Abstract
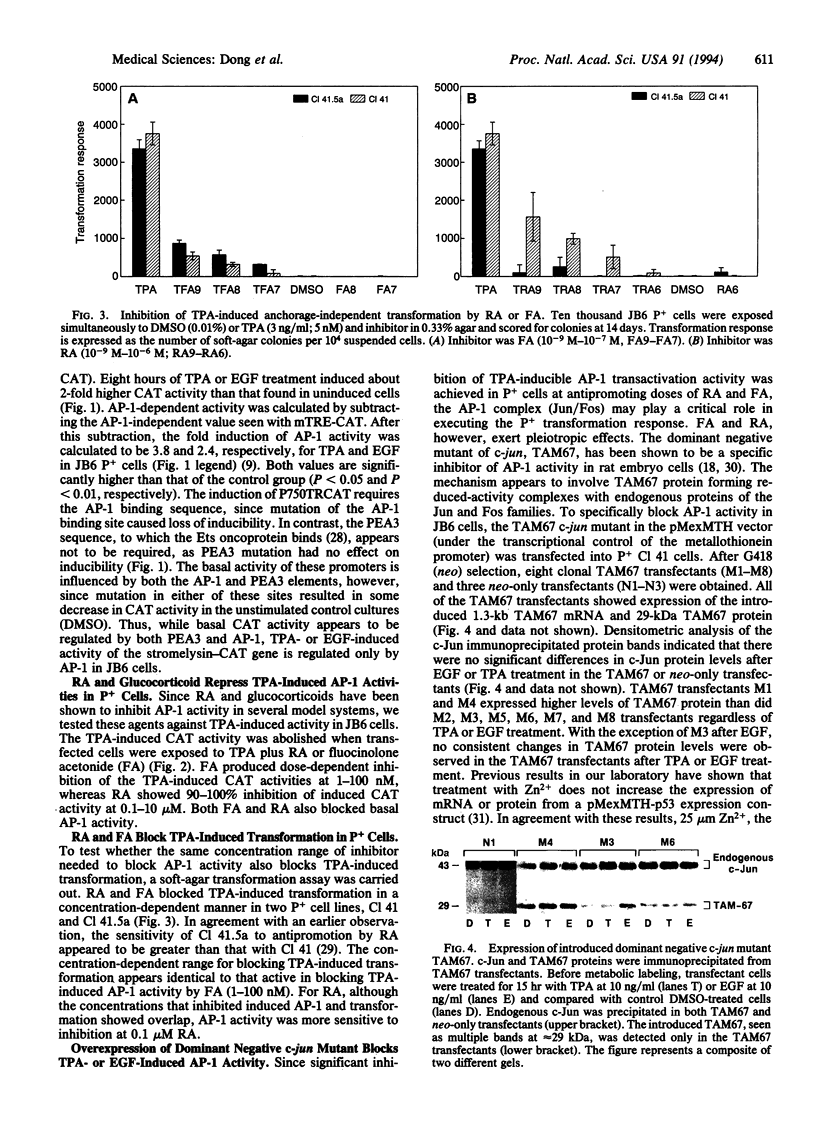
AP-1 transcriptional activity is stimulated by the transformation promoters phorbol 12-myristate 13-acetate ("12-O-tetradecanoylphorbol 13-acetate," TPA) and epidermal growth factor (EGF) in promotion-sensitive (P+) but not in promotion-resistant (P-) JB6 mouse epidermal cell lines. Although TPA stimulates expression of the jun and fos family genes, only c-jun expression shows higher elevation in P+ cells than in P- cells. The present study tests the hypothesis that induced AP-1 activity is required for tumor promoter-induced transformation in JB6 P+ cells. Both retinoic acid and the glucocorticoid fluocinolone acetonide inhibited basal and TPA-induced AP-1 activities that were tested with a stromelysin promoter-chloramphenicol acetyltransferase reporter gene in P+ cells. Since both retinoic acid and fluocinolone acetonide are active in inhibiting TPA-induced anchorage-independent transformation of P+ cells in the dose range that blocks TPA-induced AP-1 activity, their antipromoting effects may occur through inhibition of AP-1 activity. To test the hypothesis with a more specific inhibitor, stable clonal transfectants of P+ cells expressing dominant negative c-jun mutant encoding a transcriptionally inactive product were analyzed. All transfectants showed a block in TPA and EGF induction of AP-1 activity. All transfectants also showed inhibition of TPA-induced transformation, and most transfectants showed a block in EGF-induced transformation. These results indicate that AP-1 activity is required for TPA- or EGF-induced transformation. This work demonstrates that a specific block in induced AP-1 activity inhibits tumor promoter-induced transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani R., Brown P., Binétruy B., Dosaka H., Rosenberg R. K., Angel P., Karin M., Birrer M. J. The transactivating domain of the c-Jun proto-oncoprotein is required for cotransformation of rat embryo cells. Mol Cell Biol. 1991 Dec;11(12):6286–6295. doi: 10.1128/mcb.11.12.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- BOUTWELL R. K. SOME BIOLOGICAL ASPECTS OF SKIN CARCINOGENISIS. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- Ben-Ari E. T., Bernstein L. R., Colburn N. H. Differential c-jun expression in response to tumor promoters in JB6 cells sensitive or resistant to neoplastic transformation. Mol Carcinog. 1992;5(1):62–74. doi: 10.1002/mc.2940050111. [DOI] [PubMed] [Google Scholar]
- Bernstein L. R., Ben-Ari E. T., Simek S. L., Colburn N. H. Gene regulation and genetic susceptibility to neoplastic transformation: AP-1 and p80 expression in JB6 cells. Environ Health Perspect. 1991 Jun;93:111–119. doi: 10.1289/ehp.9193111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L. R., Colburn N. H. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989 May 5;244(4904):566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Alani R., Preis L. H., Szabo E., Birrer M. J. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993 Apr;8(4):877–886. [PubMed] [Google Scholar]
- Carroll R., Martarano L., Derse D. Identification of lentivirus tat functional domains through generation of equine infectious anemia virus/human immunodeficiency virus type 1 tat gene chimeras. J Virol. 1991 Jul;65(7):3460–3467. doi: 10.1128/jvi.65.7.3460-3467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn N. H., Former B. F., Nelson K. A., Yuspa S. H. Tumour promoter induces anchorage independence irreversibly. Nature. 1979 Oct 18;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Dang C. V., McGuire M., Buckmire M., Lee W. M. Involvement of the 'leucine zipper' region in the oligomerization and transforming activity of human c-myc protein. Nature. 1989 Feb 16;337(6208):664–666. doi: 10.1038/337664a0. [DOI] [PubMed] [Google Scholar]
- De Benedetti F., Falk L., Ruscetti F. W., Colburn N. H., Faltynek C. R., Oppenheim J. J. Synergistic inhibition of phorbol ester-induced transformation of JB6 cells by transforming growth factor-beta and retinoic acid. Cancer Res. 1991 Feb 15;51(4):1158–1164. [PubMed] [Google Scholar]
- Dong Z. A., Jeffrey A. M. Mechanisms of organ specificity in chemical carcinogenesis. Cancer Invest. 1990;8(5):523–533. doi: 10.3109/07357909009012077. [DOI] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Honoki K., Tsutsumi M., Tsujiuchi T., Kondoh S., Shiraiwa K., Miyauchi Y., Mii Y., Tamai S., Konishi Y., Bowden G. T. Expression of the transin, c-fos, and c-jun genes in rat transplantable osteosarcomas and malignant fibrous histiocytomas. Mol Carcinog. 1992;6(2):122–128. doi: 10.1002/mc.2940060207. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., McDonnell S., Miller D. B., Navre M., Seftor E. A., Hendrix M. J. The role of the matrix metalloproteinase stromelysin in the progression of squamous cell carcinomas. Am J Med Sci. 1991 Sep;302(3):157–162. doi: 10.1097/00000441-199109000-00008. [DOI] [PubMed] [Google Scholar]
- McDonnell S. E., Kerr L. D., Matrisian L. M. Epidermal growth factor stimulation of stromelysin mRNA in rat fibroblasts requires induction of proto-oncogenes c-fos and c-jun and activation of protein kinase C. Mol Cell Biol. 1990 Aug;10(8):4284–4293. doi: 10.1128/mcb.10.8.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. N., Diamond M. I., Yamamoto K. R. Joints in the regulatory lattice: composite regulation by steroid receptor-AP1 complexes. Cell Growth Differ. 1991 Oct;2(10):525–530. [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Nakamura K., Wendel E., Colburn N. Progression toward tumor cell phenotype is enhanced by overexpression of a mutant p53 tumor-suppressor gene isolated from nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2827–2831. doi: 10.1073/pnas.90.7.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. jun: oncogene and transcription factor. Adv Cancer Res. 1990;55:1–35. doi: 10.1016/s0065-230x(08)60466-2. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Flores P., Gutman A., Wasylyk B. PEA3 is a nuclear target for transcription activation by non-nuclear oncogenes. EMBO J. 1989 Nov;8(11):3371–3378. doi: 10.1002/j.1460-2075.1989.tb08500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yang N., Schüle R., Mangelsdorf D. J., Evans R. M. Characterization of DNA binding and retinoic acid binding properties of retinoic acid receptor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3559–3563. doi: 10.1073/pnas.88.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]




