting molecules are still cold FDG, and are fluorinated with 19F. Typical specific activities of 18F-FDG are on the order of 5 Ci/µmol. This means that there are millions of molecules fluorinated with 19F for every molecule of 18F-FDG. While cold FDG cannot be used for PET scans, it can be used for nuclear magnetic resonance (NMR) and even as a contrast agent for MRI. In particular, aspects of the metabolic pathway of FDG were worked out in 1986 by Kanazawa et al. using NMR of FDG [37]. There has been MR imaging performed using 19F of small animals, such as the brain imaging of rabbits performed in 1988 by Nakada et al [38]. One key limitation of using MRI for molecular imaging that should be noted is the reduced sensitivity relative to PET. For signal in MRI, the required molecular concentration is several orders of magnitude higher than that of PET. The structures of all molecules discussed in this review can be seen in Figure 1.
Imaging deoxyglucose distribution
In-vitro
Earlier in this review, the complex mechanism underlying the uptake of FDG and other deoxyglucose analogs into a tumor cell was discussed. While the precise direct mechanism is complicated, it was widely accepted that FDG uptake is a strong indicator of proliferation levels. This is because FDG uptake mirrors glucose uptake and glucose uptake is an indicator of proliferation. However, Higashi et al. showed that this is not necessarily the case in 1993 [39].
His group performed a comparison of FDG uptake to tritiated thymidine uptake in tumor cells. Through this comparison, they concluded that FDG uptake is strongly correlated to the number of viable tumor cells and not proliferation levels. This was in contrast with previous findings [40,41]. In this study, Higashi et al. quantified proliferation levels by defining a proliferation index. This index was defined as the percentage of cells that are in the S or G2/M phases of their cell cycle. They performed flow cytometry to determine the fractions of cells in each stage of the cell cycle. This study by Higashi et al. further demonstrates the complexity of FDG uptake.
Another in vitro study involved the development of a new microscopy technique, radioluminescence microscopy. This was work performed by Pratx et al. in 2012 [42]. During radioluminescence microscopy, a sample is placed directly on a scintillator plate that is contained in a glass-bottom dish. If the sample contains a radionuclide, its decay products can excite the scintillation material, releasing light. That light is caught through an objective underneath the glass bottom plate. Light from fluorescence emission can also be collected by the objective allowing 2 channel microscopy images to be created with radio-labeled as well as fluorescent molecules. A schematic depicting the setup for radioluminescence microscopy is shown in Figure 4.
Figure 4.
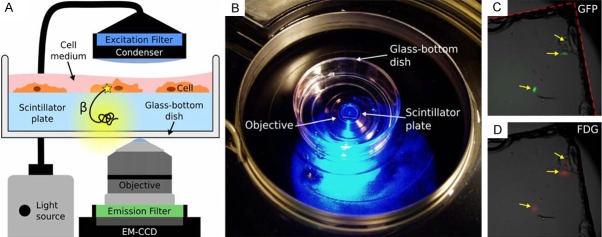
Schematic of the setup for radioluminescence microscopy as well as some early results showing the fluorescent signal from GFP and the signal from the scintillation of the decay products of FDG. Reprinted with permission from Pratx et al. 2012 [42].
Pratx et al. evaluated the use of this technique for FDG imaging by comparing the FDG signal detected with this setup to the signal from 2-NBDG. They concluded that radioluminescence microscopy is capable of quantifying radiotracer uptake at the single cell level. Some of the results from this study are shown in Figure 5. A brightfield microscope image, an image generated from the FDG signal, an image generated from the fluorescence of 2-NBDG, and an overlay between the FDG signal and the 2-NBDG fluorescence is shown [42].
Figure 5.
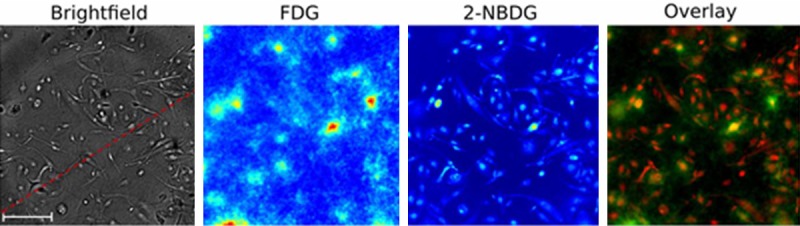
Radioluminescence microscopy results of FDG and 2-NBDG. Reprinted with permission from Pratx et al. 2012 [42]. Scale bar is 100 µm.
There are some limitations to radioluminescence microscopy. First, the resolution of the FDG signal is not sufficient to quantify the intracellular radiotracer distribution. Also, it is only possible to measure the radioactivity of single cells if they are sufficiently separated on the scintillator plate for the same reason. These issues, along with the requirement of a scintillation plate, diminish its usefulness for in vivo studies. However, as Pratx et al. assert in this work, it may be useful in assessing fluorescent analogs to PET tracers.
Another in vivo study using the glucose analogs discussed here was performed by O’Neil et al. in 2005 [43]. It was aimed at evaluating the use of 2-NBDG as a probe for glucose uptake in malignant tumor cells. This was done by comparing the uptake of 2-NBDG in non-malignant cells to tumorigenic cells. Two tumor cell lines were used in this study. They found that there was almost a 5-fold increase in 2-NBDG uptake in the tumorigenic cell lines when compared to the uptake in the non-malignant cells. This further validated the use of 2-NBDG for use as a probe for glucose uptake levels. Some key results from this study are shown in Figure 6.
Figure 6.

Uptake of 2-NBDG in tumorigenic cells (left) and an image of background fluorescence (center) along with fluorescent signal intensities from all cell lines (right). Note the 5-fold increase in 2-NBDG uptake in tumorigenic cells (left two bars in the right panel) versus non-tumorigenic (right bar in the right panel). Reprinted with permission from O’neil et al. 2005 [43].
In-vivo
In 2009, Sheth et al. performed a mouse model study aimed at the evaluation of 2-NBDG as an indicator of glucose uptake in vivo [44]. By optically imaging a whole animal for 2-NBDG fluorescence when it was well fed and when it was glucose-starved, they were able to see the relationship between 2-NBDG uptake and glucose availability. They found that the uptake of 2-NBDG increased dramatically during the glucose-starved state. This means that the localization of 2-NBDG to a tumor is not nonspecific, but depends on the glucose utilization ratio of the malignant tissue relative to the healthy tissue. This result from the study is shown in Figure 7.
Figure 7.
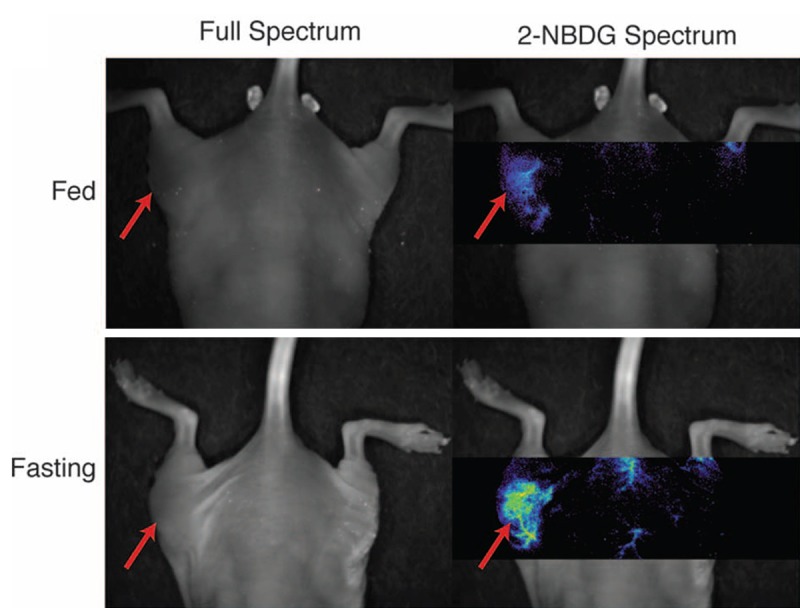
Figure showing the increased uptake of 2-NBDG in a fasting state (bottom) compared to a fed state (top). Reprinted with permission from Sheth et al. 2009 [44].
Sheth et al. also discussed the possibility of using 2-NBDG to supplement preoperative FDG-PET imaging. They suggest that 2-NBDG could be used to indicate the location of hyper-metabolic lesions during an operation within areas of interest indicated by the FDG-PET images [44].
Another study that was aimed at validating the use of fluorescent analogs to glucose in vivo was performed by Garofalakis et al. in 2012 [18]. Rather than using 2-NBDG, they used IRDye800CW 2-DG (2-DG) for this study. This molecule features an organic dye at the C-2 carbon of glucose rather than the fluorescent group found on 2-NBDG. This study by Garofalakis et al. used a comparison between fluorescence diffuse optical tomography (fDOT) images of the organic dye containing glucose analog 2-DG and PET images of FDG for their evaluation. fDOT is a technique that enables the imaging of 3D fluorescent volumes through the use of fluorescence lifetime information inside tissues [45,46]. This technique has also been called fluorescence molecular tomography (FMT).
Garofalakis et al. injected several mice with tumors with 2-DG and FDG. They then performed an FDG-PET scan and acquired an fDOT image. By registering the images to one another, they were able to directly compare the 3-dimensional distribution of FDG to that of 2-DG within the tumors contained in the model mice. An example result from this study is shown in Figure 8.
Figure 8.
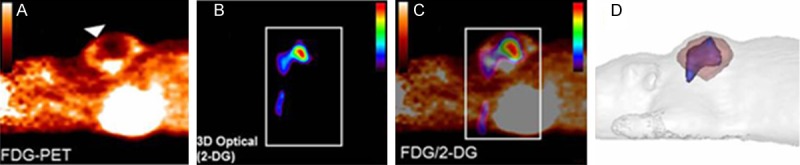
Figure showing the FDG-PET volumes (pink in the right panel) overlaid with the fDOT generated 2-DG volumes (blue in the right panel). Also shown are the original FDG-PET images (left), the 3D-optical scans of 2-DG (left center) and their overlay (right center). Reprinted with permission from Garafalakis et al. 2012 [18].
Garafalakis et al. registered the two images from PET and fDOT using a special mouse-supporting system. This system had four fiducial points on it that served as multi-modality markers, because they contained both a fluorescence signal and a PET signal. They discussed the use of fDOT and PET imaging to evaluate new fluorescent probes. As can be seen in the figure above, the images resulting from fDOT imaging are of similar resolution to those produced through PET scanning [18].
Discussion
The metabolic activity of tumors is of extreme clinical importance due to its correlation with tumor progression and staging. Tumors are heterogeneous in nature, so their characteristics vary throughout their mass. This poses a treatment problem, as one region of a tumor may respond differently to any given treatment than another. It is therefore important to map this metabolism on several scales to gain a complete picture of tumor metabolism in order to treat them consistently and effectively. The metabolism of tumors is still a very active area of research and thus there are too many metabolism related studies to review here. Instead, the focus of this review was kept to the glucose metabolism of tumors and the agents that may make multi-scale imaging of it possible.
In this review, several studies are highlighted that centered on imaging the uptake of glucose and several of its analog molecules by tumor cells. Some of these studies were performed in vitro, while others were performed in vivo, in animal tumor models. Several analogs to glucose were discussed, as well as their metabolic action within a cell. Several new imaging techniques were also discussed, and comparisons were made to the better known imaging techniques of PET scanning and optical imaging. All of these new techniques, the glucose analog molecules used, and whether they were used in vitro or in vivo are summarized in Table 1.
Table 1.
Summary of the techniques discussed in this review, along with whether they can be used in vitro or in vivo, and with which glucose analog molecules
| PET | MRI | Optical | Radioluminescence Microscopy | fDOT/FMT | |
|---|---|---|---|---|---|
| Glucose Analogs | |||||
| FDG | Yes | No | No | Yes | No |
| 18F-AFDG | Yes | No | No | Yes | No |
| 19F-FDG | No | Yes | No | No | No |
| 2-NBDG | No | No | Yes | Yes | Yes |
| IRDye800CW 2-DG | No | No | Yes | Yes | Yes |
| Pyro-2-DG | No | No | Yes | Yes | Yes |
| CyNE-2-DG | No | No | Yes | Yes | Yes |
| Cy5.5-2-DG | No | No | Yes | Yes | Yes |
| In vitro/In vivo | |||||
| In vitro | No | No | Yes | Yes | No |
| In vivo | Yes | Yes | Yes | No | Yes |
FDG has been around for over 40 years, and has been used clinically the majority of that time. It is used routinely for the diagnosis of tumors in hospitals and clinics around the world. However, despite its widespread use, only rough estimations of its intratumoral resolution can be recorded. High resolution distributions on the cellular level of FDG, or one of its analogs, in an animal model are still missing. This is due to the current state of imaging technology and the lack of a robust method to create large-scale images at cellular resolution.
The distribution of FDG in a tumor is far from homogeneous and is controlled by a complicated mechanism. That mechanism is dictated on the cellular level by two factors, GLUT protein expression and hexokinase expression. On the macroscopic level, that mechanism is dictated by tumor angiogenesis and the ability of molecules to penetrate into a tumor, among other factors. A study discussed in this review tried to simplify this picture through the use of a glucose analog, 18F-AFDG, that was independent of GLUT protein expression levels as a result of its lipophilicity [20].
Other studies discussed here used fluorescent analogs of glucose, like 2-NBDG and IRDye800CW 2-DG, to use microscopy to enhance the resolution that PET offers and to compare optical imaging data to PET images [18,32]. These studies are a step in the right direction, but more needs to be done. Treatment plans for radiotherapy are often made with target volumes that were defined in part from PET information. While this information is a step up from CT or MRI information alone, an increase in the resolution of the distribution of the PET tracer within a tumor volume would identify areas of a tumor that would benefit from increased dose.
These studies are all attempting to close the resolution gap between whole animal imaging and in vitro imaging of cells. This gap in resolution is extremely pronounced between the in vitro studies with cell cultures and the in vivo studies that use animal models. An even bigger resolution gap can be seen in the whole body clinical imaging of humans and the microscopy used for histology of tumor sections. Efforts to close this gap would enable the early detection of tumors, the high resolution monitoring of tumors during treatment, and the much more accurate assessment of different imaging agents.
Conclusion
All of the studies discussed in this review try to provide a better image of the intratumoral distribution of different glucose analogs. They do this through the use of several imaging techniques. Some of these imaging techniques are relatively new, like fDOT and radioluminescence microscopy, while others are older, like PET scanning and optical imaging. Some of these studies centered on cell cultures in vitro, while others centered on in vivo experiments in animal models. An increased knowledge of the intratumoral distribution of radiotracers like FDG will provide important clinical knowledge that will help improve treatments. But it is in the eventual closing of the resolution gap between the in vivo and in vitro studies that the true benefit of this type of information will likely be fully realized.
Acknowledgements
The authors would like to thank the Morgridge Institute for Research for their continued funding, without which this review would not be possible. They would also like to thank Stephen Graves for his useful discussions of the production of FDG. We also acknowledge Dr. Weibo Cai for useful input on the theme of the review.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Pacák J, Točík Z, Černý M. Synthesis of 2-deoxy-2-fluoro-D-glucose. J Chem Soc D. 1969:77–77. [Google Scholar]
- 3.Metter EJ, Wasterlain CG, Kuhl DE, Hanson WR, Phelps ME. FDG positron emission computed tomography in a study of aphasia. Ann Neurol. 1981;10:173–183. doi: 10.1002/ana.410100208. [DOI] [PubMed] [Google Scholar]
- 4.Phelps ME. Positron computed tomography studies of cerebral glucose metabolism in man: theory and application in nuclear medicine. Semin Nucl Med. 1981;11:32–49. doi: 10.1016/s0001-2998(81)80051-7. [DOI] [PubMed] [Google Scholar]
- 5.Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 6.Som P, Atkins HL, Bandoypadhyay D, Fowler JS, MacGregor RR, Matsui K, Oster ZH, Sacker DF, Shiue CY, Turner H, Wan CN, Wolf AP, Zabinski SV. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21:670–675. [PubMed] [Google Scholar]
- 7.Ido T, Wan CN, Casella V, Fowler JS, Wolf AP, Reivich M, Kuhl DE. Labeled 2-deoxy-D-glucose analogs. 18F-labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-fluoro-D-mannose and 14C-2-deoxy-2-fluoro-D-glucose. J Label Compd Radiopharm. 1978;14:175–183. [Google Scholar]
- 8.Thut DP, Ahmed R, Kane M, Djekidel M. Variability in myocardial metabolism on serial tumor (18)F-FDG PET/CT scans. Am J Nucl Med Mol Imaging. 2014;4:346–353. [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MD, Glaus C, Hesterman JY, Hoppin J, Puppa GHD, Kazules T, Orcutt KM, Germino M, Immke D, Miller S. Regional, kinetic [(18)F] FDG PET imaging of a unilateral Parkinsonian animal model. Am J Nucl Med Mol Imaging. 2013;3:129–141. [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen SF, Græbe M, Hag AMF, Højgaard L, Sillesen H, Kjær A. (18)F-FDG imaging of human atherosclerotic carotid plaques reflects gene expression of the key hypoxia marker HIF-1α. Am J Nucl Med Mol Imaging. 2013;3:384–392. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman JM, Gambhir SS. Molecular imaging: the vision and opportunity for radiology in the future. Radiology. 2007;244:39–47. doi: 10.1148/radiol.2441060773. [DOI] [PubMed] [Google Scholar]
- 12.Inoue O, Shukuri M, Hosoi R, Amitani M, Matsuura N, Hatazawa J, Takai N. Distinct different intra-tumor distribution of FDG between early phase and late phase in mouse fibrosarcoma. Ann Nucl Med. 2005;19:655–659. doi: 10.1007/BF02985113. [DOI] [PubMed] [Google Scholar]
- 13.Khan N, Islam MM, Mahmood S, Hossain GA, Chakraborty RK. 18F-fluorodeoxyglucose uptake in tumor. Mymensingh Med J. 2011;20:332–342. [PubMed] [Google Scholar]
- 14.Zhao S, Kuge Y, Mochizuki T, Takahashi T, Nakada K, Sato M, Takei T, Tamaki N. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46:675–682. [PubMed] [Google Scholar]
- 15.Ojili V, Tirumani SH, Chintapalli KN, Gunabushanam G. Non-invasive diagnosis of abdominopelvic masses: role of multimodality imaging. J Clin Imaging Sci. 2013;3:6. doi: 10.4103/2156-7514.106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka K, Takahashi H, Homma T, Saito M, Oh KB, Nemoto Y, Matsuoka H. A novel fluorescent derivative of glucose applicable to the assessment of glucose uptake activity of Escherichia coli. Biochim Biophys Acta. 1996;1289:5–9. doi: 10.1016/0304-4165(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 18.Garofalakis A, Dubois A, Thézé B, Czarny B, Tavitian B, Ducongé F. Fusion of [(18)F] FDG PET with fluorescence diffuse optical tomography to improve validation of probes and tumor imaging. Mol Imaging Biol. 2013;15:316–325. doi: 10.1007/s11307-012-0581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WH, Lee J, Jung DW, Williams DR. Visualizing sweetness: increasingly diverse applications for fluorescent-tagged glucose bioprobes and their recent structural modifications. Sensors (Basel) 2012;12:5005–5027. doi: 10.3390/s120405005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waki A, Fujibayashi Y, Magata Y, Yokoyama A, Sadato N, Tsuchida T, Ishii Y, Yonekura Y. Glucose transporter protein-independent tumor cell accumulation of fluorine-18-AFDG, a lipophilic fluorine-18-FDG analog. J Nucl Med. 1998;39:245–250. [PubMed] [Google Scholar]
- 21.Götz I, Grosu AL. [(18)F] FET-PET Imaging for Treatment and Response Monitoring of Radiation Therapy in Malignant Glioma Patients - A Review. Front Oncol. 2013;3:104. doi: 10.3389/fonc.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castaldi P, Leccisotti L, Bussu F, Miccichè F, Rufini V. Role of (18)F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33:1–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Hamacher K, Coenen HH, Stöcklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F] -fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27:235–238. [PubMed] [Google Scholar]
- 24.Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Mazière B. FDG accumulation and tumor biology. Nucl Med Biol. 1998;25:317–322. doi: 10.1016/s0969-8051(97)00226-6. [DOI] [PubMed] [Google Scholar]
- 25.Warburg PO. über den Stoffwechsel der Carcinomzelle. Klin Wochenschr. 1925;4:534–536. [Google Scholar]
- 26.Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974;355:77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- 27.Carruthers A. Facilitated diffusion of glucose. Physiol Rev. 1990;70:1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- 28.Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993;268:19161–19164. [PubMed] [Google Scholar]
- 29.Baldwin SA, Barros LF, Griffiths M. Trafficking of glucose transporters--signals and mechanisms. Biosci Rep. 1995;15:419–426. doi: 10.1007/BF01204346. [DOI] [PubMed] [Google Scholar]
- 30.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 31.Speizer L, Haugland R, Kutchai H. Asymmetric transport of a fluorescent glucose analogue by human erythrocytes. Biochim Biophys Acta. 1985;815:75–84. doi: 10.1016/0005-2736(85)90476-6. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka K, Saito M, Oh KB, Nemoto Y, Matsuoka H, Natsume M, Abe H. Intracellular fate of 2-NBDG, a fluorescent probe for glucose uptake activity, in Escherichia coli cells. Biosci Biotechnol Biochem. 1996;60:1899–1901. doi: 10.1271/bbb.60.1899. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Z, Levi J, Xiong Z, Gheysens O, Keren S, Chen X, Gambhir SS. Near-infrared fluorescent deoxyglucose analogue for tumor optical imaging in cell culture and living mice. Bioconjug Chem. 2006;17:662–669. doi: 10.1021/bc050345c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Zhang Z, Blessington D, Li H, Busch TM, Madrak V, Miles J, Chance B, Glickson JD, Zheng G. Pyropheophorbide 2-deoxyglucosamide: a new photosensitizer targeting glucose transporters. Bioconjug Chem. 2003;14:709–714. doi: 10.1021/bc034038n. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Li H, Liu Q, Zhou L, Zhang M, Luo Q, Glickson J, Chance B, Zheng G. Metabolic imaging of tumors using intrinsic and extrinsic fluorescent markers. Biosens Bioelectron. 2004;20:643–650. doi: 10.1016/j.bios.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Vendrell M, Samanta A, Yun SW, Chang YT. Synthesis and characterization of a cell-permeable near-infrared fluorescent deoxyglucose analogue for cancer cell imaging. Org Biomol Chem. 2011;9:4760–4762. doi: 10.1039/c1ob05519d. [DOI] [PubMed] [Google Scholar]
- 37.Kanazawa Y, Momozono Y, Ishikawa M, Yamada T, Yamane H, Haradahira T, Maeda M, Kojima M. Metabolic pathway of 2-deoxy-2-fluoro-D-glucose studied by F-19 NMR. Life Sci. 1986;39:737–742. doi: 10.1016/0024-3205(86)90022-6. [DOI] [PubMed] [Google Scholar]
- 38.Nakada T, Kwee IL, Griffey BV, Griffey RH. F-19 MR imaging of glucose metabolism in the rabbit. Radiology. 1988;168:823–825. doi: 10.1148/radiology.168.3.3136509. [DOI] [PubMed] [Google Scholar]
- 39.Higashi K, Clavo AC, Wahl RL. Does FDG uptake measure proliferative activity of human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J Nucl Med. 1993;34:414–419. [PubMed] [Google Scholar]
- 40.Minn H, Joensuu H, Ahonen A, Klemi P. Fluorodeoxyglucose imaging: a method to assess the proliferative activity of human cancer in vivo. Comparison with DNA flow cytometry in head and neck tumors. Cancer. 1988;61:1776–1781. doi: 10.1002/1097-0142(19880501)61:9<1776::aid-cncr2820610909>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Haberkorn U, Strauss LG, Reisser C, Haag D, Dimitrakopoulou A, Ziegler S, Oberdorfer F, Rudat V, van Kaick G. Glucose uptake, perfusion, and cell proliferation in head and neck tumors: relation of positron emission tomography to flow cytometry. J Nucl Med. 1991;32:1548–1555. [PubMed] [Google Scholar]
- 42.Pratx G, Chen K, Sun C, Martin L, Carpenter CM, Olcott PD, Xing L. Radioluminescence microscopy: measuring the heterogeneous uptake of radiotracers in single living cells. PloS One. 2012;7:e46285. doi: 10.1371/journal.pone.0046285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neil RG, Wu L, Mullani N. Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol Imaging Biol. 2005;7:388–392. doi: 10.1007/s11307-005-0011-6. [DOI] [PubMed] [Google Scholar]
- 44.Sheth RA, Josephson L, Mahmood U. Evaluation and clinically relevant applications of a fluorescent imaging analog to fluorodeoxyglucose positron emission tomography. J Biomed Opt. 2009;14:064014. doi: 10.1117/1.3259364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Leary MA, Boas DA, Li XD, Chance B, Yodh AG. Fluorescence lifetime imaging in turbid media. Opt Lett. 1996;21:158–160. doi: 10.1364/ol.21.000158. [DOI] [PubMed] [Google Scholar]
- 46.Hutchinson CL, Lakowicz JR, Sevick-Muraca EM. Fluorescence lifetime-based sensing in tissues: a computational study. Biophys J. 1995;68:1574–1582. doi: 10.1016/S0006-3495(95)80330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


