Abstract
Daily dietary and inhalation exposures to 16 parent polycyclic aromatic hydrocarbons (PAHs) and urinary excretion of 13 monohydroxy metabolites (OHPAHs) were monitored for 12 non-smoking university students in Beijing, China, during a controlled feeding experiment. The relationship between the urinary excretion of OHPAHs and the uptake of PAHs was investigated. The results suggest severe exposure of the subjects to PAHs via both dietary and inhalation pathways. Large increase of most urinary OHPAHs occurred after the ingestion of lamb kabob. Higher concentrations of OHPAHs were observed for female subjects, with the intakes of parent PAHs lower than those by males, likely due to the gender differences in metabolism. It appears that besides 1-PYR, metabolites of PHE could also be used as biomarkers to indicate the short-term dietary exposure to PAHs and urinary 3-BaA may serve as the biomarker for inhalation intake of high molecular weight PAHs.
Keywords: Polycyclic aromatic hydrocarbons, dietary exposure, urinary excretion, biomarkers
1. Introduction
It is well recognized that exposure to polycyclic aromatic hydrocarbons (PAHs) can cause adverse health effects including cancer (Boström et al., 2002). Relying heavily on coal and biomass fuels for energy, emission of PAHs in China accounts for one fifth of the global total (Shen et al., 2013), leading to severe contamination of environment (Liu et al., 2007a; Tao et al., 2004) and food (Xia et al., 2010). Human expose to PAHs via dietary, inhalation, and dermal contact (Bostrom et al., 2002), among which, dermal contact can be minimal for non-occupational population (Li, 2007). Due to PAHs’ low solubility, exposure through drinking water does not contribute much either. Many evidences suggest that food ingestion dominates overall PAH intake among nonsmokers who are not subject to occupational exposure (Li, 2007; Suzuki and Yoshinaga, 2007; Viau et al., 2002).
PAHs in uncooked food originate from contaminated soils, water, air, or animal feed (Phillips, 1999; Zhao et al., 2012). In general, PAH contents in meat are higher than those in cereals and vegetables (Xia et al., 2010). Relatively high PAH concentrations are often found in processed food, especially grilled and smoked meat (Alomirah et al., 2011). For example, it was reported that the total concentration of 16 parent PAHs (pPAH16) in grilled duck breast was as high as 319 ng/g, compared with 8.5 ng/g in the steamed dishes (Chen and Lin, 1997). The enriched PAHs during the process of grilling or smoking are likely to arise from the pyrolysis of fat at high temperature and adsorption of the PAHs emitted from combusted fuels (Lijinsky, 1991). It has been demonstrated that ingestion of these heavily PAH-polluted food can result in increased health risks like colorectal adenomas and postmenopausal breast cancer (Sinha et al., 1999; Steck et al., 2007).
Urinary OHPAHs have long been regarded as the biomarkers to assess internal exposure to PAHs (Jongeneelen et al., 1985). Among a variety of OHPAHs, 1-hydroxypyrene (1-PYR) is the most preferable tracer. Enhanced levels of 1-PYR have been reported in urine samples from workers occupationally exposed to PAHs, smokers, or others who have consumed PAH-polluted food and they were often found to be significantly correlated with PAH exposure (Li et al., 2012; Merlo et al., 1998; Sobus et al., 2009). However, discrepancies among different authors were sometimes reported on the relationship between PAH intake and urinary excretion, especially for population in general environmental settings (Merlo et al., 1998; Suzuki and Yoshinaga, 2007; Viau et al., 2002). The correlation between PAH intake and urinary excretion might be confounded by the inaccurate estimation of PAH exposure doses, neglection of other possible exposure routes, or inter individual variations of urinary OHPAHs (Viau et al., 2002; Scherer et al., 2000).
In this controlled study, PAHs in indoor and ambient air and daily dishes, to which a group of 12 university students exposed, were monitored during an 11-day experiment. The activity and food consumption of the subjects were strictly controlled and the relationship between parent PAH exposure and urinary OHPAH was addressed using the mean value of male and female subjects to eliminate inter-individual variability. Meanwhile, urinary excretion of OHPAHs was characterized and relative contributions of ingestion and inhalation pathways, differences in exposures and excretions between genders, excretion kinetics, and potential exposure biomarkers are discussed.
2. Methodology
2.1. Experimental design
Six male and six female university students, all non-smokers, participated in this study, and oral informed consents were obtained individually. All the students lived on campus of Peking University and commuted on foot or by bicycle between dormitories and their office/laboratory building once or twice a day (<15 minutes per trip). None of the individual subjects left the campus during the study period. Demographic profiles including age, gender, height, and weight are listed in Table S1. The study was conducted from Dec. 21 to Dec. 31, 2010. A number of popular dishes were selected and all 12 subjects had exactly the same menu including three meals and a night snack at fixed hours and 2.5 L of bottle water every day (Table S2). Lamb kabob was purchased from a street vendor near the campus, while all other food was bought from the university cafeterias where the majority of students eat their meals. Daily activities of the students were self-recorded and summarized in Table S3.
2.2. Sample collection
An aliquot of each dish selected was mixed and stored at −20°C prior to analysis. Daily (24 h) air samples were collected at four sites (laboratory, student’s offices, student’s dormitory, and outdoors) using active samplers (1.5 L/min, XQC-15E, Tianyue, China) for particulate (glass fiber filters, GFFs, 30 mm diameter) and gaseous (polyurethane foam plugs, PUFs, 22 mm diameter×76 mm, 0.024 g/m3) phase PAHs, respectively. The GFFs were baked at 450°C for 6 h and equilibrated in a desiccator (25°C) for 24 h prior to weighing and sampling. PUFs were purified by Soxhlet extraction using acetone, dichloromethane, and n-hexane successively for 8 h each. Three urine samples (100 mL each) were collected from each subject every other day (Dec. 22, 24, 26, 28, and 30) immediately before, eight hours after, and 24 hours after the lunch.
2.3. Sample extraction and analysis
The extraction and cleanup procedure of food and air samples can be found elsewhere in detail (Xia et al., 2010; 2013). Briefly, food samples were extracted with 20 mL of acetonitrile using a microwave- accelerated reaction system (CEM Mars Xpress, USA) and further extracted with n-hexane. PUFs were Soxhlet extracted with 150 mL of n-hexane/acetone mixture (1:1, v/v) for eight hours and GFFs were extracted with 25 mL of the same solvent mixture using the microwave-accelerated reaction system. The extracts were concentrated and transferred to silica/alumina chromatography columns for purification. Internal standards (NAP-d8, ACE-d10, ANT-d10, CHR-d12, and Perylene-d12, J&K Chemical, USA) were spiked before analysis.
PAHs were analyzed by a gas chromatograph using a DB-5MS capillary column, equipped with a mass spectrometer (Agilent 6890/5973, USA). The oven temperature was programmed at 80 °C for 1 min, increased to 270 °C at a rate of 5 °C/min, held for 2 min, to 290 °C at 3 °C/min, held for 1 min, then to 305 °C at 10 °C/min and held for 12 min. Helium was used as the carrier gas. Target PAHs were identified based on the retention time and qualitative ions of standards in selected ion monitoring mode and were quantified by the internal standards. Monitored PAHs included naphthalene (NAP), acenaphthene (ACE), acenaphthylene (ACY), fluorene (FLO), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CHR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), diben(a,h)anthracene (DahA), indeno(1,2,3-cd)pyrene (IcdP), and benzo(g,h,i)perylene (BghiP).
Urinary OHPAH was extracted based on the method of Fan et al (2006) with slight changes accordingly. An aliquot of 20 mL of urine sample was thawed and enzymolyzed with 40 μl of β-glucuronidase/sulfatase (G0876, Sigma-Aldrich, USA) in a mixture of 2 mL of 0.1 mol/L hydrochloric acid, and 5 mL of 0.5 mol/L sodium acetate and acetic acid buffer at 37°C for 16 h and centrifuged at 2000 rpm for 10 min. The supernatant was extracted and concentrated by solid-phase extraction using ENVITM-18 column (Supelclean, USA), which was previously activated by 5 mL of methanol (GR, Beijing Tongguang, China) and 10 mL of ultrapure water successively. The column was washed with 10 mL of ultrapure water and 10 mL of 30% methanol (v/v) in turn and the analyte was eluted with methanol (8 mL) and concentrated to 200 μL by a gentle stream of nitrogen. Another aliquot of 20 mL of urine sample was centrifuged at 2000 rpm for 10 min and the supernatant was diluted 10 times by the mobile phase solution (95% 0.02 mol/L sodium dihydrogen phosphate/5% methanol, v/v) for the measurement of urinary creatinine, according to the method of Tsikas et al (2004).
The concentrations of OHPAHs were determined by a high performance liquid chromatography equipped with XDB-C18 capillary column (4.6 × 250 mm, 5 μm) and a fluorescence detector (Agilent 1100/1200, USA). The injection volume was 20 μL. OHPAHs were separated by gradient elution (methanol/water, v/v) at a flow rate of one mL/min with 50, 90, and 50% methanol at 0–30, 30–35, and 35–45 min, respectively. The excitation wavelengths were 227, 272, 256, 240, and 275 nm and the emission wavelengths were 355, 336, 370, 387, and 430 nm for 0–13, 13–18, 18–22, 22–25, and 25–32 min, respectively. 1-hydroxynaphthalene (1-NAP), 2-hydroxynaphthalene (2-NAP), 2-hydroxyfluorene (2-FLO), 2-hydroxyphenanthrene (2-PHE), 3-hydroxyphenanthrene (3-PHE), 4-hydroxyphenanthrene (4-PHE), 9-hydroxyphenanthrene (9-PHE), 1-PYR, 3-hydroxybenzo(a)anthracene (3-BaA), 3-hydroxychrysene (3-CHR), 6-hydroxychrysene (6-CHR), 3-hydroxybenzo(a)pyrene (3-BaP), 9-hydroxybenzo(a)pyrene (9-BaP) were quantified. Urinary creatinine was detected by a variable wavelength detector (Agilent 1100, USA) at 235 nm with a retention time of 10 min.
2.4. Quality control
Reagent and procedure blanks were measured together with each batch of samples, and subtracted from the results. At least two replicates were measured for each sample. The detection limits of PAHs ranged from 0.23 to 1.42 ng/mL and 0.53 to 1.32 ng/mL for the gaseous and particulate phase air samples, respectively, and from 0.053 to 0.25 ng/g for the food samples. Method recoveries determined by spiking the sampling matrix with PAH standards (PPH-10JM, Chem Service, USA) ranged from 66 to 143% and 87 to 154% for gaseous (five duplicates) and particulate (four duplicates) phase PAHs, respectively. 2-Fluoro-1,1′-biphenyl and p-terphenyl-d14 (2.0 μg/mL, J&K Chemical, USA) were spiked before extraction as surrogates, the recoveries of which were 64–92% for food samples, 54–86% for gaseous phase PAHs, and 65–110% for particulate phase PAHs, respectively. The detection limits of OHPAHs in the urine samples ranged from 0.00165 to 0.55 μg/L. Recoveries of OHPAHs in urine samples spiked with standards were 88–123%.
2.5. Data analysis
The dietary intakes were calculated based on the measured PAH concentrations in food and the corresponding food consumptions. The daily inhalation exposures to PAHs were estimated according to the measured air concentrations, the daily activity pattern, and the respiration rate of 243.2 L/d per unit body weight (Zhang et al., 2009). The total daily intakes of individual subjects were the sums of dietary and inhalation exposures and reported as ng/kg·d body weight. Creatinine-corrected concentrations were used for urinary OHPAHs and expressed as μmol/mol creatinine. SPSS 13.0 (SPSS Inc., USA) was used for statistical analysis at a significance level of 0.05.
3. Results and discussion
3.1. PAHs in food and air
The measured pPAH16 in the food varied from 11±5.0 ng/g in banana to 350±84 ng/g in lamb kabob, which was generally within the same ranges of similar food reported in other places (see details in Table S4). For example, the concentrations of PYR in all dishes except lamb kabob (0.051 to 8.0 ng/g) were comparable to those in the daily diets of Canadians (Viau et al., 2002). The measured BaP concentrations in lamb kabob and fried steak were similar to those measured in barbecued meats (Alomirah et al., 2011; Chen and Lin, 1997). The pPAH16 in lamb kabob was also in the same range of those in meat kebab in Kuwait (Alomirah et al., 2011). Food contamination in China is regulated by National Food Safety Standard and the maximum level of BaP allowed is 5 ng/g for grain and smoked and grilled meat (PRC, 2012a), which is identical to European Commission standard (EC, 2006). Among all the food tested, BaP was only detected in lamb kabob (1.1±1.6 ng/g) and fried steak (0.9±1.2 ng/g), both of which were lower than the national standard.
The tested food can be classified into two categories in terms of composition profile (panels A and B of Fig. 1). For most food, the profiles were dominated by low to median molecular weight compounds from NAP to PYR, with the most abundant compound of PHE. Very small fractions of high molecular weight PAHs were detected. The profiles of PAHs in lamb kabob, steamed buns, Chinese cabbage and tofou, and corn with pine nuts were shown in Fig. 1A. Another category was braised pork chops and fried beef (Fig. 1B), for which NAP was the predominant compound and the percentages of high molecular weight compounds from BaA to BghiP were much higher than those in the first category. Although PAH concentration of the lamb kabob was approximately one order of magnitude higher than those of other food within the first category, the composition profiles were similar.
Fig. 1.
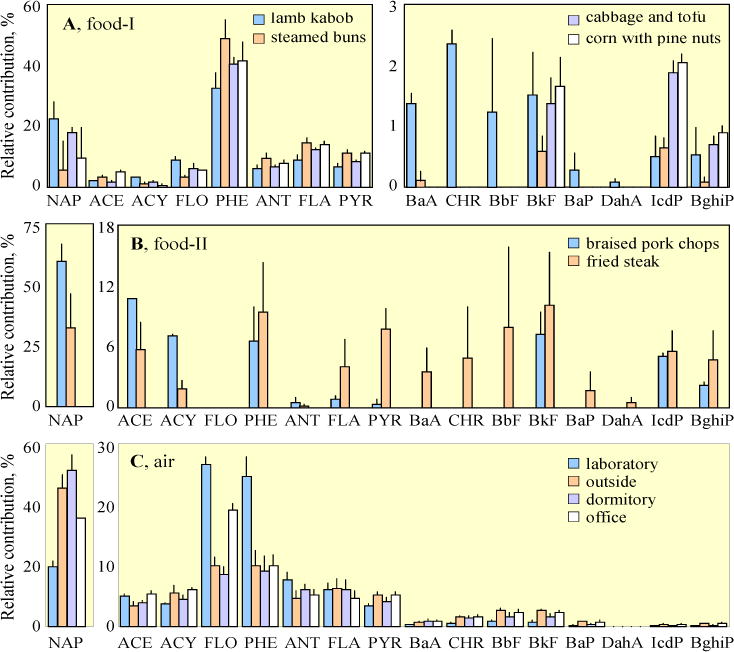
Composition profiles of the 16 PAHs in various food (A and B) and air (C) samples. The food can be classified into two categories (A and B) based on PAH profiles. Means and standard errors are shown.
Table S5 and Fig. S1 show the measured pPAH16 in the air. The daily means and standard deviations were 2800±1000, 1100±1300, 2000±1200, and 1700±850 ng/m3 at the laboratory, outdoor, dormitory, and office sites, respectively. The highest concentrations were found in laboratory where various experiments dealing with PAHs were conducted. More than 90% of PAHs in air were low and median molecular weight ones from NAP to FLA (panel C of Fig. 1). In general, the measured concentrations, with high daily variation, were of the same order of magnitude as those reported previously for ambient air in Beijing in 2005 winter (Liu et al., 2007b). The concentrations of BaP in majority of samples exceeded the national indoor (1.0 ng/m3) or ambient (2.5 ng/m3) air quality standards of China (PRC, 2002; PRC, 2012b).
3.2. Dietary and inhalation intake
The mean daily intakes of pPAH16 and BaPeq (BaP equivalent quantity, calculated based on the exposures to individual PAH compounds and toxic equivalent factors (TEFs) (Nisbet and LaGoy, 1992) for all the participants via dietary and inhalation pathways are shown in Fig. 2 (see the exposure to individual PAHs in Table S6). For pPAH16, the daily intakes from Dec. 21 through 30 varied from 1.3 to 1.8 μg/kg·d with an exception of 2.7 μg/kg·d on Dec. 28, when lamb kabob was served and the daily ingestion intake (2.3±0.34 μg/kg·d) was more than doubled of that on the other days (0.98±0.045 μg/kg·d). The large variation of inhaled pPAH16 did not contribute much to the total variation, since food ingestion accounted for 68% of the total exposure on average (Fig. 2A). The contribution was, however, lower than those reported in the literature (88–99%) (Suzuiki and Yoshinaga, 2007; Vanrooij et al, 1994; Viau et al., 2002), likely due to the severe air pollution in Beijing.
Fig. 2.
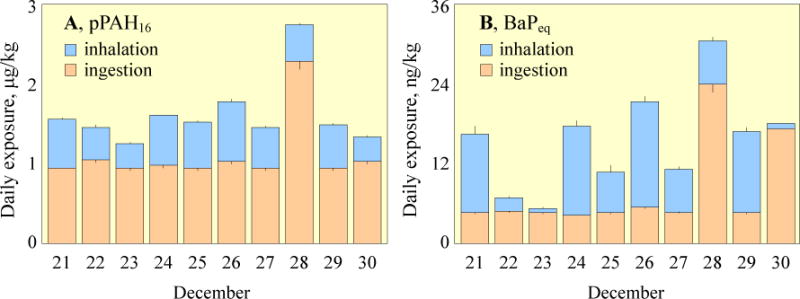
Daily exposures of the participants to pPAH16 (A) and BaPeq (B) through ingestion (orange) and inhalation (blue). The results are presented as means and standard errors of all 12 subjects.
Daily dietary intakes of BaPeq were relatively constant (4.7±0.37 ng/kg·d) except those on Dec. 28 and 30 when lamb kabob and fried beef were served. The measured concentrations of high molecular weight PAHs in fried beef and lamb kabob were similar, in spite of the significant difference in pPAH16 between them (Table S3). In comparison, daily inhalation exposures to BaPeq varied more than one order of magnitude from 0.70 to 16 ng/kg·d, accounting for 3.9 to 75% of the total, which resulted in the large variations of total BaPeq intake. The temporal trend of the inhalation exposure depended largely on daily variation of air quality and time-activity pattern of the subjects. As such, the inhalation exposures to pPAH16 and BaPeq were significantly correlated to the air concentrations in the student dormitory (p < 0.05) where they spent most of their time. The estimated inhalation intake of pPAH16 (0.51±0.14 μg/kg·d) was higher than those measured in summer (0.28 μg/kg·d) but lower than those measured in winter (1.1 μg/kg·d) for Beijing traffic police officers (Liu et al., 2007a; 2007b).
The daily dietary intake of BaPeq was estimated to be 8.5 ng/kg·d for adults in Taiyuan, China in another study conducted in 2008, which was much higher than the result in this study except those on Dec. 28 and 30 (Xia et al., 2010). Such differences are expected since Taiyuan, a coke and iron-steel industry center, is among the most severely PAH contaminated cities in China (Xia et al., 2010; 2013). It is reasonable to find much lower dietary exposure to pPAH16 (0.096 ng/kg·d) and BaPeq (2.3 ng/kg·d) reported for male adults in Catalonia, Spain in 2008 compared to the current study (Martorell et al., 2010), as the emission level of PAHs in Spain was much lower than that in China (Shen et al., 2013).
3.3. Urinary excretion of OHPAHs
Fig. 3A shows the urinary OHPAHs as means and standard errors for male and female participants over the experimental period. Large differences, spanning six orders of magnitude, were observed among different OHPAHs. 1-NAP was the highest in all samples (67±85 μmol/mol creatinine), while concentrations of 3-BaP (0.00010±0.0011 μmol/mol creatinine) was extremely low. The monohydroxy metabolites of NAP, FLO, PHE, PYR, and BaA were detected in 93–98% of the samples, with the exception of 1-NAP (80%). In general, the concentrations of low molecular weight metabolites were higher than those of high molecular weight ones, except that relatively high levels of 6-CHR were observed. In a survey conducted in 2001–2002, aiming at establishing the reference range concentrations for the U.S. population, it was also found that the urinary OHPAH concentrations were negatively correlated with the molecular weight (Li et al., 2008). This pattern is different from the intake composition profile of the corresponding parent PAHs, the differences among which were much smaller (Fig. 3B). One reason is that not all monohydroxy metabolites were measured due to unavailability of standards. Another possible explanation is the difference in solubility and bioavailability among the compounds, leading to different absorption rates and metabolic pathways. The low molecular weight metabolites of 2–3 ring PAHs tend to be excreted in the urine, while more higher molecular weight ones are excreted in feces (Ramesh et al., 2004). Based on the results of several animal tests, it was found that the fractions of the metabolites of PHE and PYR excreted through urine were much higher than those of BaP, which was poorly absorbed and mainly excreted as 3-BaP in feces (Grova et al., 2002; Marie et al., 2010; Viau et al., 1999). Regardless of the large differences, metabolites of NAP, FLO, PHE, PYR, and BaA were mostly significantly correlated to one another (p < 0.05, except 1-NAP with 3-BaA and 4-PHE with 3-BaA).
Fig. 3.
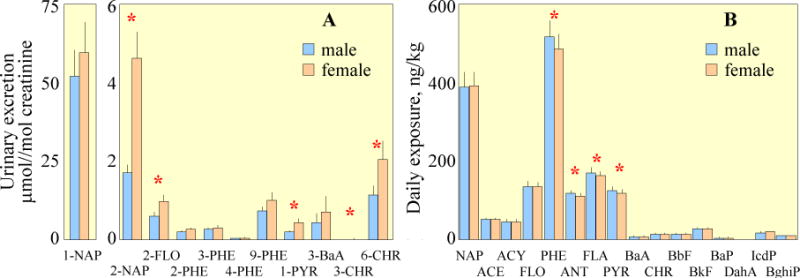
Concentrations of individual OHPAHs in urine samples (A) and daily intakes of parent PAHs (B). The results are shown as means and standard errors of male and female participants and those with statistical significance (p < 0.05) between them are marked with “*” for individual compounds.
The experiment was designed in a way that dishes with different PAH concentrations were only served at lunch every another day (Table S2). In this case, the measured OHPAHs in the urine samples collected right before and 8 h after the lunches are regarded as the background and the post-exposure levels, respectively (Table S7), for the convenience of comparison with other literature (Chien and Yeh, 2010; Li et al., 2012). The mean background concentration of 1-PYR in this study was 0.23±0.15 μmol/mol creatinine, which is lower than 0.87 μmol/mol creatinine for nonsmokers selected from five districts of Beijing (Zhao et al., 1992), but higher than most data reported in other countries (Suzuki and Yoshinaga, 2007; Roggi et al., 1997). For example, the geometric mean of the background urinary 1-PYR (0.18 μmol/mol creatinine) was one order of magnitude higher than that reported for Americans older than 20 (Li et al., 2008). Moreover, the mean post-lunch level of 1-PYR was 0.52±0.81 μmol/mol creatinine, which is even comparable to those for workers with occupational PAHs exposure or smokers without occupational intake in Italy (Merlo et al., 1998; Roggi et al., 1997). Similar results were observed for several other metabolites, including 1-NAP, 2-FLO, 2-PHE, 3-PHE, 9-PHE, 3-BaA, and 3-BaP (Forster et al., 2008; Grainger et al., 2006; Gundel et al., 2000; Lafontaine et al., 2006; Li et al., 2008; Preuss et al., 2005). It is surprised to see that the concentration of 9-PHE was the highest among all the PHE metabolites detected in this study. Since 1-PHE, which was not included in this study, is often reported as one of the most abundant metabolites of PHE (Gundel et al., 2000; Li et al., 2008), and 1-PHE and 9-PHE can be co-eluted in HPLC method (Fan et al., 2012; Kuusimaki et al., 2004). It is possible that the 9-PHE detected in our study was the combination of 1-PHE and 9-PHE, as they could probably be co-eluted. It is also surprised to see that the concentrations of 6-CHR were notably higher than those reported in the literature, which can not be explained at this stage.
The measured urinary OHPAHs among the 12 subjects varied widely. The average CV of all OHPAHs except BaP metabolites (very low detection rates) was as high as 96%, and the inter-individual variation (CV) was 34–74% for 1-PYR, which is similar to those reported in the literature (Chien and Yeh, 2010; Viau et al., 2002). It is believed that the large variation in urinary excretion of OHPAHs among individuals is due to a number of factors including smoking, diet, environmental tobacco smoke, urban traffic, air quality, bioavailability of PAHs, genetic polymorphism in metabolic enzymes, and the efficiency of enterohepatic cycling (Alexandrie et al., 2000; Coco et al., 2007; Vanrooij and Veeger, 1994; Viau et al., 2002). In this controlled experiment, the influences of smoking and traffic can be excluded. Since the variation in the daily intake among the individuals (CV=20%) was relatively small, the large variation in OHPAHs excretion was likely due to difference in metabolism among subjects.
In terms of gender difference, the average concentrations of individual OHPAHs from the six female subjects were all higher than those from the six male ones (Fig. 3A), although only several OHPAH compounds were significant (those with “*” in the figure). In comparison, the gender difference in parent PAH intake was opposite with higher intakes of most PAHs by male subjects (PHE, ACY, FLA, and PYR are significant at 0.05 level) (Fig. 3B). It is likely that gender-related differences in the metabolism of PAHs do exist, since the probability of such a difference would be less than 0.006% statistically, if there was no such difference. Enhanced susceptibility of women to carcinogenic PAHs has been demonstrated before (Perera, 1997). Uppstad et al (2011) reported that significantly higher levels BaP-DNA adducts and CYP1A1 gene expression were observed in lung adenocarcinoma cell lines from females compared to those from males after exposure to BaP (p < 0.05). Significantly higher levels of 1-NAP, 2-NAP, and 1-PHE for female US population (Li et al., 2008) and significantly higher 1-PYR for female traffic police officers in Italy (Merlo et al., 1998) have also been reported. However, the gender difference was not always observed (Cocco et al., 2007; Roggi et al., 1997), likely due to large individual variations caused by many factors mentioned above. Additional studies, excluding or strictly controlling interference factors, are needed to demonstrate the gender difference in the urinary excretion of OHPAHs.
3.4. Relationship between the parent PAH intake and OHPAH excretion
Fig. 4A shows the temporal variations of urinary OHPHEs and 1-PYR as means and standard errors for all participants over the study period. The concentrations of urinary metabolites of NAP, FLO, PHE, and PYR, peaked approximately eight hours after the ingestion of lamb kabob, which was similar to the variation pattern of daily dietary PAH intake (Fig. 2A). The post-exposure levels of 1-PYR via ingestion of lamb kabob were three times higher than those observed in a similar feeding experiment of barbecued meats (Chien and Yeh, 2010). It is because that the intake of PYR was 5200 ng on average by eating lamb kabob alone, more than twice of that by the barbecued meats in Chien and Yeh’s study. Increases in the urinary excretion of 3-BaA and 3-CHR were also observed after the ingestion of lamb kabob with an obvious lag compared with the metabolites originated from lower molecular weight compounds. For example, the maximum value of 3-CHR was not reached till 48 h after the ingestion (Fig. S2). Similar results were also reported for the excretion of 3-BaP for workers exposed to PAHs, the lag time for the maximum level between 3-BaP and 1-PYR was 10–17 h (Gendre et al., 2002).
Fig. 4.
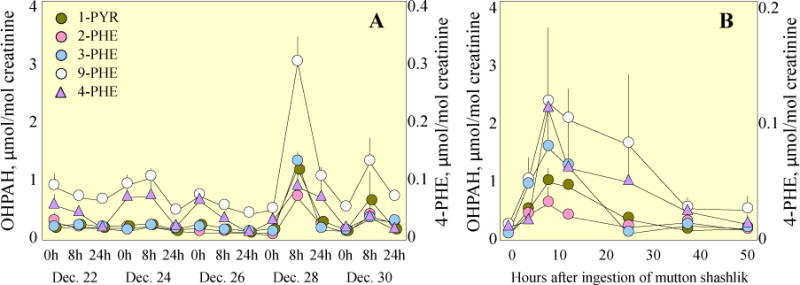
Temporal variations of the excretion of OHPHEs and 1-PYR. A) Time trends of urinary 2-PHE, 3-PHE, 4-PHE, 9-PHE, and 1-PYR over the experimental period. Means and standard errors of the measurements for all participants are shown. B) Time trends of 2-PHE, 3-PHE, 4-PHE, 9-PHE, and 1-PYR before and after the ingestion of mutton shashilik. Results are presented as the means and standard errors of the two selected participants.
In this study, two individual subjects were chosen to collect urine samples at relatively high temporal resolution. The time trends of urinary OHPHEs and 1-PYR after the consumption of lamb kabob are shown in Fig. 4B. Similar temporal patterns were found for all other OHPAHs except metabolites of BaA, CHR, and BaP. After the ingestion of a high dose of parent PAHs, urinary excretion of OHPAHs increased sharply first, reached peak values at 8–12 h, depending on individual compounds, and then decreased gradually to background level approximately 36 h after the exposure. It appears that the sampling time for studying a single-dose exposure is critical and 8–12 h is recommended. The elimination after the peak appeared to be a typical first-order process, in accordance with those in the literature (Chien and Yeh, 2010; Li et al., 2012). The calculated half-lives varied from 10 h for 1-NAP, 15 h for 1-PYR to 26 h for 2-PHE (see details in S7). Both the time needed to reach the peaks and the half-lives estimated in our study were longer than most of those reported previously (Chien and Yeh, 2010; Li et al., 2012). It is likely due to the fact that in addition to the lamb kabob ingestion, other food with less PAHs though, were taken as dinner in the evening and breakfast and the subjects also continuously exposed to high levels of ambient air PAHs.
Because of the similarity in time trends between the parent PAH intake and OHPAH excretion, it is anticipated logically that the level of a urinary OHPAH should be associated with intake quantity of its corresponding parent PAH. In this study, significant correlations (p < 0.05) were found between urinary excretions of 2-PHE, 3- PHE, 9-PHE, and 1-PYR in 8-h samples and the exposure levels of their corresponding parent PAHs, as well as the total intake of pPAH16 (Table S8). 4-PHE, 9-PHE, and 1-PYR in 24-h samples were also found to be significantly correlated with dietary intake of PAHs (p < 0.05). It was noted that most OHPAHs appeared to be more significantly correlated (relatively low p values) with dietary exposures than with total intakes. This is reasonable since food ingestion dominated the uptakes of PHE and PYR. Moreover, if the quantities of PAHs ingested in the lunch eight hours before sampling, instead of daily total intakes were used, the p values further decreased. It suggests that urinary OHPAHs in 8-h samples were mainly originated from the PAHs ingested at lunch. However, these metabolites of low-median molecular weight PAHs can not serve as biomarkers for the exposures to carcinogenic PAHs, quantified as BaPeq, which was dominated by inhalation intake. Urinary 3-BaA in 8-h samples was significantly correlated with daily inhalation exposure to BaPeq and pPAH16 (p < 0.05), although only marginally significant correlation was found between 3-BaA and inhaled BaA (p = 0.075). Except 3-BaA, no positive correlation between urinary OHPAHs and inhalation exposure to PAHs was found.
Fig. 5 shows several typical examples of the relationship between urinary excretion and PAHs intake. For metabolites of low and median molecular weight PAHs such as 2-PHE and 1-PYR (panels A and B of Fig. 5), the most significant correlations were found between urinary OHPAHs and dietary intake of their corresponding parent PAHs at lunch eight hours before. Panel C and D of Fig. 5 shows the relationship between urinary 3-BaA and daily respiration intake of pPAH16 and BaPeq on the sampling day, respectively.
Fig. 5.
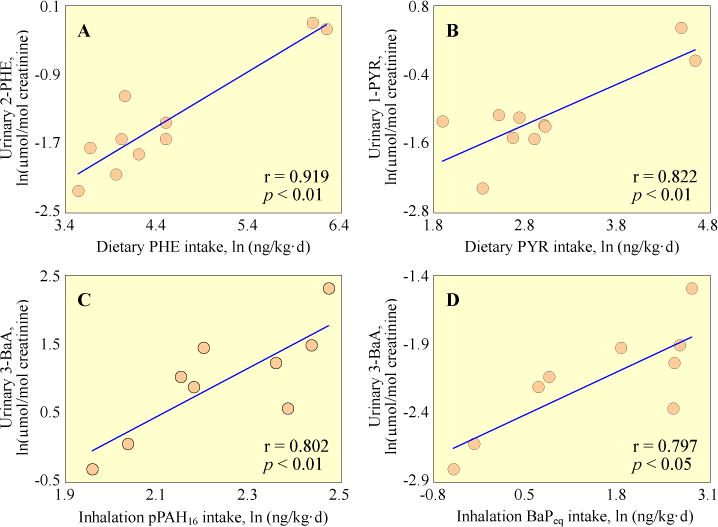
Correlations of urinary OHPAHs with dietary exposure to their corresponding parent PAHs. A) 2-PHE vs. dietary PHE intake eight hours before; B) 1-PYR vs. dietary PYR intake eight hours before; C) 3-BaA vs. daily inhalation pPAH16 intake; D) 3-BaA vs. daily inhalation BaPeq intake. Means of all male and female subjects are used. Ln-transformed data are presented.
In most previous studies, significant correlation between inhaled PAHs and urinary excretion was found for smokers and workers with occupational exposure (Alexandrie et al, 2000; Merlo et al., 1998; Roggi et al., 1997), but not for those exposed to ambient conditions (Leroyer et al., 2010; Suzuki and Yoshinaga, 2007). For example, urinary 1-PYR was significantly correlated with dietary PAH intake, but not with inhaled PAHs for non-smoking university students from Japan under normal feeding conditions (Suzuki and Yoshinaga, 2007). Urinary 1-PYR is often referred to be the surrogate biomarker for PAH exposure (Jongeneelen et al., 1985; Roggi et al., 1997), for 1-PYR has relatively high detection levels and the exposure to PYR correlates well with that to total PAHs. Based on the results of our controlled experiment, it appears that urinary metabolites of PHE, besides PYR, can also serve as biomarkers for the short-term dietary intake of PAHs of non-smoking population without occupational exposure (Table S8). Urinary 3-BaA has been suggested to be the biomarker for internal exposure to carcinogenic PAHs in a study concerning workers in a fireproof stone producing plant, because of its relatively high concentrations in the urine and the fact that it was significant correlated with urinary 1-PYR (Gundel et al., 2000). Based on the results obtained in this study, 3-BaA can also be used as a biomarker for non-occupational inhalation exposure to carcinogenic PAH.
4. Conclusion
This study is among a few investigations on the urinary excretion of OHPAHs and the relationship with parent PAH intake in China. As a controlled experiment, the dependence of urinary excreted OHPAHs on inhaled and dietary intake of parent PAH can be better characterized. High dose exposure to PAHs via both dietary and inhalation pathways by the subjects was observed, especially after the consumption of lamb kabob, which resulted in extraordinarily high levels of PAH intake and large increase in most urinary excretion of OHPAHs. It appears that gender difference in metabolism of PAH is likely to exist, since higher concentrations of urinary OHPAHs were found for female subjects with lower intake of parent PAHs than males. It was also demonstrated that apart from 1-PYR, metabolites of PHE can serve as the biomarker of short-term dietary intake of PAHs and urinary 3-BaA may be used to indicate the inhalation exposure to high molecular weight PAHs.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation of China (Grant No.41101490, 41130754, and 41001343). We thank Dr. Yu Zhiqiang of Guangzhou Institute of Environmental Geochemistry for training the students for OHPAH analysis and Dr. Duan Xiaoli for providing several OHPAH standards. R.M. Coveney of the University of Missouri-Kansas City commented on a draft of the manuscript.
Footnotes
The authors declare no completing financial interest.
Supporting materials associated with this article are provided and available free of charge via the internet.
References
- Alexandrie AK, Warholm M, Carstensen U, Axmon A, Hagmar L, Levin JO, Ostman C, Rannug A. CYP1A1 and GSTM1 polymorphisms affect urinary 1-hydroxypyrene levels after PAH exposure. Carcinogenesis. 2000;21:669–676. doi: 10.1093/carcin/21.4.669. [DOI] [PubMed] [Google Scholar]
- Alomirah H, Al-Zenki S, Al-Hooti S, Zaghloul S, Sawaya W, Ahmed N, Kannan K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control. 2011;22:2028–2035. [Google Scholar]
- Boström CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environment Health Perspecitves. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Lin YS. Formation of polycyclic aromatic hydrocarbons during processing of duck meat. Journal of agricultural and food chemistry. 1997;45:1394–1403. [Google Scholar]
- Chien YC, Yeh CT. Amounts and proportion of administered pyrene dose excreted as urinary 1-hydroxypyrene after dietary exposure to polycyclic aromatic hydrocarbons. Archives of Toxicology. 2010;84:767–776. doi: 10.1007/s00204-010-0570-4. [DOI] [PubMed] [Google Scholar]
- Chien YC, Yeh CT. Excretion kinetics of urinary 3-hydroxybenzo[a]pyrene following dietary exposure to benzo[a]pyrene in humans. Archives of Toxicology. 2012;86:45–53. doi: 10.1007/s00204-011-0727-9. [DOI] [PubMed] [Google Scholar]
- Cocco P, Moore PS, Ennas MG, Tocco MG, Ibba A, Mattuzzi S, Meloni M, Monne M, Piras G, Collu S, Satta G, Zucca M, Scarpa A, Flore C. Effect of urban traffic, individual habits, and genetic polymorphisms on background urinary 1-hydroxypyrene excretion. Annals of Epidemiology. 2007;17:1–8. doi: 10.1016/j.annepidem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- European Commission. Setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union L. 2006:364/22–364/24. [Google Scholar]
- Fan RF, Dong YL, Zhang WB, Wang Y, Yu ZQ, Sheng GY, Fu JM. Fast simultaneous determination of urinary 1-hydroxypyrene and 3-hydroxybenzo [a] pyrene by liquid chromatography–tandem mass spectrometry. Journal of Chromatography B. 2006;836:92–97. doi: 10.1016/j.jchromb.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Fan R, Ramage R, Wang D, Zhou J, She J. Determination of ten monohydroxylated polycyclic aromatic hydrocarbons by liquid–liquid extraction and liquid chromatography/tandem mass spectrometry. Talanta. 2012;93:383–391. doi: 10.1016/j.talanta.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Forster K, Preuss R, Roßbach B, Bruning T, Angerer J, Simon P. 3-Hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occupational and Environmental Medicine. 2008;65:224–229. doi: 10.1136/oem.2006.030809. [DOI] [PubMed] [Google Scholar]
- Gendre C, Lafontaine M, Morele Y, Payan JP, Simon P. Relationship between urinary levels of 1-hydroxypyrene and 3-hydroxybenzo[a]pyrene for workers exposed to polycyclic aromatic hydrocarbons. Polycyclic Aromatic Compounds. 2002;22:761–769. [Google Scholar]
- General Administration of Quality Supervision, Inspection and Quarantine of P.R.C., Ministry of Health of P.R.C., State Environmental Protection Administration of P.R.C. GB/T 1883–2002. Beijing: 2002. Indoor Air Quality Standard of PRC. [Google Scholar]
- Grainger J, Huang WL, Patterson DG, Jr, Turner WE, Pirkle J, Caudill SP, Wang RY, Needham LL, Sampson EJ. Reference range levels of polycyclic aromatic hydrocarbons in the US population by measurement of urinary monohydroxy metabolites. Environmental Research. 2006;100:394–423. doi: 10.1016/j.envres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Grova N, Feidt C, Laurent C, Rychen G. [14C] Milk, urine and faeces excretion kinetics in lactating goats after an oral administration of [14C] polycyclic aromatic hydrocarbons. International Dairy Journal. 2002;12:1025–1031. [Google Scholar]
- Gundel J, Schaller KH, Angerer J. Occupational exposure to polycyclic aromatic hydrocarbons in a fireproof stone producing plant: biological monitoring of 1-hydroxypyrene, 1-, 2-, 3- and 4-hydroxyphenanthrene, 3-hydroxybenz(a)anthracene and 3-hydroxybenzo(a)pyrene. International Archives of Occupational and Environmental Health. 2000;73:270–274. doi: 10.1007/s004200050427. [DOI] [PubMed] [Google Scholar]
- Jongeneelen FJ, Anzion RBM, Leijdekkers ChM, Bos RP, Henderson P. Th1-Hydroxypyrene in human urine after exposure to coal tar derived product. International Archives of Occupational and Environmental Health. 1985;57:47–55. doi: 10.1007/BF00383545. [DOI] [PubMed] [Google Scholar]
- Kuusimäki L, Peltonen Y, Mutanen P, Peltonen K, Savela K. Urinary hydroxy-metabolites of naphthalene, phenanthrene and pyrene as markers of exposure to diesel exhaust. International archives of occupational and environmental health. 2004;77:23–30. doi: 10.1007/s00420-003-0477-y. [DOI] [PubMed] [Google Scholar]
- Lafontaine M, Champmartin C, Simon P, Delsaut P, Brentano C. 3-Hydroxybenzo[a]pyrene in the urine of smokers and non-smokers. Toxicology Letters. 2006;162:181–185. doi: 10.1016/j.toxlet.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Leroyer A, Jeandel F, Maitre A, Howsam M, Deplanque D, Mazzuca M, Nisse C. 1-Hydroxypyrene and 3-hydroxybenzo [a] pyrene as biomarkers of exposure to PAH in various environmental exposure situations. Science of the Total Environment. 2010;408:1166–1173. doi: 10.1016/j.scitotenv.2009.10.073. [DOI] [PubMed] [Google Scholar]
- Li XR. PhD Dissertation. Peking University; 2007. Spatial distribution pattern of emission, dispersion and exposure of polycyclic aromatic hydrocarbons in Tianjin, China. [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG., Jr Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environmental Research. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjodin A. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chemical Research in Toxicology. 2012;25:1452–1461. doi: 10.1021/tx300108e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijinsky W. The formation and occurrence of polynuclear aromatic hydrocarbons associated with food. Mutation Research. 1991;259:251–261. doi: 10.1016/0165-1218(91)90121-2. [DOI] [PubMed] [Google Scholar]
- Liu YN, Tao S, Dou H, Zhang TW, Zhang XL, Dawson R. Exposure of traffic police to polycyclic aromatic hydrocarbons in Beijing, China. Chemosphere. 2007a;66:1922–1928. doi: 10.1016/j.chemosphere.2006.07.076. [DOI] [PubMed] [Google Scholar]
- Liu YN, Tao S, Yang YF, Dou H, Yang Y, Coveney RM. Inhalation exposure of traffic police officers to polycyclic aromatic hydrocarbons (PAHs) during winter in Beijing, China. Science of the Total Environment. 2007b;383:98–105. doi: 10.1016/j.scitotenv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Marie C, Bouchard M, Heredia-Ortiz R, Viau C, Maitre A. A toxicokinetic study to elucidate 3-hydroxybenzo(a)pyrene atypical urinary excretion profile following intravenous injection of benzo(a)pyrene in rats. Journal of Applied Toxicology. 2010;30:402–410. doi: 10.1002/jat.1511. [DOI] [PubMed] [Google Scholar]
- Martorell I, Perello G, Marti-Cid R, Castell V, Llobet JM, Domingo JL. Polycyclic aromatic hydrocarbons (PAH) in foods and estimated PAH intake by the population of Catalonia Spain: Temporal trend. Environment International. 2010;36:424–432. doi: 10.1016/j.envint.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Merlo F, Andreassen A, Weston A, Pan CF, Haugen A, Valerio F, Reggiardo G, Fontana V, Garte S, Puntoni R, Abbondandolo A. Urinary excretion of 1-hydroxypyrene as a marker for exposure to urban air levels of PAH. Cancer Epidemiology, Biomarkers and Prevention. 1998;7:147–155. [PubMed] [Google Scholar]
- Ministry of Health of P.R.C. GB 2762-2012. Beijing: 2012. National food safety standard, maximum levels of contaminants in foods. [Google Scholar]
- Nisbet ICT, LaGoy PK. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs) Regulatory Toxicology and Pharmacology. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]
- Perera FP. Environment and cancer: who are susceptible? Science. 1997;278:1068–1073. doi: 10.1126/science.278.5340.1068. [DOI] [PubMed] [Google Scholar]
- Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutation Research. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Preuss R, Drexler H, Bottcher M, Wilhelm M, Bruning T, Angerer J. Current external and internal exposure to naphthalene of workers occupationally exposed to poclycyclic aromatic hydrocarbons in different industries. International Archives of Occupational and Environmental Health. 2005;78:355–362. doi: 10.1007/s00420-004-0593-3. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. International Journal of Toxicology. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Roggi C, Minoia C, Sciarra GF, Apostoli P, Maccarini L, Magnaghi S, Cenni A, Fonte A, Nidasio GF, Micoli G. Urinary 1-hydroxypyrene as a marker of exposure to pyrene: an epidemiological survey on a general population group. The Science of the Total Environment. 1997;199:247–254. doi: 10.1016/s0048-9697(97)05458-2. [DOI] [PubMed] [Google Scholar]
- Scherer G, Frank S, Riedel K, Meger-Kossien I, Renner T. Biomonitoring of exposure to polycyclic aromatic hydrocarbons of nonoccupationally exposed persons. Cancer Epidemiology, Biomarkers & Prevention. 2000;9:373–380. [PubMed] [Google Scholar]
- Shen HZ, Huang Y, Wang R, Zhu D, Li W, Shen GF, Wang B, Zhang YY, Chen YC, Lu Y, Chen H, Li TC, Sun K, Li BG, Liu WX, Liu JF, Tao S. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environmental science and technology. 2013;47:6415–6424. doi: 10.1021/es400857z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Chow WH, Kulldorff M, Denobile J, Butler J, Garcia-Closas M, Weil R, Hoover RN, Rothman N. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Research. 1999;59:4320–4324. [PubMed] [Google Scholar]
- Sobus JR, McClean MD, Herrick RF, Waidyanatha S, Onyemauwa F, Kupper LL, Rappaport SM. Investigation of PAH biomarkers in the urine of workers exposed to hot asphalt. The Annals of Occupational Hygiene. 2009;53:551–560. doi: 10.1093/annhyg/mep041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State Environmental Protection Administration of P.R.C., General Administration of Quality Supervision, Inspection and Quarantine of P.R.C., Ministry of Health of P.R.C. GB 3095-2012. Beijing: 2012. Ambient Air Quality Standard of PRC. [Google Scholar]
- Steck SE, Gaudet MM, Eng SM, Britton JA, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD. Cooked meat and risk of breast cancer—lifetime versus recent dietary intake. Epidemiology. 2007;18:373–382. doi: 10.1097/01.ede.0000259968.11151.06. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. International Archives of Occupational and Environmental Health. 2007;81:115–121. doi: 10.1007/s00420-007-0188-x. [DOI] [PubMed] [Google Scholar]
- Tao S, Cui YH, Xu FL, Li BG, Cao J, Liu WX, Schmitt G, Wang XJ, Shen WR, Qing BP, Sun R. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables form Tianjin. The Science of the Total Environment. 2004;320:11–24. doi: 10.1016/S0048-9697(03)00453-4. [DOI] [PubMed] [Google Scholar]
- Tsikas D, Wolf A, Frolich JC. Simplified HPLC method for urinary and circulating creatinine. Clinical Chemistry. 2004;50:201–203. doi: 10.1373/clinchem.2003.024141. [DOI] [PubMed] [Google Scholar]
- Uppstd H, Osnes GH, Cole KJ, Phillips DH. Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer. 2011;71:254–270. doi: 10.1016/j.lungcan.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Vanrooij JGM, Veeger MMS, Bodelier-Bade MM, Scheepers PTJ, Jongeneelen FJ. Smoking and dietary intake of PAH as sources of interindividual variability in the baseline excretion of 1-hydroxypyrene in urine. International Archives of Occupational and Environmental Health. 1994;66:55–65. doi: 10.1007/BF00386580. [DOI] [PubMed] [Google Scholar]
- Viau C, Bouchard M, Carrier G, Brunet R, Krishnan K. The toxicokinetics of pyrene and its metabolites in rats. Toxicology Letters. 1999;108:201–207. doi: 10.1016/s0378-4274(99)00090-9. [DOI] [PubMed] [Google Scholar]
- Viau C, Diakite A, Ruzgyte A, Tuchweber B, Blais C, Bouchard M, Vyskocil A. Is 1-hydroxylpyrene a reliable bioindicator of measured dietary polycyclic aromatic hydrocarbons under normal conditions? Journal of Chromatography B. 2002;778:165–177. doi: 10.1016/s0378-4347(01)00465-0. [DOI] [PubMed] [Google Scholar]
- Xia ZH, Duan XL, Qiu WX, Liu D, Wang B, Tao S, Jiang QJ, Lu B, Song YX, Hu XX. Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Science of the Total Environment. 2010;408:5331–5337. doi: 10.1016/j.scitotenv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Xia ZH, Duan XL, Tao S, Qiu WX, Liu D, Wang YL, Wei SY, Wang B, Jiang QJ, Lu B, Song YX, Hu XX. Pollution level, inhalation exposuer and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environmental Pollution. 2013;173:150–156. doi: 10.1016/j.envpol.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Tao S, Shen HZ, Ma JM. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21063–21067. doi: 10.1073/pnas.0905756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Dong YH, Wang H. Residues of organochlorine pesticides and polycyclic aromatic hydrocarbons in farm-raised livestock feeds and manures in Jiangsu, China. The Science of the Total Environment. 2012:450–451. doi: 10.1016/j.scitotenv.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Zhao ZH, Quan WY, Tian DH. Experiments on the effects of several factors on the 1-hydroxypyrene in human urine as an indicator of exposure to polycyclic aromatic hydrocarbons. The Science of the Total Environment. 1992;113:197–207. doi: 10.1016/0048-9697(92)90001-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


