Abstract
Objective:
Although malnutrition (body mass index (BMI)<18.5kg/ m2) has been associated with impaired health status in patients with chronic obstructive pulmonary disease (COPD), the effects of body composition (body fat and protein percentage) in patients with COPD have not been clearly demonstrated.
Materials and Methods:
A total of 180 stable patients with COPD at the stages of moderate, severe, very severe, and 50 healthy subjects were included in this prospective study. All subjects underwent a clinical evaluation, spirometry tests, anthropometric measurements and blood analysis.
Results:
Frequency of underweight was higher in COPD (11.7%) patients than the control group (8%). The frequency of underweight increased as the severity of COPD worsens. There was body decomposition (protein or fat depletion) in not only all underweight patients but also some normal/overweight COPD patients, as well as in the healthy subjects. Deterioration in FEV1 (L), and FEV1/FVC was more evident in underweight patients with protein and fat depletion compared to normal/overweight patients (p=0.004, and p=0.005). Inspiratory and expiratory respiratory muscle power was lower in under-weight patients with depletion than in normal/overweight patients (p=0.02, and p=0.01). DLCO and DLCO/VA were significantly lower in underweight patients than in normal/overweight patients (p=0.003, and p=0.004), they were also lower in normal/overweight patients with depletion than in normal/overweight patients with no depletion (p=0.01, and p=0.07). Normal/overweight patients with protein depletion had the most frequent number of exacerbations than others (p=0.04).
Conclusion:
These results show that the body decomposition is important in patients with COPD. Assessment of body composition should be a part of nutritional assessment besides BMI in patients with COPD.
Keywords: Chronic obstructive pulmonary disease (COPD), malnutrition, anthropometry, measurement
Özet
Amaç:
Malnütrisyonun (beden kitle indeksi (BKİ)<18.5kg/m2) kronik obstrüktif akciğer hastalığı (KOAH) olanlarda sağlık durumunda bozulmaya yol açtığı bulunmasına rağmen, beden kompozisyonun (vücut yağ ve protein oranı) KOAH özelliklerine etkisi tam olarak ortaya konmamıştır.
Gereç ve Yöntem:
Toplam 180 orta, ağır ve çok ağır evredeki stabil KOAH hastası ve 50 sağlıklı hasta bu prospektif çalışmaya alındı. Tüm hastalara klinik değerlendirme, spirometrik testler, antropometrik değerlendirme ve kan analizleri yapıldı.
Bulgular:
Malnütrisyon prevalansı KOAH grubunda (11,7%, n:21) kontrol grubundan (%8, n:4) daha sıktı. Malnütrisyon sıklığı KOAH şiddeti kötüleştikçe arttı. Giderek azalan sırada olmak üzere kas deposu, viseral protein ve yağ deposundan oluşan beden kompozisyonunda değişiklik sadece tüm zayıf, bazı normal ve fazla kilolu KOAH hastalarında değil aynı zamanda sağlıklılarda da vardı. FEV1 (L) ve FEV1/FVC’de düşüklük zayıf ve protein ve yağ oranı düşük hastalarda normal ve fazla kilolu hastalara gore daha belirgindi (p=0,004 ve p=0,005). İnspiratuvar ve ekspiratuvar kas gücü, zayıf ve protein ve yağ oranı düşük hastalarda normal ve fazla kilolu hastalara göre daha belirgindi (p=0,02 ve p=0,01). Difüzyon kapasitesi (DLCO) ve DLCO/ alveolar hacim oranı sadece zayıf ve protein ve yağ oranı düşük hastalarda değil (p=0,003 ve p=0,004) aynı zamanda normal ve fazla kilolu hastalardan protein ve yağ oranı düşük olanlarda olmayanlara göre daha düşüktü (p=0,01 ve p=0,07). Normal ve fazla kilolu hastalardan protein ve yağ oranı düşük olanlar diğerlerine gore daha sık KOAH atağı geçiriyor bulundu (p=0,04).
Sonuç:
Bu sonuçlar beden kompoziyonu değişikliğinin KOAH’ta önemli olduğunu göstermektedir. KOAH’lı hastalarda BKİ yanında beden kompozisyonu incelemesi nütrisyonel değerlendirmenin bir parçası olmalıdır.
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as a persistent airflow limitation that is not fully reversible with some extra-pulmonary effects such as cachexia and skeletal muscle wasting [1]. Nutritional abnormalities including weight loss and skeletal muscle dysfunction are reported in chronic diseases as a cause or result. Between 19–60% of patients with COPD are classified as malnourished [2]. Frequent exacerbations, increased resting energy expenditure (REE), inactivity, poor diet, inflammation and hypoxia are suggested as the causes for the deterioration in nutritional status, and it may result in impaired exercise intolerance and poor health status in patients with COPD [1].
Malnutrition is frequently used to mean underweight. If malnutrition, defined by BMI, had been the only part of nutritional assessment in patients with COPD, all studies conducted on nutritional support would have successful outcomes in malnourished patients. Nevertheless, a recent meta-analysis of randomized trials found that nutritional support had no effect on anthropometric measures, lung function, or functional exercise capacity in patients with stable COPD [3]. Furthermore, two patients in the same weight may look completely different from each other because they have different body compositions. Body composition is used to describe body fat and protein percentages in human body, and can be measured by anthropometric measurements using caliper, which provides an indirect evaluation of nutritional status. Fat free mass, which represents skeletal muscle mass, has been reported to decrease in malnourished patients with COPD, and skeletal muscle weakness is associated with wasting of fat-free mass but not with airflow obstruction in patients with COPD [4]. Although several studies have examined the contribution of nutritional impairment in decreased lung functions and respiratory muscle strength in patients with COPD [2, 5, 6], the effects of body composition on pulmonary functions have not been clearly demonstrated.
The aim of this study was to evaluate the effect of malnutrition and body composition in with COPD patients in stable condition, and to assess the relationship between the nutritional indices and pulmonary parameters.
Materials and Methods
A prospective study was conducted on patients with stable COPD in a tertiary hospital. The local ethics committee approved the research. Oral COPD patients in stable condition aged between 40 and 75 years were included for a two-year term. COPD was diagnosed according to the guideline and associated with the presence of a forced expiratory volume in one second (FEV1) 80% below the reference value, a ratio of FEV1/forced vital capacity (FVC) less than 70%, and a history of smoking, passive smoking or exposure to biomass [1]. Severity of COPD was classified as moderate (50%≤FEV1 <80% predicted), severe (30%≤FEV1 <50% predicted), very severe (FEV1 <30% predicted) [1]. The patients, who were required to be in clinically stable stage of COPD, are defined not to experience an exacerbation 2 months prior to the study [1]. Patients with concomitant diseases that may affect nutritional status (heart failure, liver cirrhosis, uncontrolled diabetes, chronic renal failure, uncontrolled thyroid disease, neoplasm, and uncontrolled chronic cor pulmonale) were excluded. The control group was consisted of healthy subjects, who are caregivers of patients, with no known disease, and have normal spirometric values. Subjects with a history of recent infection, surgery, trauma, and systemic diseases that could affect nutritional status were excluded.
All subjects underwent clinical and nutritional assessment, blood analysis and pulmonary function tests in approximately 5 days. Demographic characteristics, smoking status and COPD history were recorded. Smoking index was defined as the number of cigarettes smoked per day multiplied by the number of smoking years. The presence of emphysema was detected on computed tomography. Modified British Medical Research Council (MRC) questionnaire was used to measure disability due to breathlessness [7]. Severity of exacerbations was classified as mild (exacerbation treated with antibiotics but no systemic corticosteroid), moderate - severe (exacerbation treated with parenteral corticosteroids, and or hypoxemia but no carbon dioxide retention or acidosis) [8].
Anthropometric parameters including body weight (kg), height (m), body mass index (BMI, kg/m2), mid-upper arm length (AMC, cm), triceps skin fold thickness (TSF, mm) were measured. The international World Health Organization (WHO) classification of adult weight according to BMI was as follows; underweight (BMI<18.50), normal range (18.50–24.99), overweight (BMI≥25) [9]. The AMC was measured using a non-stretchable tape at the midpoint of non-dominant arm, between the olecranon and acromion. The TSF was measured using an adipometer skin fold caliper with graduated 0–10 mm at the width of a fold of skin taken over the triceps muscle at the same midpoint of the upper arm. Mid arm muscle circumference (MAMC, cm), mid arm muscle area (MAMA, cm2), mid arm fat area (MAFA, cm2) were calculated from the formulas as mentioned before [2]. The cut off points for normality of these values were calculated with the percentiles for the population of 18–65 years, according to WHO tables [10]. ‘Muscle protein depletion’ was defined if one of the MAMA, AMC or MAMC was below the 25th percentile (≤P25). ‘Fat store depletion’ was defined if TSF or MAFA was less than the 25th percentile.
Blood for arterial gas analysis (PaO2, PaCO2) and for venous (protein stores) were obtained from all subjects. Values under the normal range were accepted as depletion of that analysis. ‘Visceral protein depletion’ was defined serum albumin or transferrin’s being under the normal range. ‘Protein depletion’ was accepted if there was either muscle or visceral protein depletion.
FEV1 and FVC were measured using the helium dilution technique (Vmax 229 pulmonary function, Sensor Medics, Bilthoven-Holland), and the FEV1/FVC ratio was calculated. The single-breath diffusing capacity test was used to determine the diffusing capacity of the lung for carbon monoxide (DLCO) and diffusing capacity divided by the alveolar volume (DLCO/VA) [11]. Respiratory muscle function was measured with maximal pressures, generated in the mouth after full inspiration (PImax) and full expiration (PEmax) by electronic pressure gauge, according to the method of Black and Hyatt [12].
Calculations were done using the SPSS-statistical computer software. Two group analyses were compared applying parametric (t-test) or nonparametric analyses (Mann-Whitney test), whereas three group comparisons were done applying parametric (one way ANOVA) or nonparametric analyses (Kruskal-Wallis) depending on the normality of the distribution of variables. Categorical variables (frequencies) were analysed using the χ2 test, whereas small sample sizes were analysed using the Fisher’s exact test. If the sample size of the categorical variable was smaller than 5, the statistical difference wasn’t calculated. The results were considered significant where is p<0.05 (two sided).
Results
The sample comprised 180 patients with COPD and 50 healthy controls aged between 40 and 90 years, and 84.3% of them were male. Almost all the study subjects were smokers (91.6%) (Min-max: 3–79 packet/years), and the average BMI of the study group was 23.3±3.9 kg/m2. Both groups had similar average ages, gender ratios, average smoking index, and BMIs. Pulmonary function parameters and PaO2 were significantly lower in the COPD group than the control.
The frequency of patients, who are underweight, was higher in the COPD group, and frequency of normal-weight patients was higher in the control group. The frequency of patients with underweight increased as the severity of COPD worsens (Table 1). Fat store depletion was observed only in patients with COPD with a ratio of 10.6%, and no difference between the COPD subgroups. MAFA was the most affected fat related parameter, whereas TSF showed least fat depletion. The frequency of overall muscle protein depletion and depletion in muscle protein indicators of MAMA, AMC, and MAMC were significantly higher in the COPD group than in the control. Muscle protein indicators were close to each other among the COPD severity groups. A total of 43 patients with COPD had low plasma concentrations of at least one of the visceral proteins - albumin or transferrin. There were no healthy subjects with visceral protein depletion.
Table 1.
The relationship between anthropometric assessment, blood analysis and COPD disease and severity in COPD patients compared to control subjects with principal cut off points
| Characteristics n (%) | COPD Very severe (n:40) | COPD Severe (n:95) | COPD Moderate (n:45) | p | COPD Total (n:180) | Control Group (n:50) | p |
|---|---|---|---|---|---|---|---|
| BMI classification; | |||||||
| Underweight | 6 (15) | 11 (11.6) | 4 (8.9) | 21 (11.7) | 4 (8) | ||
| Normal weight | 22 (55) | 56 (58.9) | 27 (60) | 105 (58.3) | 32 (64) | ||
| Overweight | 12 (30) | 28 (29.5) | 14 (31.1) | 54 (30) | 14 (28) | ||
| Fat store depletion | 4 (10) | 10 (10.5) | 5 (11.1) | 19 (10.6) | - | ||
| TSF: ≤P25 | 1 (2.5) | - | 1 (2.2) | 2 (1.1) | - | ||
| MAFA: ≤P25 | 4 (10) | 10 (10.5) | 5 (11.1) | 19 (10.6) | - | ||
| Muscle protein depletion | 26 (65) | 55 (57.9) | 28(62.2) | 0.71 | 109 (60.6) | 8 (16) | <0.001 |
| MAMA : ≤P25 | 17 (42.5) | 37 (38.9) | 19 (42.2) | 0.89 | 73 (40.6) | 6 (12) | <0.001 |
| AMC: ≤P25 | 21 (52.5) | 44 (46.3) | 24 (53.3) | 0.67 | 89 (49.4) | 8 (16) | <0.001 |
| MAMC: ≤P25 | 12 (30) | 28 (29.5) | 15 (33.3) | 0.89 | 55 (30.6) | 1 (2) | |
| Visceral protein depletion | 7 (15.6) | 26 (27.4) | 7 (15.6) | 0.30 | 43 (23.9) | - | |
| Albumin ≤3.5 g/dL | 9(22.5) | 22 (23.2) | 7 (15.6) | 0.57 | 38 (21.1) | - | |
| Transferrin ≤2g/L | 1(2.5) | 8 (8.4) | - | 9 (5) | - | ||
| Prealbumin <0.2 g/L | 21 (52.5) | 44 (46.3) | 18 (40) | 0.51 | 83 (46.1) | 1 (2) |
BMI: body mass index; AMC: mid-upper arm length; MAMC: mid arm muscle circumference; TSF: triceps skin fold; MAMA: mid arm muscle area; MAFA: mid arm fat area; ≤P25: under %25 percentile
The stratification of body composition by using protein and fat stores besides BMI groups was a significant difference between COPD and control groups (Figure 1). There was either a protein or a fat depletion in almost all under-weight patients with COPD-except 2 patients-, and controls, whereas mixed type -fat and protein-of depletion was absent in healthy subjects. Somewhat, there was also protein or fat depletion in normal and overweight subjects which was only 10% in the control group and in more than half of patients with COPD.
Figure 1.
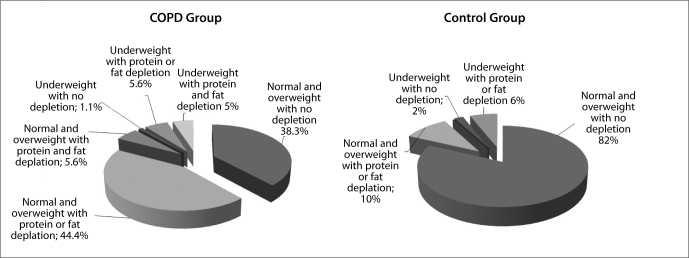
Stratification of body composition by using protein and fat stores between COPD and control groups.
Patients with COPD were stratified according to the body composition (Table 2). Thin patients with no depletion were excluded in order to be able to make statistics, since they were only two. Deterioration in pulmonary function tests was more evident in underweight patients with protein and fat depletion compared to normal/overweight patients with and without depletion, which was statistically significant in only FEV1 (L), and FEV1/FVC (p=0.004, and p=0.005). PImax and PEmax were lower in underweight patients with depletion than in normal-overweight patients with no depletion and with the depletion (p=0.02, and p=0.01).
Table 2.
The Relationship between body composition and pulmonary function tests, respiratory pressures, diffusing capacity and arterial blood gas analysis in COPD patients
| Normal/overweight without depletion (n: 69) | Normal/overweight with protein and/or fat depletion (n:90) | Underweight with protein and fat depletion (n:19) | p | |
|---|---|---|---|---|
| Pulmonary function tests: | ||||
| FEV1, L | 1.3±0.6 | 1.3±0.6 | 0.8±0.3 | 0.004 |
| FEV1, % | 40.6±14.1 | 41.5±16.6 | 37.2±15.9 | 0.55 |
| FVC, L | 2.9±6.5 | 2.1±0.7 | 1.8±0.6 | 0.37 |
| FVC, % | 58.7±15.9 | 58.2±16.8 | 52.4±15.1 | 0.31 |
| FEV1/FVC | 52.6±10.3 | 54.8±10.9 | 46.1±8.2 | 0.005 |
| Maximal respiratory pressures: | ||||
| PImax (cm H20) | 58.8±16.2 | 51.8±18.5 | 50.8±11.2 | 0.02 |
| PEmax (cm H20) | 74.5±27.7 | 67.9±26.7 | 53.7±12.9 | 0.01 |
| Diffusing capacity of the lung | ||||
| DLCO | 48.0±20.2 | 39.8±19.7 | 32.9±6.3 | 0.003 |
| DLCO/VA | 74.6±30.3 | 66.8±24.7 | 52.6±6.2 | 0.004 |
| Arterial blood gas analysis: | ||||
| PaO2 (mm Hg) | 62.7±12.8 | 61.7±15.3 | 56.5±14.2 | 0.25 |
| PaCO2 (mm Hg) | 38.9±40.6 | 40.6±8.9 | 44.5±14.4 | 0.13 |
| COPD characteristics: | ||||
| Emphysema,¥ | 56 (81.2) | 71 (78.9) | 13 (68.4) | 0.72 |
| MRC score | 2.04±1.20 | 2.12±1.21 | 2.37±1.25 | 0.30 |
| Number of exacerbations/last year | 1.81±1.02 | 2.37±1.25 | 1.31±0.88 | 0.04 |
| Severity of past exacerbations,¥: | 0.56 | |||
| Mild | 35 (50.7) | 49 (54.4) | 7 (36.8) | |
| Moderate and severe | 34 (49.3) | 41 (45.6) | 12 (63.2) |
COPD: chronic obstructive pulmonary disease; DLCO: diffusing capacity of the lung for carbon monoxide; DLCO/VA: diffusing capacity divided by the alveolar volume; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; PaO2: partial pressure of oxygen in arterial blood; PaCO2: partial pressure of carbon dioxide in arterial blood
DLCO and DLCO/VA were significantly lower in not only underweight patients than in normal/overweight patients (p=0.003, and p=0.004), but also in normal/overweight patients with depletion than in normal-overweight patients with no depletion (p=0.01, and p=0.07, not shown in the table). There was no significant difference for PaO2 and PaCO2 among the patients. There was no significant difference between groups in means of COPD characteristics. Normal-overweight patients and protein depletion had the most frequent number of exacerbations, whereas underweight patients had the most frequent ratio of severe exacerbations.
Discussion
The current results showed that in a group of Turkish COPD population, ratio of malnutrition was 11.7%. The weight loss value in stable COPD outpatients was smaller. National −20%-, and international reports as 19.1%, 29%, and 35% [2, 5, 6, 13]. A comparison of findings in previous studies with our findings was difficult because the criterion for malnutrition was different in each study. We used the definition of the WHO expert consultation, where BMI is lower than 18.5 kg/m2 that indicates underweight. However, the experts also agreed the needs for developing different BMI cut off points for different ethnic groups [9]. In this study, frequency of malnutrition was higher in the patients with COPD than in healthy ones. This result isn’t surprising anymore [14], and malnutrition has been accepted as one of the comorbidity of COPD in the recent years. Because, illness is a stress in the body, which ensues a catabolic phase, COPD is a chronic disease with a chronic catabolism and acute attacks by an ongoing infection or respiratory failure that all may result with malnutrition [15, 16]. Therefore, overall frequency of malnutrition increases as the disease severity increases, as in this study.
We used anthropometric measurements to assess protein and fat stores, corrected with the reference values, and opted to use the ratios of percentiles lower than 25% in an attempt to show the early stage of malnutrition. In this study, there was a protein or fat depletion in almost all under-weight patients with COPD and controls, whereas mixed type -fat and protein- of depletion was absent in healthy ones. Furthermore, the muscle store (60.6%) has been the compartment most frequently affected following visceral protein (23.9%) and fat store depletion (10.6%) in patients with COPD. This issue was mentioned also in other studies as malnutrition led muscle wasting rather than deficiencies in the other body stores, and body fat was reported to be affected, though to a lesser extent than muscle store in patients with COPD [2, 5, 6]. Following a stress, for the caloric requirement carbohydrate from liver and muscle glycogen are the first consumed stores in the body. If the stress is prolonged, metabolism of skeletal protein supplies substrate for energy production, following fat metabolization [15]. Similarly, the depletion ratios in this study confirmed the order of store consumptions in the body, and the presence of fat store depletion suggested a prolonged catabolic phase in COPD.
In this study, body decomposition was also seen in normal-weight or overweight patients with COPD, and even in some healthy subjects. A similar result was found previously, and they explained depletion in normal-weight by the extension of normal weight limits from 20 to 18.5 kg/m2. On the other hand, these results suggest that distribution disorders in the body composition occur before being underweight.
In this study, patients with COPD had significant depletions in visceral protein stores. These results are not surprising, since during the catabolic stage of chronic illness, serum concentrations of catecholamine, glucocorticoid, and glucagon are increased and insulin is decreased, all of which favor protein wasting, and lipolysis [15, 17].
Body weight loss has been reported to be associated with impairment of pulmonary functions [18], reduction of respiratory muscle strength [19], and diffusing capacity of the lung [20]. Our results were consistent with these previous reports, moreover body composition as evaluated by protein and/or fat depletion was found to be different in means of respiratory muscle strength and lung diffusing capacity, but not with pulmonary functions. PImax and PEmax were reduced in patients with protein-depleted composition. It has been known that fat free mass is reduced in malnourished patients leading to lower muscle strength and that muscle wasting can affect pulmonary functions [21]. The relative protection in pulmonary functions in protein-depleted patients might be explained by the order of the impairment in fat free mass and lung functions. In conclusion, underweight had negative effects on pulmonary function tests, expiratory muscle strength, and the ratio of DLCO/VA, whereas body decomposition with depletion in protein and/or fat stores reduced the inspiratory/expiratory muscle strength, and the DLCO. Both underweight and body decomposition decreased the PaO2, and increased the PaCO2. This result implicates that the respiratory muscle strength can also be reduced in patients without malnutrition but with disturbed body composition, and the impairment first develops on the respiratory muscle strength following pulmonary function tests. Therefore, it is essential to evaluate body composition as well as body weight in patients with COPD.
In the present study, DLCO and DLCO/VA were reduced in normal/overweight patients with depletion as well as in underweight patients. A series of articles have demonstrated that malnutrition causes reduction in DLCO and DCLO/VA, by causing gas-change unit enlargement and alveolar loss, which is defined as ’nutritional emphysema’ [22]. ’Nutritional emphysema’ in addition to classic emphysema in patients with COPD may be the reason for the higher reduction in pulmonary function tests of underweight patients. There were no significant differences for PaO2 and PaCO2 among the patients. The recruitment of patients with different distribution of emphysema, and COPD severity could have been a confounding factor in the interpretation of these results. There was no significant difference between groups in means of COPD characteristics. Normal/overweight patients with protein depletion had the frequent number of exacerbations, whereas underweight patients had the most frequent ratio of severe exacerbations.
Presence of emphysema and the MRC dyspnoea score didn’t change with body composition. The higher frequency of exacerbation rate in normal/overweight patients might be due to protein depletion, whereas malnutrition might be the reason of severe exacerbations which might be due to impaired immunologic defence and decreased respiratory muscle strength which altered ventilatory drive [23].
Even though anthropometric parameters are simple, cheap, and quick measures, they have some limitations [2]. Firstly, anthropometric measures are less sensitive than other measures such as bioelectric impedance and dual energy x-ray absorption. They overestimate the lean body mass when compared to bioelectric impedance [24], and secondly reproducibility of the anthropometric values from one observer to another is lower.
In conclusion, stable patients with COPD show frequent alterations in body composition besides malnutrition. Malnutrition and body decomposition were both related to impairment in respiratory muscle strength, and diffusing capacity of the lung. These results indicate that body composition should be a part of nutritional assessment besides BMI.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ankara University Faculty of Medicine.
Informed Consent: Informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.A., B.E.G., A.B.; Design - T.A., B.E.G., A.B.; Supervision - T.A., B.E.G.; Materials - T.A., B.E.G., A.B.; Data Collection and/or Processing - A.B.; Analysis and/or Interpretation - T.A., B.E.G., A.B.; Literature Review - T.A., B.E.G., A.B.; Writing - T.A., B.E.G., AB.; Critical Review - T.A., B.E.G., A.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Global initiative for chronic obstructive lung disease (GOLD) Global strategy for the diagnosis, management and prevention of chronic pulmonary disease. 2011.
- 2.Soler JJ, Sanches L, Román P, et al. Frequency of malnutrition in outpatients with stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2004;40:250–8. doi: 10.1016/s1579-2129(06)70095-7. http://dx.doi.org/10.1016/S0300-2896%2804%2975516-7. [DOI] [PubMed] [Google Scholar]
- 3.McDonald C. ACP Journal Club. review: nutritional supplementation has uncertain effects on patient-important outcomes in COPD. Ann Intern Med. 2013;21:158. doi: 10.7326/0003-4819-158-10-201305210-02005. JC5. [DOI] [PubMed] [Google Scholar]
- 4.Engelen MPKJ, Schols AMWJ, Does JD, et al. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–8. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 5.Schols AMWJ, Soeters PB, Dingemans AMC, et al. Frequency and characteristics ofnutritional depletion in patients with stable COPD eligible forpulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–6. doi: 10.1164/ajrccm/147.5.1151. http://dx.doi.org/10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 6.Sahebjami H, Doers JT, Render ML, et al. Anthropometric and pulmonary function test profiles of outpatients with stable chronic obstructive pulmonary disease. Am J Med. 1993;94:469–74. doi: 10.1016/0002-9343(93)90080-9. http://dx.doi.org/10.1016/0002-9343%2893%2990080-9. [DOI] [PubMed] [Google Scholar]
- 7.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. http://dx.doi.org/10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;21:46–53. doi: 10.1183/09031936.03.00078002. http://dx.doi.org/10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 9.WHO. BMI classification. Global Database on Body Mass Index. 2006.
- 10.WHO. Multicentre growth reference study group WHO Child Growth Standards: Head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfoldfor-age: Methods and development. Geneva: World Health Organization; 2007. pp. 57–154. [Google Scholar]
- 11.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. http://dx.doi.org/10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 12.Black LE, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 13.Coskun O, Ugurman F, Akkalyoncu B, et al. The effect of nutriton on the pulmonary functions in COPD. Solunum Hastalıkları. 2005;16:153–60. [Google Scholar]
- 14.Deveci F, Tuğ T, Turgut T, et al. Nutritional status, pulmonary functions, and exercise performance in COPD cases. Tüberküloz ve Toraks Dergisi. 2005;53:330–9. [PubMed] [Google Scholar]
- 15.Murray MJ, Coursin DB, Pearl RG, et al. Critical Care Medicine: Perioperative management. 2nd edition. Lippincott: Williams&Wilkins; 2002. pp. 176–85. [Google Scholar]
- 16.Telluoglu E, Bicmen C, Meral AR, et al. Association of biochemical nutritional parameters and the severity of the disease in patients with COPD. Izmir G H Dergisi. 2012;3:157–63. [Google Scholar]
- 17.Gocmen H, Ediger D, Uzaslan E, et al. The relationships of serum prealbumin levels wth parameters that indicate severity of disease and emphysema pattern in patients with stable chronic obstructive pulmonary disease. EAJM. 2010;42:105–10. doi: 10.5152/eajm.2010.31. http://dx.doi.org/10.5152/eajm.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Openbrier DR, Irwin MM, Rogers RM, et al. Nutritional status and lung function in patients with emphysema and chronic bronchitis. Chest. 1983;83:17–22. doi: 10.1378/chest.83.1.17. http://dx.doi.org/10.1378/chest.83.1.17. [DOI] [PubMed] [Google Scholar]
- 19.Braun SR, Keim NL, Dixon RM, et al. The prevalence and determinants of nutritional changes in chronic obstructive pulmonary disease. Chest. 1984;86:558–63. doi: 10.1378/chest.86.4.558. http://dx.doi.org/10.1378/chest.86.4.558. [DOI] [PubMed] [Google Scholar]
- 20.Salepçi B, Eren A, Çağlayan B, et al. The effect of body mass index on functional parameters and quality of life in COPD patients. Tüberküloz ve Toraks Dergisi. 2007;55:342–9. [PubMed] [Google Scholar]
- 21.Akkoca O, Demir G, Saryal S, et al. The effect of hyperinflation on respiratory muscles and breathing pattern in COPD. Tüberküloz ve Toraks Dergisi. 2003;51:244–52. [PubMed] [Google Scholar]
- 22.Massaro D, De Carlo Massaro G. Hunger disease and pulmonary alveoli. Am J Respir Crit Care Med. 2004;170:723–4. doi: 10.1164/rccm.2408002. http://dx.doi.org/10.1164/rccm.2408002. [DOI] [PubMed] [Google Scholar]
- 23.Pingleton SK. Nutrition in chronic critical illness. Clin Chest Med. 2001;22:149–63. doi: 10.1016/s0272-5231(05)70031-9. http://dx.doi.org/10.1016/S0272-5231%2805%2970031-9. [DOI] [PubMed] [Google Scholar]
- 24.Schols AMWJ, Wouters EFM, Soeters PB, et al. Body composition by bioelectrical impedance analysis compared to deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;53:421–4. doi: 10.1093/ajcn/53.2.421. [DOI] [PubMed] [Google Scholar]
- 25.Gocmen H, Ediger D, Uzaslan E, et al. The relationships of serum prealbumin levels wth parameters that indicate severity of disease and emphysema pattern in patients with stable chronic obstructive pulmonary disease. EAJM. 2010;42:105–10. doi: 10.5152/eajm.2010.31. http://dx.doi.org/10.5152/eajm.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]


