Abstract
Background
Fetal aortic valvuloplasty (FAV) can be performed for severe mid-gestation aortic stenosis (AS) in an attempt to prevent progression to hypoplastic left heart syndrome (HLHS). A subset of patients has achieved a biventricular (BV) circulation after FAV. The postnatal outcomes and survival of the BV patients, compared to those managed as HLHS, have not been reported.
Methods and Results
We included 100 patients who underwent FAV for severe mid-gestation AS with evolving HLHS from March 2000 to January 2013. Patients were categorized based on postnatal management as BV or HLHS. Clinical records were reviewed. Eighty-eight fetuses were live-born, and 38 had a BV circulation (31 from birth, 7 converted after initial univentricular palliation). Left-sided structures, namely aortic and mitral valve sizes and LV volume, were significantly larger in the BV group at the time of birth (p-values <0.01). After a median follow-up of 5.4 years, freedom from cardiac death among all BV patients was 96±4% at 5 years and 84±12% at 10 years, which was better than HLHS patients (log-rank p=0.04). There was no cardiac mortality in patients with a BV circulation from birth. All but 1 of the BV patients required postnatal intervention; 42% underwent aortic and/or mitral valve replacement. On most recent echocardiogram, the median LV end-diastolic volume z-score was +1.7 (range: -1.3, +8.2), and 80% had normal ejection fraction.
Conclusions
Short- and intermediate-term survival among patients who underwent FAV and achieved a BV circulation postnatally is encouraging. However, morbidity still exists, and on-going assessment is warranted.
Keywords: Fetal, valvuloplasty, aortic stenosis, hypoplastic left heart syndrome, outcome
Introduction
Hypoplastic left heart syndrome (HLHS) is a form of congenital heart disease consisting of underdeveloped left-sided heart structures that are unable to support the systemic circulation. Staged univentricular palliation, with the right ventricle serving as the systemic pumping chamber, has transformed HLHS from a lethal condition to a survivable one.1-3 However, perioperative mortality and long-term morbidity remain significant challenges.4-7
Several natural history studies have demonstrated that fetuses with severe aortic stenosis (AS) at mid-gestation, with physiologic aberrations such as left ventricular (LV) dilation and/or dysfunction and retrograde flow in the transverse aortic arch, progress to having HLHS by the time of birth. 8-12 In such patients, fetal aortic valvuloplasty (FAV) can be performed at mid-gestation in an attempt to prevent evolution to HLHS and allow postnatal survival with a biventricular (BV) circulation.13 Significant changes in LV function and left heart hemodynamics, as well as improved growth of the aortic and mitral valves, have been observed after successful FAV,14, 15 and a subset of patients has survived postnatally with a BV circulation.13,15, 16
We have previously reported the patient selection criteria, procedural aspects, and predictors of technical success and postnatal BV outcome for FAV at our institution.12, 13, 15, 17-19 However, the postnatal outcomes of patients who achieved a BV circulation after fetal intervention, compared to those managed as HLHS, have not been described. The aim of this study is to report the short- and intermediate-term survival and clinical status of the first 100 patients to undergo FAV at our institution, with an emphasis on the patients who achieved a BV circulation.
Methods
Patients
We included consecutive patients who underwent attempted FAV at our institution from March 2000 to January 2013. As previously reported, our original patient selection criteria included fetuses with severe valvar AS and physiologic aberrations consistent with evolving HLHS, such as LV dysfunction and retrograde flow in the transverse aortic arch.12, 13 In 2009, a multivariable threshold scoring system was devised to allow better discrimination of fetal candidates with salvageable left hearts for a BV circulation postnatally.15 Our selection criteria were revised at that time to include fetuses with less hypoplastic left-sided structures and higher LV pressures. The criteria regarding the physiologic aberrations indicative of evolution to HLHS have not changed over the course of our experience.
The technical aspects of the procedure, as well as the management and outcomes of fetal hemodynamic instability, have been previously described.17-19 For the purposes of the current study, technical success was defined as balloon inflation across the aortic valve with improved antegrade flow. Patients who underwent fetal cardiac interventions for other diagnoses or indications, including atrial septoplasty for established HLHS with an intact or highly restrictive atrial septum20 or FAV for AS with severe mitral regurgitation and hydrops21 were excluded.
Patients were categorized according to postnatal status at most recent follow-up or prior to death as having a BV or univentricular circulation, i.e. HLHS. A BV circulation was defined as the LV pumping the full cardiac output systemically without a shunt or other palliative strategy. A functionally univentricular circulation or HLHS was defined as any stage of palliation to enable the right ventricle to pump systemically, with the LV handling only a portion or none of the cardiac output, such as after the Stage 1 (Norwood), bidirectional Glenn, or Fontan procedures.
Follow-up postnatal clinical records and echocardiograms were gathered from all centers at which patients were managed after birth through July 2013. Each institution made the decision as to whether patients were managed as BV or univentricular based on clinical assessment. There was no standardized postnatal treatment algorithm. A small number of patients were initially managed with a univentricular palliation strategy but later underwent conversion to a BV circulation. All echocardiograms were reviewed by a single investigator to ensure consistency. The study was approved by the Institutional Review Boards at Boston Children’s Hospital and Brigham and Women’s Hospital with a waiver of informed consent.
Data Analysis
Data are reported as median (minimum, maximum) or frequency (%). We compared demographic characteristics; technical success of FAV; echocardiographic dimensions at birth; number of cardiac procedures, including interventional catheterizations and surgeries; and most recent anthropometric parameters between the BV and HLHS patients. Catheterizations in which the only intervention was occlusion of aorto-pulmonary collaterals were not considered interventional procedures for the purposes of this study. Continuous parameters were assessed for normality using the Shapiro-Wilk test. Variables were compared using the two-sided t-test, two-sample test of proportions, chi-square analysis, and/or Fisher’s exact test where appropriate. Odds ratios (OR) are presented with 95% confidence intervals (CI). To compare outcome by patient order of intervention in quartiles, ANOVA was used.
An intention-to-treat survival analysis was performed among all patients who underwent FAV from the time of fetal diagnosis, considered the date of fetal intervention (there were no deaths between diagnosis and intervention), with stratification by technical success of the procedure. In addition, a landmark analysis was performed, with survival to birth as the landmark criterion, and the date of birth as time 0. This postnatal analysis was performed with stratification by BV and HLHS outcome and with two event definitions: 1) any death; and 2) cardiac death. An analysis of cardiac death was also performed with stratification by initial postnatal management as BV or HLHS. In analyses of freedom from cardiac death, patients who died of non-cardiac causes were censored event-free at the time of death. Both the intention-to-treat and landmark analyses are depicted with Kaplan-Meier figures, with the log-rank test used to compare survival between groups. SPSS version 20.0 (Armonk, NY: IBM Corp) was used for statistical analysis.
Within the BV group only, a cross-sectional descriptive assessment was performed with a focus on the following postnatal variables: cardiac procedures, including interventional catheterizations and surgeries; presence of a permanent pacemaker; current or prior use of a gastrostomy tube; medications; and most recent echocardiographic findings. Hemodynamic data from the cardiac catheterization laboratory were not reported since catheterization was most commonly undertaken to perform an intervention or for evaluation prior to surgery, both of which would have resulted in significant changes in hemodynamics. Furthermore, some patients either had no postnatal cardiac catheterization or none since the time of neonatal aortic valvuloplasty.
Results
Procedural and Prenatal Outcomes
FAV for severe AS and evolving HLHS was attempted in 100 patients from March 2000 to January 2013. The median gestational age at intervention was 23.8 weeks (19.3, 32.0). The procedure was technically successful in 77 cases. There were 11 fetal demises after FAV (11%): 4 within 24 hours of the procedure and the remainder at a median of 11 days (4, 39) following the procedure, which included the delivery of 2 infants who were non-viable secondary to prematurity, as described previously.15, 19 One mother elected to terminate pregnancy after an unsuccessful intervention. There were no maternal deaths or significant complications related to the fetal intervention.
Estimated survival for the entire cohort of fetuses was 80±4% at 1 year after FAV and 75±5% at 5 years. Survival over time was better for patients who underwent a technically successful procedure than for those in whom the procedure was technically unsuccessful (log-rank p=0.03; Figure 1).
Figure 1.
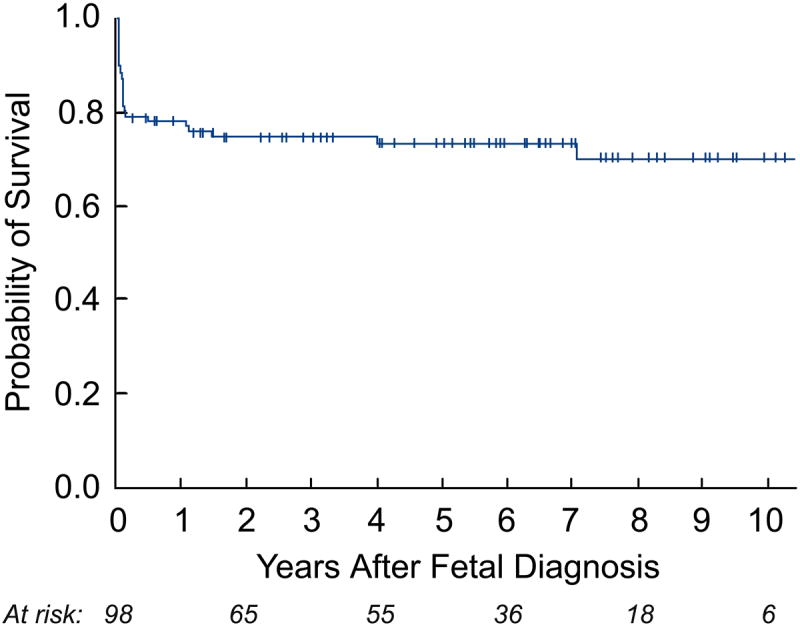
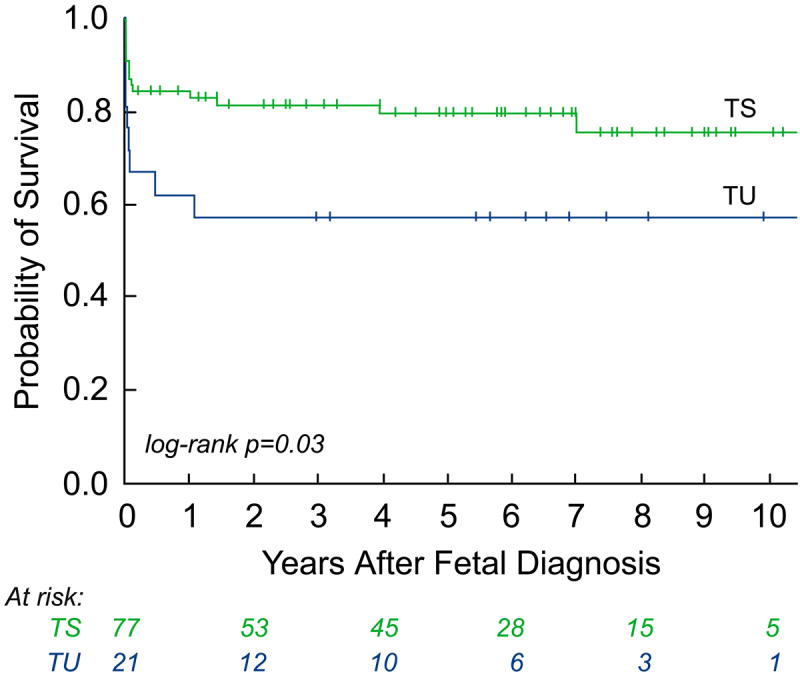
Kaplan-Meier curves depicting intention-to-treat analyses from the date of fetal intervention: a) survival of all fetuses; and b) survival of all fetuses stratified by technical success – technically successful (TS) versus technically unsuccessful (TU).
Postnatal Management: Outcome Groups
Of the 88 live-born patients, 57 neonates were initially managed with a univentricular palliative strategy (i.e., HLHS) and 31 with a BV circulation. In the HLHS group, 54 patients proceeded to have Stage 1 surgery. Of the 3 patients who did not undergo Stage 1 palliative surgery, 1 received comfort measures only, 1 died from sepsis, and 1 received a heart transplant and died in the early post-operative period. There were 4 additional neonatal deaths in the HLHS group following Stage 1 surgery (7.4% of those who underwent Stage 1). The overall neonatal mortality in the HLHS group, including the 3 patients who did not undergo Stage 1, was 12.3%. No neonatal deaths occurred in the BV cohort, yielding a significant difference in neonatal mortality between the two groups (p=0.04).
Seven patients in the HLHS group were converted to a BV circulation at a median age of 32 months (18 days, 6.2 years): 1 after Stage 1 surgery, 4 after the bidirectional Glenn procedure, and 2 after the Fontan operation. The technical process and short-term outcomes of staged left heart rehabilitation at our center, in both fetal intervention and other borderline left heart patients, have been described previously.22-24 Thus, the total number of patients managed with a BV circulation at latest follow-up was 38, which was 43% of all live-born patients (Figure 2).
Figure 2.
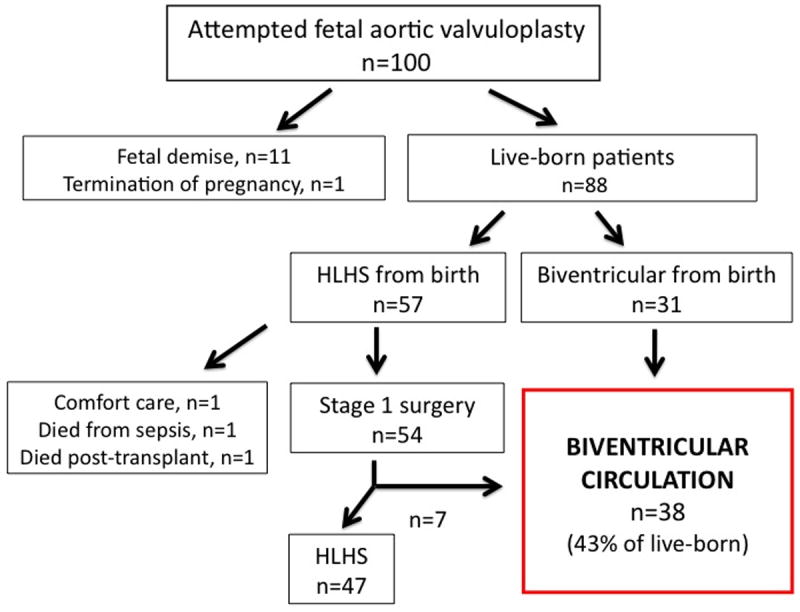
Flow diagram summarizing postnatal management and outcomes for the entire 100 patient cohort. (HLHS=hypoplastic left heart syndrome)
Thirty-five (92%) of the patients with a BV circulation had a technically successful FAV. Therefore, among the 77 technically successful interventions, 45% resulted in a BV outcome, and among the 70 technically successful interventions that proceeded to live birth, 50% had a BV outcome. Among all live-born patients, those who underwent a technically successful intervention were significantly more likely to have a BV outcome than those in whom the FAV was unsuccessful (OR 5.0 [95% CI 1.3-18.8], p=0.01). There was a progressive increase in the likelihood of BV outcome over the course of our experience and following the implementation of the revised patient selection criteria in 2009; however, these differences were not statistically significant (p-values 0.44 and 0.19, respectively; Supplemental Figure 1).
Three patients had a technically unsuccessful intervention but achieved a BV repair. These procedures were performed later in gestation: 2 at 28 weeks and 1 at 32 weeks. Two of the patients were initially managed as HLHS and subsequently were converted to a BV circulation: 1 had a hybrid Stage 1 procedure with aortic valvotomy prior to BV repair, and 1 was converted after the Fontan operation. The third patient had a Ross procedure with mitral valve replacement and endocardial fibroelastosis (EFE) resection in the neonatal period.
Comparison of Outcomes between BV and HLHS Groups
Among live-born patients, gestational age and weight at birth did not differ between the BV and HLHS groups. Prior to any postnatal intervention, left heart structures at birth were significantly larger in patients ultimately managed as BV compared to those managed as HLHS (Table 1). 25 Figure 3 demonstrates examples of fetal and neonatal echocardiograms from both outcome groups.
Table 1.
Demographic characteristics and dimensions of left heart structures on the first neonatal echocardiogram among patients with hypoplastic left heart syndrome (HLHS) or a biventricular circulation (BV)
| HLHS (n=43) |
BV* (n=38) |
p-value | |
|---|---|---|---|
| Gestational age at birth (weeks) | 38.7 (29.4, 41.1) | 37.9 (30.6, 41.1) | 0.57 |
| Birth weight (kg) | 3.0 (1.4, 4.4) | 3.2 (2.0, 4.1) | 0.44 |
| Aortic valve annulus diameter z-score† | -3.7 (-6.5, -1.4) | -2.5 (-4.0, -0.5) | <0.001 |
| Aortic root diameter z-score | -2.6 (-4.1, +0.4) | -1.1 (-3.2, +1.1) | <0.001 |
| Ascending aorta diameter z-score | -1.4 (-4.2, +1.2) | 0.3 (-2.0, +2.0) | <0.001 |
| Mitral valve lateral diameter z-score | -2.3 (-4.5, +1.0) | -1.5 (-4.2, +2.6) | 0.002 |
| Mitral valve anteroposterior diameter z-score | -2.4 (-3.8, -0.4) | -1.0 (-2.4, +1.9) | <0.001 |
| LV end-diastolic dimension z-score | -2.1 (-6.2,+1.9) | -0.5 (-5.2,+4.7) | <0.001 |
| Long-axis LV length z-score | -3.8 (-6.5, -0.9) | -1.2 (-4.9, +3.0) | <0.001 |
| LV end-diastolic volume z-score | -2.8 (-9.2, +1.4) | -1.5 (-4.3, +6.6) | <0.001 |
Data are represented as median (minimum, maximum). LV=left ventricle.
The BV group includes patients who were initially managed with univentricular palliation and subsequently converted to a BV circulation.
Z-scores were calculated from Boston Children’s Hospital data using previously validated models.25
Figure 3.
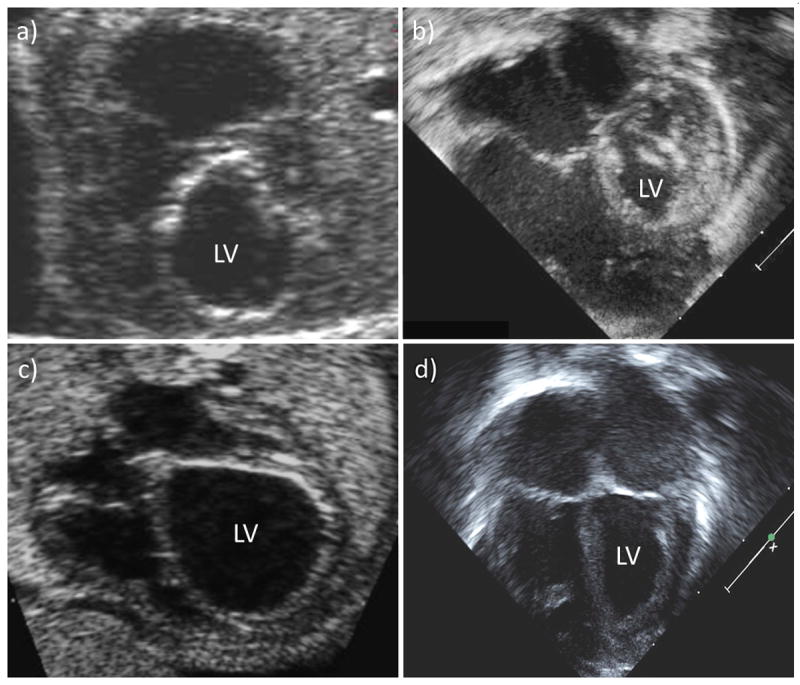
Pre-intervention fetal and neonatal echocardiograms of (a, b) a patient who underwent technically unsuccessful fetal aortic valvuloplasty (FAV) and was managed with a univentricular strategy, and (c, d) a patient who underwent technically successful FAV and was managed as biventricular postnatally. (LV=left ventricle)
During a median postnatal follow-up of 5.4 years (2 months, 13.2 years), a total of 14 deaths occurred. In the BV group, there were 3 deaths: 1 non-cardiac at 18 months of age (due to a motor vehicle accident) and 2 cardiac at 4 years and 7 years of age. Both of the cardiac deaths occurred in patients who were converted to a BV circulation after initial univentricular palliation. One patient experienced sudden death at home 16 months after conversion, and the other died from ventricular assist device complications while awaiting heart transplantation 9 months after conversion. In the HLHS cohort, a total of 11 deaths, 1 non-cardiac and 10 cardiac, occurred, including the 7 deaths in the neonatal period.
As depicted in the landmark analysis Kaplan-Meier curves in Figure 4, postnatal survival (p=0.07) and freedom from cardiac death (p=0.04) were worse among HLHS patients compared to those with a BV outcome at latest follow-up. The steeper early hazard in the HLHS group was followed by near convergence of the curves at 7 years. If patients were categorized based on initial postnatal management as BV or HLHS, with patients who were converted to a BV circulation later in life included in the HLHS cohort, then there were no cardiac deaths in the BV group. In this case, freedom from cardiac death was significantly different (p=0.009), favoring the BV group, and there was no convergence at follow-up. Among surviving patients, the median age at latest follow-up was 4.8 years (2 months, 11.5 years) in the BV group (n=35) and 4.7 years (2 weeks, 13.2 years) in the HLHS cohort (n=39). The HLHS survivors underwent more cardiac surgeries than the BV survivors (3 [2, 6] vs. 1 [0, 8]; p=0.02); however, there was no significant difference in the number of interventional catheterizations performed (2 [0, 8] vs. 1 [0, 6]; p=0.29).
Figure 4.
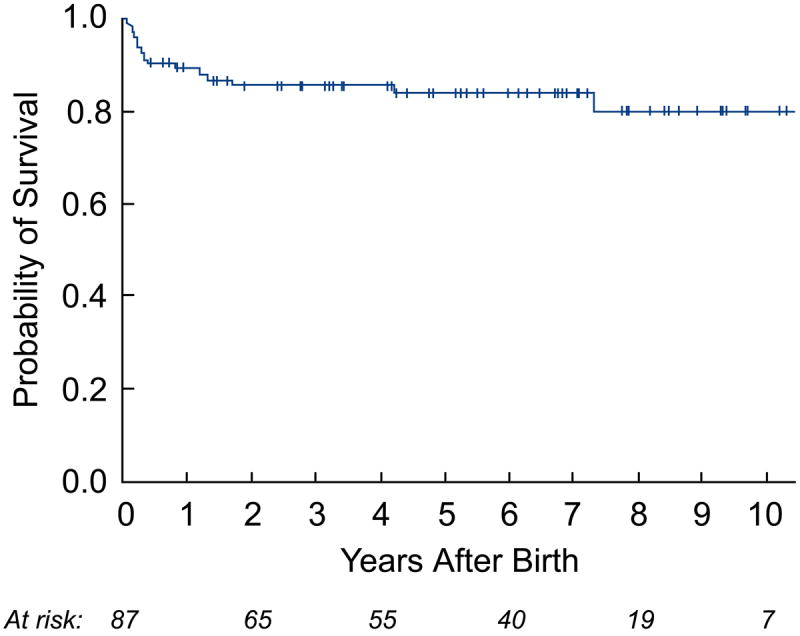
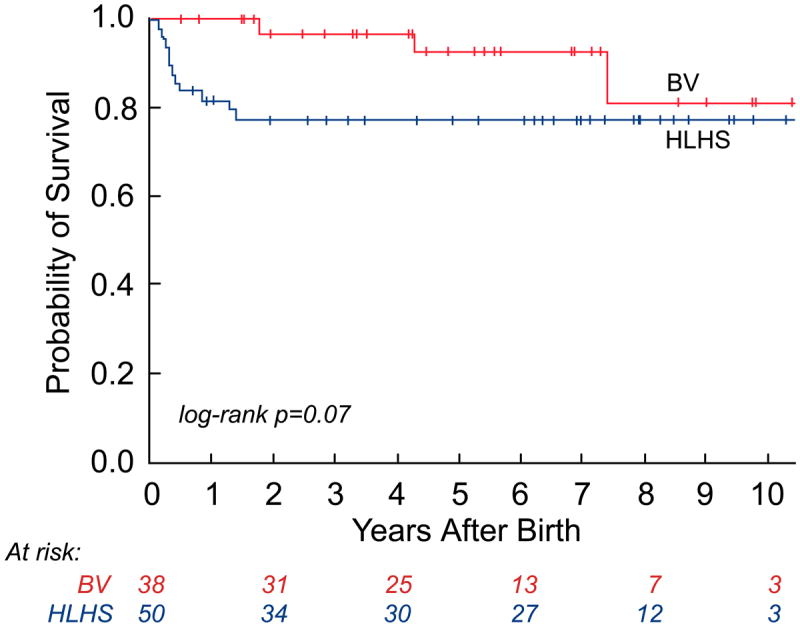
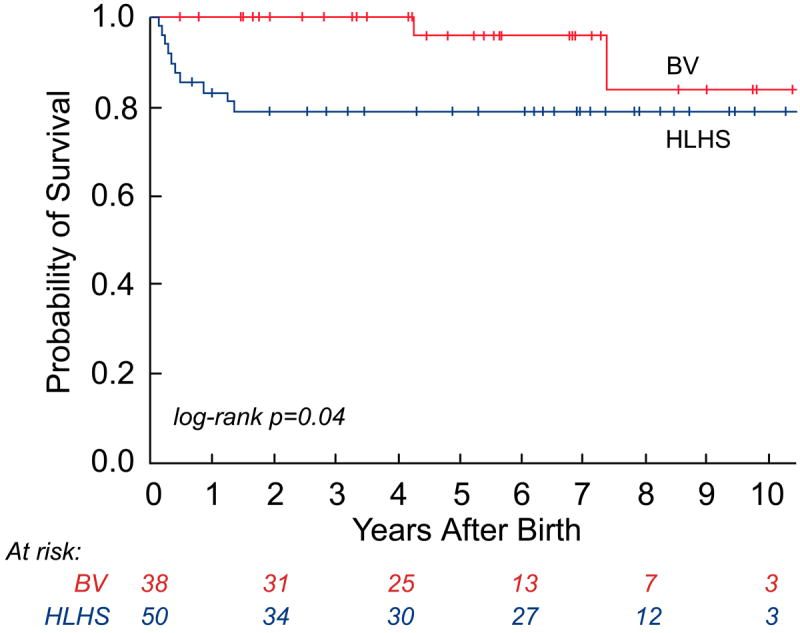
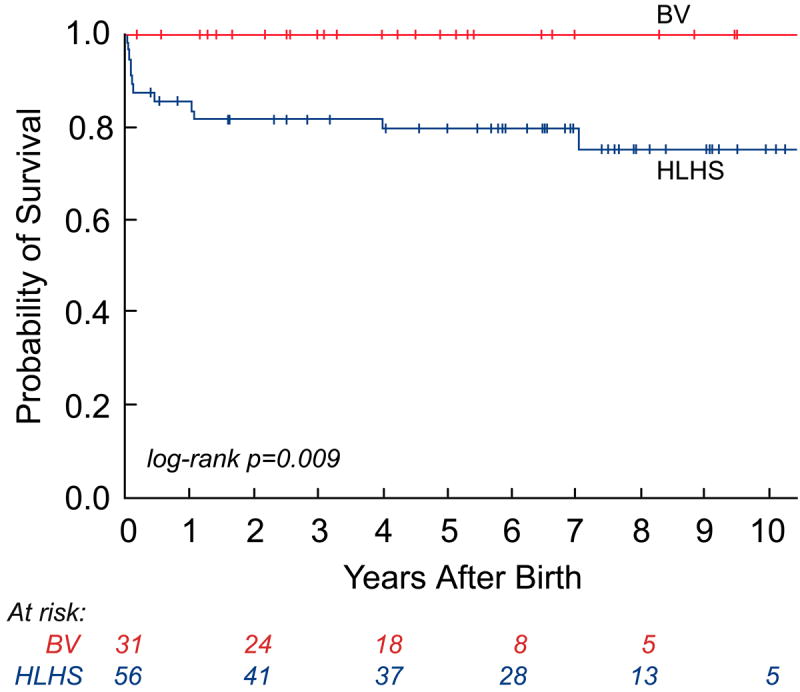
Kaplan-Meier curves depicting survival from the time of birth: a) all-cause mortality in the live-born cohort; b) all-cause mortality between hypoplastic left heart syndrome (HLHS) and biventricular (BV) outcome groups; c) cardiac mortality between HLHS and BV outcome groups (excluding the 2 non-cardiac deaths: 1 in the HLHS and 1 in the BV outcome group); and d) cardiac mortality between HLHS and BV outcome groups based on the initial postnatal management strategy.
At latest follow-up, the median height percentile of the BV survivors was 20.4 (0.1, 95.0), and the median weight percentile was 18.5 (0.1, 93.0). These percentiles were not significantly different from the HLHS survivors: 25.6 (0.1, 89.7) and 26.0 (0.1, 78.4) for height and weight, respectively. There was also no statistically significant difference between the proportion of patients below the 5th percentile for weight in the BV and HLHS groups (14% vs. 18%).
Procedures and Follow-up Status in the BV Outcome Group
Among the 38 patients in the BV group (survivors [n=35] and non-survivors [n=3]), 37 (97%) had at least 1 postnatal catheterization or surgery (Table 2). At least 1 interventional catheterization was performed in 58% of patients in the neonatal period and in 74% by latest follow-up. The median number of interventional catheterizations was 1 (0, 7). Similarly, 55% of patients underwent ≥1 cardiac surgical procedure in the neonatal period and 84% at latest follow-up. Six patients did not have cardiac surgery. Including initial univentricular palliation, the median number of cardiac surgeries was 2 (0, 8).
Table 2.
Major interventional catheterizations and surgeries in patients managed with a biventricular (BV) circulation from birth (n=31) and in patients who underwent conversion to a BV circulation after initial univentricular palliation (n=7)
| Patients (%) | Performed in neonatal period | Total performed | |
|---|---|---|---|
| BV from birth (n=31) | |||
| Interventional catheterizations | |||
| Balloon dilation of aortic valve | 28 (90%) | 24 | 40 |
| Balloon dilation of mitral valve* | 6 (19%) | 1 | 10 |
| Balloon dilation of aortic arch | 4 (13%) | 1 | 4 |
| Surgeries | |||
| Mitral valve repair | 18 (58%) | 9 | 29 |
| Endocardial fibroelastosis resection | 17 (55%) | 6 | 22 |
| Aortic valve repair | 14 (45%) | 3 | 19 |
| Ross procedure (pulmonary autograft) | 12 (39%) | 4 | 12 |
| Subaortic resection or Konno | 9 (29%) | 2 | 10 |
| Mitral valve replacement | 5 (16%) | 0 | 6 |
| Coarctation repair | 4 (13%) | 4 | 4 |
| Aortic valve replacement (mechanical) | 1 (3%) | 1 | 1 |
| Converted to BV circulation (n=7) | |||
| Interventional catheterizations | |||
| Balloon dilation of aortic valve | 4 (57%) | 3 | 7 |
| Balloon dilation of mitral valve | 1 (14%) | 0 | 1 |
| Balloon dilation of aortic arch | 1 (14%) | 0 | 1 |
| Staged Palliative Surgeries | |||
| Stage 1 (Norwood) | 7 (100%) | 7 | 7 |
| Bidirectional Glenn | 6 (86%) | -- | 6 |
| Fontan | 2 (29%) | -- | 2 |
| Other Surgeries | |||
| Endocardial fibroelastosis resection | 6 (86%) | 4 | 19 |
| Mitral valve repair | 6 (86%) | 3 | 18 |
| Aortic valve repair | 6 (86%) | 4 | 14 |
| Mitral valve replacement | 3 (43%) | 0 | 5 |
| Ross procedure (pulmonary autograft) | 2 (29%) | 0 | 2 |
| Subaortic resection or Konno | 1 (14%) | 0 | 1 |
Listed in order of most common procedures performed.
Native or surgically implanted Melody valve in the mitral position.
Valve replacements were among the most common surgical procedures performed. The primary mode of aortic valve replacement was the Ross procedure (pulmonary autograft), which was performed in 14 patients at a median age of 11 months (7 days, 6.2 years), including 4 in the neonatal period. One patient had a mechanical aortic valve replacement at 5.2 years. Eight patients underwent mitral valve replacement at a median age of 17.5 months (4, 72 months), either with a tissue or mechanical prosthesis (n=5) or a modified Melody valve (Medtronic Inc., Minneapolis, MN) implanted surgically (n=3).26 Three patients who initially had a tissue prosthesis underwent a repeat mitral valve replacement with either a mechanical valve (n=2) or a modified Melody valve (n=1) at a median age of 6.7 years. Seven patients (18%) had both aortic and mitral valve replacements. All patients that underwent valve replacement had prior attempts at balloon dilation and/or surgical repair of the affected valve.
In addition to catheterization and surgical management, 3 patients had a permanent pacemaker placed for sinus node dysfunction, high-grade atrioventricular block, and/or cardiac resynchronization therapy after BV conversion. Sixteen patients (42%) had a gastrostomy tube placed at some point for poor feeding and/or weight gain, 5 of which were subsequently removed. Among survivors (n=35), 71% were on cardiac medications at latest follow-up, including 3 (9%) who were managed on pulmonary vasodilator therapy with sildenafil. No patients were treated with chronic oxygen therapy.
Echocardiographic parameters among survivors at latest follow-up are displayed in Table 3. Notably, among patients with native aortic or mitral valves, the median annulus diameters measured within the normal range. The median LV end-diastolic volume was also within the normal range; no patients had an LV end-diastolic volume z-score less than -2, while 13 (37%) had a z-score greater than +2. The estimated right ventricular pressure was ≤ ½ systemic in 89% of patients.
Table 3.
Echocardiographic parameters of patients surviving with a biventricular outcome at latest follow-up (n=35)
| Dimensions | |
|---|---|
| Native aortic valve annulus diameter z-score† | -0.6 (-3.9, +3.0) |
| Native ascending aorta diameter z-score | 1.5 (-1.9, +5.0) |
| Native mitral valve lateral diameter z-score | +0.1 (-3.1, +2.3) |
| Native mitral valve anteroposterior diameter z-score | -0.3 (-3.4, +1.9) |
| LV end-diastolic dimension z-score | +1.4 (-1.6, +7.9) |
| LV long-axis length z-score | +1.1 (-1.2, +3.2) |
| LV end-diastolic volume z-score | +1.7 (-1.3, +8.2) |
| Valve Function | |
|
| |
| Aortic valve gradient* | |
| Native (22) | Max 31 (0, 60); Mean 21 (0, 32) |
| Ross (13) | Max 0 (0, 22); Mean 0 (0, 12) |
| Aortic regurgitation, mild or less | |
| Native (22) | 18 (77%) |
| Ross (13) | 13 (100%) |
| Mitral valve gradient | |
| Native (28) | Mean 4 (0, 16) |
| Prosthetic (7) | Mean 6 (4, 7) |
| Mitral regurgitation, mild or less | |
| Native (28) | 25 (89%) |
| Prosthetic (7) | 7 (100%) |
| Ventricular Function and Pressure | |
|
| |
| LV ejection fraction > 55% | 28 (80%) |
| LV shortening fraction > 30% | 29 (83%) |
| Right ventricular pressure ≤ ½ systemic | 31 (89%) |
Data are represented as median (minimum, maximum) or number of patients (%). LV=left ventricle.
Aortic and mitral stenosis maximum instantaneous (max) and mean Doppler gradients presented in mm Hg.
Z-scores were calculated from Boston Children’s Hospital data using previously validated models.25
Discussion
We have previously reported the rationale for fetal intervention for severe mid-gestation AS with evolving HLHS, our patient selection criteria, and aspects of the procedure, including predictors of technical success and a postnatal BV outcome.12, 13, 15, 17, 18 The current study builds upon this work by reporting the postnatal outcomes and short- and intermediate-term survival of the first 100 patients who underwent FAV at our institution. We particularly focused on patients with a BV circulation, since achieving a BV outcome postnatally is the goal of fetal intervention for this disease. As formerly described15 and further demonstrated in the present study, a substantial proportion of patients who underwent technically successful FAV were able to achieve a BV circulation. Among all live-born patients, cardiac survival was better in the BV group compared to the HLHS cohort at latest follow-up, primarily due to differences in neonatal mortality. Importantly, there were no cardiac deaths among patients managed as BV from birth.
The goal of FAV is to alter left heart physiology in utero prior to the development of growth arrest and severe left heart hypoplasia. We previously documented that FAV does, in fact, modify left heart physiology and promote relative growth of the aortic and mitral valves.14, 15 In the present study, we found that left heart dimensions at birth, prior to any postnatal intervention, were significantly larger in the BV group than in the HLHS cohort. Even though our center favors a BV approach for neonates with borderline left heart hypoplasia, the differences in left heart dimensions support the argument that institutional bias was not a major determinant of outcome.
In the cohort of patients who had a BV outcome after FAV, there was on-going disease burden due to abnormal valvar and myocardial function. In particular, there was significant aortic and mitral valve disease, often characterized by a combination of stenosis and regurgitation and the need for valve intervention and/or replacement. Not surprisingly, the inherently abnormal aortic valve remained problematic, as indicated by the 32 patients (84% of the cohort) who underwent at least 1 postnatal balloon valvuloplasty and the 15 patients (39%) who underwent aortic valve replacement. Eight patients (21%) also underwent mitral valve replacement.
While valves may be replaced, the development of the abnormal LV myocardium may be a more difficult problem to overcome. At most recent follow-up, BV patients generally had LV volume and systolic function within the normal range, although more than one-third had ventricular dilatation. While the abnormal myocardium may play a role in this process, sequelae from EFE resection and/or valvar disease may also be contributing factors. Invasive hemodynamic data were not available to assess ventricular end-diastolic pressure, but diastolic dysfunction, with lower mitral annular and septal E’ tissue velocities and higher mitral inflow E/E’ ratios, has previously been characterized in these patients.27 We found normal right ventricular pressure as assessed by echocardiography in 89% of patients; however, the development of elevated pulmonary artery pressure secondary to left atrial hypertension from mitral valve disease and/or LV diastolic dysfunction will need to be monitored over time.
Of the 38 patients with a BV outcome, 7 were initially managed with univentricular palliation prior to being converted to a BV circulation. Consistent with the strategy of rehabilitating the left heart at our institution, these patients had modifications at the time of the staged palliative procedures to promote continued LV growth.22-24 The achievement of a BV circulation with this aggressive approach may, on the one hand, offer insights into the potential for LV rehabilitation, but, on the other hand, may potentially engender as yet undetermined long-term morbidity. Since 2 of the 7 patients managed with this strategy died, on-going assessment of this approach, with more rigorous selection criteria, will need to be sought.
While the long-term morbidity of the BV cohort is yet to be determined, there are substantial data regarding the significant long-term morbidities, impaired quality of life, and premature death in patients with HLHS and a Fontan circulation.6, 7, 28-33 Although heart transplantation is an option for select patients with failing Fontan physiology, survival is significantly lower among patients with a prior Fontan operation compared to patients with other forms of congenital heart disease or cardiomyopathy.34, 35 Thus, even if there are substantial on-going issues in patients with a BV circulation after FAV, the potential for improved long-term prognosis and survival compared to patients with a univentricular circulation is the real question. A multicenter, case-control study to systemically study outcomes over time may help address this question, with patients treated with FAV and a BV rehabilitation strategy at one center matched to patients treated with staged univentricular palliation at another center.
As previously reported by our group,13, 15, 19 there is a risk of fetal demise associated with the procedure. Despite demise in 11% of fetuses in our experience, the 10 year-survival of the entire cohort was 72%, which is comparable to, if not better than, the 10-year survival reported for patients with HLHS in other contemporary series.5, 36, 37 Importantly, patients who underwent a technically successful intervention had significantly better survival than those who did not. Given that patients with a technically successful intervention were 5 times more likely to have a BV outcome than those with an unsuccessful intervention, this finding is consistent with our postnatal survival analysis between the BV and HLHS groups.
The 3 patients that had a technically unsuccessful fetal intervention but still went on to achieve a BV circulation postnatally warrant further discussion. These patients underwent attempted interventions later in gestation than most of the cohort at 28-32 weeks. This highlights the fact that although we understand the natural history leading to HLHS in mid-gestation fetuses, the natural history of fetuses diagnosed with AS later in gestation is less clear and deserves further investigation. The later gestational age notwithstanding, all 3 of the patients were born with borderline left heart structures that were incapable of supporting the systemic circulation without aggressive interventions. As detailed previously, 2 of these patients initially underwent univentricular palliation, and the third had replacement of both the aortic and mitral valves in the neonatal period, along with EFE resection.
The current study highlights the concept behind fetal therapy, which has evolved from open surgery for non-cardiac defects to minimally invasive, ultrasound-guided therapy for cardiac defects: namely, that by modifying a developing lesion in utero, postnatal outcome and/or survival may be improved. It is important to recognize that among patients with HLHS, only the subset of patients with mitral and aortic stenosis are likely to present with a dilated LV at mid-gestation and be potential candidates for FAV. In addition, a question to consider is why all of our patients did not respond as desired to FAV and achieve a BV circulation postnatally despite meeting our selection criteria. Most likely, a contributing factor in some of the patients was that they were referred for FAV at a point in the disease process when, despite maintenance of LV size, the ability to reverse the progression to HLHS was not possible. Had the procedure been performed earlier in the disease process, the left heart may have been capable of recovery. Since the disease evolves at slightly different points in gestation – and at distinct rates – from fetus to fetus, we believe such patients should be referred for FAV as soon as AS is detected to enhance the possibility of a BV outcome. At the same time, given the complex and somewhat controversial nature of this endeavor, we must continue to strive to understand the subtleties of the natural history and to refine our patient selection for those in whom a BV outcome can be realistically achieved.
Limitations
One limitation of this study was its retrospective, cross-sectional design and the relatively small cohort of patients, although this is the largest reported series of patients who have undergone attempted fetal cardiac intervention to date. Some live-born patients did not return to our institution after birth, and, consequently, postnatal management decisions may have been made in a non-uniform fashion. Recent directly measured hemodynamic data, which are of particular interest in this patient population, were not available for 36 out of the 38 BV patients. Although most patients underwent catheterization at some point, in virtually all cases catheterization was undertaken to perform interventions and/or prior to surgery, which would have altered the hemodynamic parameters of interest. There is additional important follow-up information that we were not able to obtain for this study, including data regarding other functional outcomes, such as cardiopulmonary exercise capacity and neurodevelopmental status. We plan to study these outcomes in the future.
Conclusions
The current report gives reason for optimism, not only in terms of the substantial proportion of patients who achieved a BV circulation after successful FAV but also for the excellent short- and intermediate-term survival of those managed as BV from birth. This survival was significantly different than patients managed as HLHS; however, the BV patients still carried a substantial burden of cardiac disease. Critical evaluation of longer-term outcomes will be needed to understand fully the benefits and shortcomings of fetal intervention for mid-gestation AS with evolving HLHS. Much of the success of the strategy is likely to depend on how the abnormal myocardium evolves over time. It remains to be seen which type of circulation and ventricle is better in the long-term – a univentricular circulation with a systemically-functioning right ventricle or a BV circulation with a rehabilitated LV.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Freud is funded by the National Institutes of Health (T32-HL757229) and the Kenrose Kitchen Foundation. The Ellianna Grace Foundation also supported this study.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, Ghanayem NS, Frisbee SJ, Litwin SB. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: Lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–89. [PubMed] [Google Scholar]
- 2.Mitchell ME, Ittenbach RF, Gaynor JW, Wernovsky G, Nicolson S, Spray TL. Intermediate outcomes after the fontan procedure in the current era. J Thorac Cardiovasc Surg. 2006;131:172–180. doi: 10.1016/j.jtcvs.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Delmo Walter EM, Hubler M, Alexi-Meskishvili V, Miera O, Weng Y, Loforte A, Berger F, Hetzer R. Staged surgical palliation in hypoplastic left heart syndrome and its variants. J Card Surg. 2009;24:383–391. doi: 10.1111/j.1540-8191.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, Kaltman JR, Laussen PC, Lewis AB, Lurito KJ, Minich LL, Ohye RG, Schonbeck JV, Schwartz SM, Singh RK, Goldberg CS. Interstage mortality after the norwood procedure: Results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafane J, Bhatt AB, Peng LF, Johnson BA, Marsden AL, Daniels CJ, Rudd NA, Caldarone CA, Mussatto KA, Morales DL, Ivy DD, Gaynor JW, Tweddell JS, Deal BJ, Furck AK, Rosenthal GL, Ohye RG, Ghanayem NS, Cheatham JP, Tworetzky W, Martin GR. Hypoplastic left heart syndrome: Current considerations and expectations. J Am Coll Cardiol. 2012;59:S1–42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ., 3rd Contemporary outcomes after the fontan procedure: A pediatric heart network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams IA, Sleeper LA, Colan SD, Lu M, Stephenson EA, Newburger JW, Gersony WM, Cohen MS, Cnota JF, Atz AM, Williams RV, Margossian R, Powell AJ, Stylianou MP, Hsu DT. Functional state following the fontan procedure. Cardiol Young. 2009;19:320–330. doi: 10.1017/S1047951109990382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25:341–343. doi: 10.1016/0167-5273(89)90226-x. [DOI] [PubMed] [Google Scholar]
- 9.Danford DA, Cronican P. Hypoplastic left heart syndrome: Progression of left ventricular dilation and dysfunction to left ventricular hypoplasia in utero. Am Heart J. 1992;123:1712–1713. doi: 10.1016/0002-8703(92)90834-i. [DOI] [PubMed] [Google Scholar]
- 10.Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997;77:205–210. doi: 10.1136/hrt.77.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCaffrey FM, Sherman FS. Prenatal diagnosis of severe aortic stenosis. Pediatr Cardiol. 1997;18:276–281. doi: 10.1007/s002469900174. [DOI] [PubMed] [Google Scholar]
- 12.Makikallio K, McElhinney DB, Levine JC, Marx GR, Colan SD, Marshall AC, Lock JE, Marcus EN, Tworetzky W. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: Patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- 13.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 14.Selamet Tierney ES, Wald RM, McElhinney DB, Marshall AC, Benson CB, Colan SD, Marcus EN, Marx GR, Levine JC, Wilkins-Haug L, Lock JE, Tworetzky W. Changes in left heart hemodynamics after technically successful in-utero aortic valvuloplasty. Ultrasound Obstet Gynecol. 2007;30:715–720. doi: 10.1002/uog.5132. [DOI] [PubMed] [Google Scholar]
- 15.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arzt W, Wertaschnigg D, Veit I, Klement F, Gitter R, Tulzer G. Intrauterine aortic valvuloplasty in fetuses with critical aortic stenosis: Experience and results of 24 procedures. Ultrasound Obstet Gynecol. 2011;37:689–695. doi: 10.1002/uog.8927. [DOI] [PubMed] [Google Scholar]
- 17.Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, Wilkins-Haug LE, Marx GR, Lock JE. Aortic valvuloplasty in the fetus: Technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539. doi: 10.1016/j.jpeds.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins-Haug LE, Tworetzky W, Benson CB, Marshall AC, Jennings RW, Lock JE. Factors affecting technical success of fetal aortic valve dilation. Ultrasound Obstet Gynecol. 2006;28:47–52. doi: 10.1002/uog.2732. [DOI] [PubMed] [Google Scholar]
- 19.Mizrahi-Arnaud A, Tworetzky W, Bulich LA, Wilkins-Haug LE, Marshall AC, Benson CB, Lock JE, McElhinney DB. Pathophysiology, management, and outcomes of fetal hemodynamic instability during prenatal cardiac intervention. Pediatr Res. 2007;62:325–330. doi: 10.1203/PDR.0b013e318123fd3a. [DOI] [PubMed] [Google Scholar]
- 20.Marshall AC, van der Velde ME, Tworetzky W, Gomez CA, Wilkins-Haug L, Benson CB, Jennings RW, Lock JE. Creation of an atrial septal defect in utero for fetuses with hypoplastic left heart syndrome and intact or highly restrictive atrial septum. Circulation. 2004;110:253–258. doi: 10.1161/01.CIR.0000135471.17922.17. [DOI] [PubMed] [Google Scholar]
- 21.Vogel M, McElhinney DB, Wilkins-Haug LE, Marshall AC, Benson CB, Juraszek AL, Silva V, Lock JE, Marx GR, Tworetzky W. Aortic stenosis and severe mitral regurgitation in the fetus resulting in giant left atrium and hydrops: Pathophysiology, outcomes, and preliminary experience with pre-natal cardiac intervention. J Am Coll Cardiol. 2011;57:348–355. doi: 10.1016/j.jacc.2010.08.636. [DOI] [PubMed] [Google Scholar]
- 22.Emani SM, Bacha EA, McElhinney DB, Marx GR, Tworetzky W, Pigula FA, del Nido PJ. Primary left ventricular rehabilitation is effective in maintaining two-ventricle physiology in the borderline left heart. J Thorac Cardiovasc Surg. 2009;138:1276–1282. doi: 10.1016/j.jtcvs.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emani SM, McElhinney DB, Tworetzky W, Myers PO, Schroeder B, Zurakowski D, Pigula FA, Marx GR, Lock JE, del Nido PJ. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J Am Coll Cardiol. 2012;60:1966–1974. doi: 10.1016/j.jacc.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Kalish BT, Banka P, Lafranchi T, Tworetzky W, Del Nido P, Emani SM. Biventricular conversion after single ventricle palliation in patients with small left heart structures: Short-term outcomes. Ann Thorac Surg. 2013;96:1406–12. doi: 10.1016/j.athoracsur.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 25.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 26.Abdullah I, Ramirez FB, McElhinney DB, Lock JE, del Nido PJ, Emani S. Modification of a stented bovine jugular vein conduit (melody valve) for surgical mitral valve replacement. Ann Thorac Surg. 2012;94:e97–98. doi: 10.1016/j.athoracsur.2012.02.101. [DOI] [PubMed] [Google Scholar]
- 27.Friedman KG, Margossian R, Graham DA, Harrild DM, Emani SM, Wilkins-Haug LE, McElhinney DB, Tworetzky W. Postnatal left ventricular diastolic function after fetal aortic valvuloplasty. Am J Cardiol. 2011;108:556–560. doi: 10.1016/j.amjcard.2011.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, Blaufox AD. Arrhythmias in a contemporary fontan cohort: Prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010;56:890–896. doi: 10.1016/j.jacc.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valente AM, Bhatt AB, Cook S, Earing MG, Gersony DR, Aboulhosn J, Opotowsky AR, Lui G, Gurvitz M, Graham D, Fernandes SM, Khairy P, Webb G, Gerhard-Herman M, Landzberg MJ. The calf (congenital heart disease in adults lower extremity systemic venous health in fontan patients) study. J Am Coll Cardiol. 2010;56:144–150. doi: 10.1016/j.jacc.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal DN, Friedman AH, Kleinman CS, Kopf GS, Rosenfeld LE, Hellenbrand WE. Thromboembolic complications after fontan operations. Circulation. 1995;92:II287–293. doi: 10.1161/01.cir.92.9.287. [DOI] [PubMed] [Google Scholar]
- 31.Caruthers RL, Kempa M, Loo A, Gulbransen E, Kelly E, Erickson SR, Hirsch JC, Schumacher KR, Stringer KA. Demographic characteristics and estimated prevalence of fontan-associated plastic bronchitis. Pediatr Cardiol. 2013;34:256–261. doi: 10.1007/s00246-012-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rychik J. Protein-losing enteropathy after fontan operation. Congenit Heart Dis. 2007;2:288–300. doi: 10.1111/j.1747-0803.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 33.Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348–1352. doi: 10.1016/j.jtcvs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Lamour JM, Kanter KR, Naftel DC, Chrisant MR, Morrow WR, Clemson BS, Kirklin JK. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol. 2009;54:160–165. doi: 10.1016/j.jacc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Davies RR, Sorabella RA, Yang J, Mosca RS, Chen JM, Quaegebeur JM. Outcomes after transplantation for “failed” fontan: A single-institution experience. J Thorac Cardiovasc Surg. 2012;143:1183–1192 e1184. doi: 10.1016/j.jtcvs.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 36.Lee MG, Brizard CP, Galati JC, Iyengar AJ, Rakhra SS, Konstantinov IE, Pflaumer A, d’Udekem Y. Outcomes of patients born with single-ventricle physiology and aortic arch obstruction: The 26-year melbourne experience. J Thorac Cardiovasc Surg. 2013 Sep 24; doi: 10.1016/j.jtcvs.2013.07.076. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Weintraub RG, Bullock A, Celermajer DS, d’Udekem Y. The extracardiac conduit fontan procedure in australia and new zealand: Hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg. 2014 Feb 26; doi: 10.1093/ejcts/ezu015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


