Abstract
Importance
Type 2 diabetes is common, and treatment of blood glucose is a mainstay of diabetes management. However, the benefits of intensive glucose treatment take many years to manifest, while treatment burden begins immediately. Because guidelines often fail to consider treatment burden, many patients with diabetes may be overtreated.
Objective
We examined how treatment burden affects the benefits of intensive vs. moderate glycemic control in patients with type 2 diabetes.
Design
We estimated the effects of A1c reduction on diabetes outcomes and overall quality-adjusted life years (QALYs) using a Markov simulation model. Model probabilities were based on estimates from randomized trials and observational studies.
Setting
US adults with type 2 diabetes
Participants
Simulated patients based on patients with type 2 diabetes drawn from the National Health and Nutrition Examination Study.
Interventions
Glucose lowering with oral agents or insulin in type 2 diabetes
Main Outcome measures
QALYs and reduction in risk of microvascular and cardiovascular diabetes complications.
Results
Assuming a low treatment burden (0.001, or 0.4 lost days per year), treatment that lowers A1c by 1 point provided benefits ranging from 0.77–0.91 QALYs for patients diagnosed at age 45 to 0.08–0.10 QALYs for those diagnosed at age 75. An increase in treatment burden (0.01, or 3.7 days lost per year) resulted in A1c lowering causing more harm than benefit in those aged 75. Across all ages, patients who view treatment as more burdensome (0.025–0.05) experienced a net loss in QALYs from treatments to lower A1c.
Conclusions
Improving glycemic control can provide substantial benefits, especially for younger patients; however, for most patients over age 50 with an A1c below 9% on metformin, further glycemic treatment usually offers at most modest benefits. Further, the magnitude of benefit is enormously sensitive to patients’ views of the treatment burden, and even very small treatment adverse effects result in net harm in older patients. The current approach of broadly advocating intensive glycemic control for millions of patients should be reconsidered; instead, treating A1c’s less than 9% should be individualized based on estimates of benefit weighed against the patient’s views of the burdens of treatment.
Introduction
Intensive glycemic control is a standard of care for many organizations, and achieving an A1c of <7% is a quality measure often used to profile physicians and health plans.1,2 Lowering A1c delays the onset and slows the progression of early microvascular disease.3,4 However, trials have found no significant reductions in clinically-relevant endpoints such as visual loss, end-stage renal disease, and amputation, with 10 years of improved glycemic control.3 Observational studies and disease modeling suggest that benefits in these major outcomes will eventually accrue, but typically take two or more decades to manifest.5,6 Effects on macrovascular endpoints, such as heart attacks and strokes, have varied widely between trials, but meta-analyses suggest that glycemic control may convey a small reduction in non-fatal events.7–12
Whenever treatments have limited or delayed benefits, the burden and risks of treatment become particularly important.13–20 Most glycemic medications have unwanted effects, such as weight gain, hypoglycemia or gastrointestinal (GI) side effects.3,21 Moreover, clinicians are now faced with an expanding arsenal of diabetes treatments that have varying side effects and a risk of treatment-related harm, such as that found in the ACCORD trial and suggested by meta-analyses of rosiglitazone.18–20 Treatment burden and side effects can have an appreciable negative impacts on patient quality of life.13,22–24 Decisions made in chronic diseases often lead to lifelong therapy, allowing these undesired effects to accrue over a long period. These considerations have led many guidelines to recommend consideration of patient preferences, age, and health status when setting glucose management targets in patients with type 2 diabetes.2,16,25 However, operationalization of these concepts is limited due to the lack of quantitative estimation of the benefits and burdens of treating various A1c levels with different glycemic medications. We sought to quantify the advantages of using a tailored approach for intensifying glycemic control and to examine thresholds at which treatment decisions become sensitive to the level of treatment burden (quantified as “disutility”, a small loss in quality of life).
Methods
Overview
We used an updated version of a previously published Markov model of diabetes outcomes to examine the benefits of glycemic control.6,15,26–28 The model considers microvascular and cardiovascular diabetes complications, specifically examining the impact of risk factor levels on their development and progression. We have previously published estimates for benefits from blood pressure and lipid treatment,15 and focus here on quantifying the benefits of A1c reduction. As we are challenging existing paradigms of treatment, we chose somewhat optimistic assumptions of the benefits of glycemic control as detailed below.
Model parameters
Detailed model specifications are available in the technical appendix; we describe key parameter estimates briefly here.
We modeled risk of early microvascular and neuropathic diabetes complications primarily using estimates drawn from the UK Prospective Diabetes Study (UKPDS),3 as a patient-level meta-analysis for microvascular complications has yet to be published, and other trials have short-term follow-up. We modeled progression through the intermediate steps as measured in the UKPDS: risk of progression to photocoagulation; risk of microalbuminuria and proteinuria; and risk of neuropathy. The relationship between these risks and A1c are defined by assuming a constant relative risk across the spectrum of A1c (Table 1). This implies a log-linear relationship between A1c and microvascular outcomes, a well-established finding in observational studies.29–31 Estimates for rates of progression from intermediate to end-stage microvascular complications were drawn from clinical trials, and from observational studies when necessary.32–40
Table 1.
Base assumptions about key model parameters*
| Parameter | Value | References |
|---|---|---|
| Risk of photocoagulation | 29% reduction per 0.9% change in A1c | 3 |
| Risk of neuropathy | 19% reduction per 0.9% change in A1c | 3 |
| Risk of microalbuminuria | 33% reduction per 0.9% change in A1c | 3 |
| Risk of CHD event | 15% reduction per 1.0% change in A1c | 7–11,29 |
| Utility of visual loss | 0.69 (0.40–0.85) | 43,44 |
| Utility of ESRD | 0.61 (0.40–0.80) | 43,68 |
| Utility of Myocardial Infarction | 0.88 (0.70 – 0.95) | 69 |
| Utility of Stroke | 0.64 (0.40–0.85) | 70 |
| Utility of amputation | 0.60 (0.40 – 0.80) | 43,44,68 |
For more detail, see technical report
Pre-treatment risks of coronary heart disease (CHD) and stroke were estimated using the Framingham risk estimator reported by Anderson et al.41 Estimates of the distribution of risk factors in the US diabetes population were obtained from the 2009–2010 NHANES survey data.42 These risks were then input into the model in a non-stationary fashion (changing with time).
There is substantial uncertainty around the relationship between CHD risk and A1c level. A series of meta-analyses have converged around a consensus that lowering A1c reduces the risk of non-fatal CHD events, but not cardiovascular or total mortality.7–11 We therefore assumed that CHD risk is reduced by 15% per 1 percentage point change in A1c. A 15% CHD reduction is the effect size seen in rigorous observational studies12 and is similar to the non-statistically significant reduction in MI risk seen in the UKPDS 33 and in the long-term follow-up study of the UKPDS.3,5
Quantifying and comparing the impact of disease complications and treatment burdens on overall patient quality of life is generally done using the concept of utility, measured on a scale with 1 being perfect health, and 0 being death. For example, a commonly used health utility for visual loss is 0.6943,44 – blindness is estimated to reduce quality of life compared to perfect health by 31% (disutility = 0.31), equivalent to about 113 days of high-quality life lost per year. We generally followed prior models in selecting health utility values for disease complications (table 1).39,43,45
We did not specify a baseline burden of treatment; rather, we examined the effects across a range, based on analyses of glycemic treatment disutilities.22–24 Insulin is the best studied; disutility estimates range between .02 and .12, equivalent to the loss of 7 to 44 days of quality of life per year, while for oral therapies the reported treatment burdens are smaller and are driven primarily by side effects. For example, a weight gain of 3%, which occurs in most patients taking sulfonylureas and thiazolidinediones, has an average attributed disutility of 0.04, and GI side effects, which can occur in patients taking metformin, also have a disutility of 0.04. We examined a conservative range of treatment disutility, from 0.001 to 0.05 (i.e., a utility range of 0.95 to 0.999 for those in otherwise normal health, or a loss of 0.3 days to 18 days of high quality life per year). This range of estimates was based largely on existing data, such as that outline for insulin above, or for the act of taking a daily pill (0.001).46,47 In order to remain optimistic about our estimates of treatment benefit, we chose not to discount future events, nor to consider out of pocket costs or the potential for adverse events inherent in using newer treatments.
We examined the effects of a treatment that lowers A1c by 1%, a typical response to glucose lowering therapies.48 We examined how starting A1c levels affected the benefits of A1c lowering, but we assumed that A1c levels above 9% would be routinely treated. We also examined two specific treatment scenarios. In the first, a newly diagnosed 45 year old with an A1c of 8.5% is started on metformin, and their A1c is reduced by 1.5 points to 7.0%. Using a side effect profile based on clinical reports, we assumed that persistent GI side effects occurred in 10% of individuals, with a disutility of 0.04 for those who experience the effects (for an average loss of 0.004, or 1.46 days of high-quality life per year).23 Minor hypoglycemia was assumed in 0.4% each year, with a disutility of 0.01;22,23,49 in combination with GI side effects, this produces a total average disutility of 0.00404, or about 1.47 days lost of high-quality life per year. In a second scenario, we examined the impact of switching to insulin in this patient if their A1c increased to 9.0% over a 10 year period, similar to that seen in the UKPDS; insulin was assumed to reduce A1c by 1.0%.3,50 Daily injections themselves have an estimated disutility of about 0.03.22 After adding in a disutility for an average 0.5% weight gain per year over 10 years (a cumulative disutility of 0.007 per year), a minor hypoglycemia rate of 2% per year (disutility 0.01), and a major hypoglycemia rate of 0.2% per year (disutility 0.03),3,23 insulin therapy had a total average disutility of 0.0372, or 13.6 days lost of high-quality life per year.
We conducted sensitivity analyses of all variables in the model across a broad range of assumptions. The key variables we identified as critical were age at diagnosis, pre-treatment A1c level, and treatment disutility. We also found that any benefits from reduction in CHD events due to lower rates albuminuria were important, particularly if it was assumed that these benefits were additive with the assumed 15% reduction in CHD events with a 1 point reduction in A1c.
Our primary outcome was quality-adjusted life years (QALYs); we also examined the risk reductions in individual endpoints for our two treatment scenarios. We tested model predictions by comparing them to reported outcomes in the literature. While there are no reports of observed quality-adjusted life-years, we were able to compare life expectancy predictions to those based on UKPDS actuarial projections across age and A1c strata.51 We additionally compared model predictions of complication rates to those seen in the STENO-2 study.52
Results
Our model predictions of life expectancy aligned well with those predicted from the UKPDS, as did estimates of individual complication rates seen in the STENO-2 study (see technical report).
In our best-case scenario (improving A1c lowers CHD risk, and treatment has minimal patient burden/side effects), we found substantial benefits to lowering A1c, particularly among younger individuals (Supporting Table 1). For example, in a 45 year old, lifetime treatment from an A1c of 8.5% to 7.5% produces a gain of 0.906 QALYs. This benefit is smaller in older individuals, declining to a gain of 0.269 QALYs at age 65 and 0.104 QALYs at age 75. Although these benefits are slightly less in a patient with a starting A1c of 7.5%, as long as treatment risks and treatment burden remain very small (disutility of 0.001, equivalent to 0.3 days of high-quality life lost per year), all age groups receive some benefit.
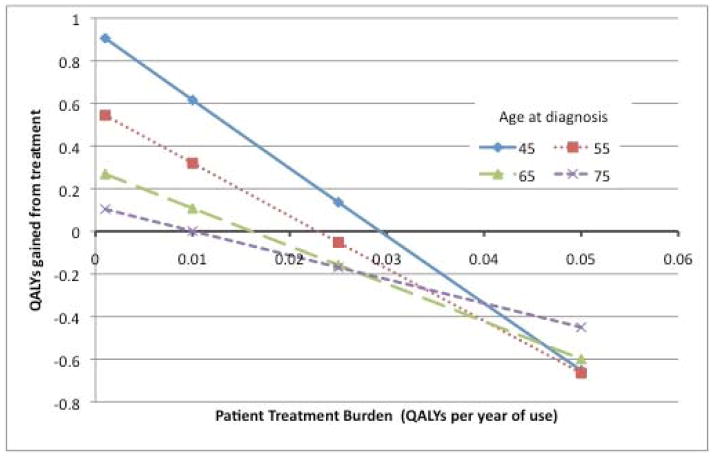
The patient perception of the level of treatment burden has a profound impact on the net benefits of A1c lowering (Figure 1). For example, in an otherwise favorable scenario (diabetes onset at age 45, and a 15% risk reduction in CHD per unit decrease in A1c), a high treatment burden of 0.05 (equivalent to 18.2 days of high-quality life lost per year, a level often reported by people on insulin22–24) outweighs all benefits of glycemic control. Indeed, the model predicts that patients will lose between 0.653 and 0.818 QALYs even when treatments improve glycemic control by 1%. The treatment burden at which reducing A1c by 1 point results in net harm ranges between 0.01 and 0.05, depending on other key factors like patient age and pre-treatment A1c (Supporting table 1).
Figure 1.
QALYs gained or lost by age and treatment burden
This figure relates the QALYs gained or lost by a treatment that leads to a 1% reduction in A1c (from 8.5% to 7.5%) across 4 age groups and views of the burden of treatment.
To provide a sense of the relative efficiency and the required duration of treatment, we also estimated the number of QALYs gained per 100 years of treatment. These estimates can be seen in Table 2. In the best case scenario, 3.47 QALYs are gained per 100 treatment-years when reducing an A1c from 8.5% to 7.5% with a low burden/side effect treatment started in a 45 year old.
Table 2.
QALYs gained per 100 years of treatment
| Disutility | ||||
|---|---|---|---|---|
|
| ||||
| 0.001 | 0.01 | 0.025 | 0.05 | |
| Age | ||||
| A1c 7.5 to 6.5% | ||||
| 45 | 2.85 | 1.76 | −0.06 | −3.05 |
| 55 | 2.15 | 1.06 | −0.73 | −3.68 |
| 65 | 1.43 | 0.37 | −1.37 | −4.26 |
| 75 | 0.83 | −0.19 | −1.87 | −4.67 |
| Age | ||||
| A1c 8.5 to 7.5% | ||||
| 45 | 3.47 | 2.36 | 0.52 | −2.50 |
| 55 | 2.65 | 1.56 | −0.26 | −3.24 |
| 65 | 1.78 | 0.72 | −1.05 | −3.96 |
| 75 | 1.04 | 0.01 | −1.69 | −4.50 |
Assuming a 15% reduction in CHD risk per 1 point reduction in A1c
We also examined two representative treatment scenarios, one examining the impact of starting metformin and another of starting insulin (Table 3). Metformin was assumed to be started at diagnosis, and reduced A1c from 8.5 to 7.0%. Metformin, which has relatively small treatment disutility due to lack of weight gain and minimal risks of hypoglycemia, produces benefits across the age spectrum (ranging from 0.148 QALYs in a 75 year old to 1.2 QALYs in a 45 year old). The reductions in individual endpoints are also shown in table 3; for example, the absolute risk reduction in ESRD risk is almost 10 times greater for a 45 year old (0.065) than in a 75 year old (0.007).
Table 3.
Treatment scenarios*
| A. Initiation of metformin at diagnosis
| |||||
|---|---|---|---|---|---|
| Age | Lifetime QALY gain | ARR ESRD | ARR Visual loss | ARR amputation | ARR First MI |
| 45 | 1.200 | .065 | .021 | .027 | .026 |
| 55 | 0.714 | .042 | .016 | .022 | .040 |
| 65 | 0.359 | .021 | .010 | .015 | .037 |
| 75 | 0.148 | .007 | .005 | .008 | .027 |
| B. Switch to insulin at 10 years after diagnosis
| |||||
|---|---|---|---|---|---|
| Age at diagnosis | Lifetime QALY gain | ARR ESRD | ARR Visual loss | ARR amputation | ARR First MI |
| 45 | −0.495 | .013 | .004 | .004 | .010 |
| 55 | −0.380 | .007 | .002 | .003 | .008 |
| 65 | −0.239 | .003 | .001 | .002 | .006 |
| 75 | −0.102 | 0.001 | .000 | .001 | .003 |
Metformin was assumed reduce A1c from 8.5 to 7.0% and had a mean disutility of 0.004. Insulin was started after 10 years of oral therapy, reduced A1c from 8.5 to 7.5%, and had a mean disutility of 0.037. For derivation of these, see Model Parameters. Abbreviations: ARR = absolute risk reduction; ESRD = end-stage renal disease
In our second example, insulin was started after 10 years of oral agents after a gradual rise in A1c from 7.0 to 9.0%; insulin reduced A1c back to 8.0%.50 In contrast to the consistent benefits of metformin, the switch to insulin produces a negative effect on QALYs across all age groups; that is, the side effects and burdens of treatment, based on literature estimates and accumulated over time, outweigh the benefits of improved glycemic control. In addition to insulin’s higher treatment burden, the treatment benefit is lower in this scenario because of smaller A1c reduction relative to metformin and because the patient is now 10 years older, reducing the time available to achieve benefit. For example, the absolute risk reduction in ESRD is 0.013 when initiating insulin at age 55, as compared to the 0.065 from starting metformin 10 years earlier.
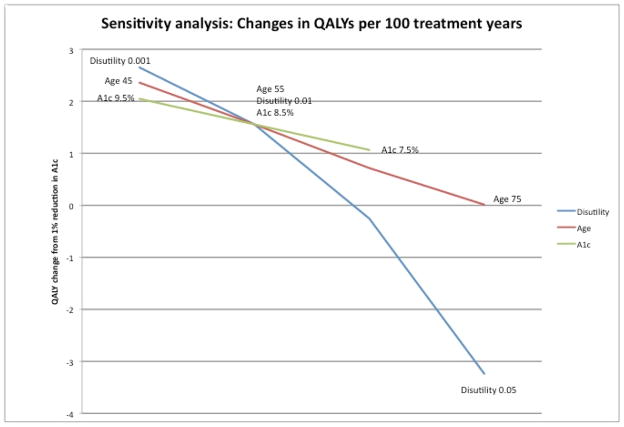
Figure 2 shows the results of varying key parameters in our sensitivity analyses. The estimates of QALYs gained per year of treatment are shown for a representative patient, 55 years old with an A1c of 8.5%, a treatment disutility of 0.01, and with an expected 15% reduction in CHD per 1 point reduction in A1c. Each parameter is varied across a reasonable range; larger effects are demonstrated by a greater change in QALYs across the ranges of the variables. The importance of treatment burden relative to the other variables is apparent.
Figure 2.
Sensitivity analysis
This figure demonstrates the variability in gains in QALYs from a 1% reduction in A1c for various age, utility, and starting A1c values.
Further sensitivity analyses showed that our results were robust to changes in most other model parameters (see technical appendix). A key additional parameter was the relationship between albuminuria and cardiovascular events. If albuminuria is causally related to higher CHD rates, and this effect is additive to the assumed 15% reduction posited for a 1% change in A1c, then the benefits of glycemic control are larger (Supporting table 2). However, it is more likely that any effect of prevention of albuminuria is already captured by the assumption that A1c reduction leads to a reduction in CHD.
Discussion
A growing body of research has accepted that the benefit of diabetes treatment is complicated by variation in patient clinical circumstances and treatment burden, so that no single A1c target is appropriate for all patients.14–17,25,53,54 However, operationalization of this concept is limited; our results help to inform the decision making process for patients and providers. We found that once moderate A1c control (9%) is achieved, patient views of the burdens of treatment are the most important factor in the net benefit of glucose lowering treatments. Thus, higher quality decision-making is best achieved by individualizing treatment decisions (“What are the burdens and benefits of starting a new medication on this patient?”), not solely by individualizing A1c targets (“What should this patient’s A1c target be?”).55
Although in this paper we used QALYs to facilitate comparing different potential disease complications and treatment burdens, clinicians may find our results for outcome-specific (heart attack, ESRD, etc.) absolute risk reductions to be more useful for thinking about individual patient decisions. These estimates of the potential benefits of A1c reduction can provide clinicians a means of considering and balancing treatment benefits with the burdens of glucose lowering treatments. Although there is no consensus on the optimal approach to implementing shared decision-making in practice, having fairly concrete estimates of treatment benefit is particularly necessary because evidence suggests that most clinicians vastly overestimate treatment benefits, and few consider treatment burden explicitly.56
In addition to providing information to aid clinical decision making, our results challenge the wisdom of the current A1c centered approach to quality measures and clinical research. Instead of current recommendations and performance measures based on achievement of specific A1c goals, our results suggest that quality of diabetes care is more accurately defined by assessing whether high benefit treatment is provided and whether informed decision-making process is used when potential benefits are more modest.57 Implementing such measures will be more challenging than current approaches, but accessing high priority care is already feasible through medical record review. With the spread of the electronic medical record, measures of high benefit care can be automated, since A1c, current diabetes medications and age are readily available in most EMRs. In addition to identification of whether high value treatment is provided appropriately, the EMR can also facilitate and document shared decision-making, without making treatments with uncertain net benefit into standards of care.58,59 Eddy has argued that an intervention should be considered a ‘standard’ only if there is ‘virtual unanimity among patients [emphasis ours] about the overall desirability [of treatment]”.60 Our results show that given variability in individual preferences, it is unlikely that there will be “virtual unanimity” for most glycemic treatment decisions. For example, even for a 45 year old with an A1c of 8.5%, insulin therapy can easily result in net harm for someone with a moderate dislike of insulin treatment (disutility = 0.05).24
Our finding that older patients experience smaller benefits from glycemic control is not unexpected,6,14,39,45,61 but the degree is noteworthy. We estimate that the expected gain in quality-adjusted life years for a 1 point change in A1c in a 75-year old is 0.06 years (22 days), even with the favorable assumption that glycemic control’s cardiovascular benefit extends to the elderly.
Glycemic medications continue to be approved and marketed based almost entirely on whether they help achieve A1c targets. Our findings provide further reason to favor evaluating diabetes medications with clinically-relevant endpoints, rather than A1c alone, as has been suggested in recent trials, and argues against using new, expensive medications with minimal safety data on the basis of achieving A1c targets.55,62 Our findings also support the importance of developing and approving medications that are both safe and have fewer side effects and inconvenience, as for many patients, treatment burden is the primary consideration in determining the net benefit of treatment.
Given how influential treatment burden is in our study, it is important to note that these burdens are not easy to quantify.22–24 For this reason, we ran the models using treatment burden as a variable, ascribing particular values to particular treatments only for the purpose of the scenarios in Table 3. However, we were conservative in our estimates; one study reported an average disutility for insulin (0.12) that was more than twice the level at which all of the patient groups have a net loss of quality of life with treatment.24 Although the act of taking a pill has little to no burden for most patients, side effects such as weight gain and hypoglycemia can confer significant burden.
The main limitation of our study relates to inherent uncertainties in the literature. Our results are robust to the ranges found in the literature for disease progression and for treatment effects. There are some scenarios that we do not consider, such as assuming a greater than average CHD benefit from treatment of younger patients. But our optimistic assumption of 15% CHD reduction per 1% A1c lowering, and our lack of discounting, are likely to result in our results being somewhat biased towards favoring glucose lowering treatment, particularly in light of data accruing from meta-analyses7–11, from FDA approval standards, and from outcome studies of incretin-based and other therapies.63–66
In our base case we did not model potential increase in mortality seen in ACCORD.18 Inclusion of this in scenarios for the most intensive targets (A1c 7.5% to 6.5%) led to net harm in all patients, but the implications of ACCORD remain controversial. We also did not model any direct medication effects, such as the mortality benefit from metformin observed in the UKPDS that was independent of A1c reduction.21 Finally, we used median survival and since the health of the median person is quite good in all age groups examined in our study, it is important to note that our results by age mainly apply to those in relatively good health. Those with reduced life expectancy due to comorbidities are likely to receive less benefit than those reported in the tables.
There are important known mediators of diabetes complications other than A1c. Our results show that the benefits of blood pressure and statin therapy dwarf those for glycemic control; for example, in previous analyses using the same model, we found that simvastatin, prescribed at 20 mg/d in the highest-benefit patients, led to over 30 QALYs gained per 100 years of treatment.15 This is more than 8 times higher than the highest-benefit group achieves with glycemic control (Table 2).
In summary, we found that net treatment benefits of glycemic treatments vary widely depending on a patient’s age at diagnosis, their pre-treatment A1c, and most importantly, a patient’s view of the burden of the specific treatment being considered. Because of this, using A1c treatment targets alone to guide patient decision-making is a fundamentally flawed strategy; instead, each glycemic treatment decision should be individualized, based mostly on patients’ views of the burdens of therapy, with age and initial level of glycemic control important but secondary considerations. Thus, shared decision-making, where patient preferences are specifically elicited and considered, appears to be the best approach to making most decisions about glycemic management in patients with type 2 diabetes. This study provides a starting point to the implementation of such an approach. To make optimal decisions, clinicians and patients will need decision support and incentives to engage in discussions with their patients that incorporate their values.67 Currently, we are failing our patients by not recognizing that their preferences and views of treatment burden are the most important factor in helping patients make glycemic treatment decisions that are best for them.
Supplementary Material
Acknowledgments
Financial Support was provided via grants from the Department of Veterans Affairs HSR&D (IIR 06-253) and Quality Enhancement Research Initiative (QUERI DIB 98-001), and the Michigan Center for Diabetes Translational Research (NIDDK of The National Institutes of Health [P60 DK-20572]). The funding agencies were not involved in the design and conduct of the study; collection, management, analysis, or interpretation of the data; in preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Disclosures: No conflicts on interest regarding this work.
References
- 1.National Committee for Quality Assurance. HEDIS 2013 [Internet] 2013 Available from: http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures/HEDIS2013.aspx.
- 2.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes care. 2013;36 (Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 4.Accord Study Group, Accord Eye Study Group. Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. The New England journal of medicine. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England journal of medicine. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Annals of internal medicine. 1997;127:788–95. doi: 10.7326/0003-4819-127-9-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 8.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. Bmj. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yudkin JS, Richter B, Gale EA. Intensified glucose lowering in type 2 diabetes: time for a reappraisal. Diabetologia. 2010;53:2079–85. doi: 10.1007/s00125-010-1864-z. [DOI] [PubMed] [Google Scholar]
- 10.CONTROL GROUP. Turnbull FM, Abraira C, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–98. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 11.Hemmingsen B, Lund SS, Gluud C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. Bmj. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Annals of internal medicine. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 13.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. Journal of general internal medicine. 2005;20:479–82. doi: 10.1111/j.1525-1497.2005.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang ES, Shook M, Jin L, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new-onset diabetes. Diabetes care. 2006;29:259–64. doi: 10.2337/diacare.29.02.06.dc05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timbie JW, Hayward RA, Vijan S. Variation in the net benefit of aggressive cardiovascular risk factor control across the US population of patients with diabetes mellitus. Archives of internal medicine. 2010;170:1037–44. doi: 10.1001/archinternmed.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Geriatrics Society. Choosing wisely: Five things physicians and patients should question [Internet] 2013 Available from: http://americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/choosingwisely.
- 17.Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with D. Guidelines for improving the care of the older person with diabetes mellitus. Journal of the American Geriatrics Society. 2003;51:S265–80. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 18.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Archives of internal medicine. 2010;170:1191–201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 22.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. The European journal of health economics: HEPAC: health economics in prevention and care. 2011;12:219–30. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 23.Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2007;16:1251–65. doi: 10.1007/s11136-007-9226-0. [DOI] [PubMed] [Google Scholar]
- 24.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes care. 2007;30:2478–83. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554–9. doi: 10.7326/0003-4819-154-8-201104190-00007. [DOI] [PubMed] [Google Scholar]
- 26.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA: the journal of the American Medical Association. 2000;283:889–96. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 27.Schmittdiel J, Vijan S, Fireman B, Lafata JE, Oestreicher N, Selby JV. Predicted quality-adjusted life years as a composite measure of the clinical value of diabetes risk factor control. Medical care. 2007;45:315–21. doi: 10.1097/01.mlr.0000254582.85666.01. [DOI] [PubMed] [Google Scholar]
- 28.Rosen AB, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-effectiveness of full medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Annals of internal medicine. 2005;143:89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- 29.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA: the journal of the American Medical Association. 1988;260:2864–71. [PubMed] [Google Scholar]
- 31.The Diabetes Control and Complications Trial Research Group. The Relationship of Glycemic Exposure (HBA1c) to the Risk of Development and Progression of Retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 32.Early Treatment Diabetic Retinopathy Study Research Group. Early Photocoagulation for Diabetic Retinopathy: ETDRS Report Number 9. Ophthalmology. 1991;98:766–85. [PubMed] [Google Scholar]
- 33.Ferris FL. How effective are treatments for diabetic retinopathy? JAMA. 1993;269:1290–1. [PubMed] [Google Scholar]
- 34.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 35.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 36.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 37.Ballard DJ, Humphrey LL, Melton LJ, 3d, et al. Epidemiology of persistent proteinuria in type II diabetes mellitus. Population-based study in Rochester, Minnesota. Diabetes. 1988;37:405–12. doi: 10.2337/diab.37.4.405. [DOI] [PubMed] [Google Scholar]
- 38.Humphrey LL, Ballard DJ, Frohnert PP, Chu CP, O’Fallon WM, Palumbo PJ. Chronic renal failure in non-insulin-dependent diabetes mellitus. A population-based study in Rochester. Minnesota Ann Intern Med. 1989;111:788–96. doi: 10.7326/0003-4819-111-10-788. [DOI] [PubMed] [Google Scholar]
- 39.The CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of Intensive Glycemic Control, Intensified Hypertension Control, and Serum Cholesterol Level Reduction for Type 2 Diabetes. JAMA. 2002;287:2542–51. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 40.Diabetes in America. 2. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995. [Google Scholar]
- 41.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. American heart journal. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data [Internet] 2013 Available from: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes09_10.aspx.
- 43.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. I. Model construction and assumptions [see comments] Diabetes Care. 1997;20:725–34. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- 44.Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. JAMA: the journal of the American Medical Association. 1996;276:1409–15. [PubMed] [Google Scholar]
- 45.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia [see comments] Diabetes Care. 1997;20:735–44. doi: 10.2337/diacare.20.5.735. [DOI] [PubMed] [Google Scholar]
- 46.Augustovski FA, Cantor SB, Thach CT, Spann SJ. Aspirin for primary prevention of cardiovascular events. J Gen Intern Med. 1998;13(12):824–35. doi: 10.1046/j.1525-1497.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. 2006;144(5):326–36. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 48.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes care. 2010;33:1859–64. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Current medical research and opinion. 2006;22:1523–34. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 50.Hayward RA, Manning WG, Kaplan SH, Wagner EH, Greenfield S. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA: the journal of the American Medical Association. 1997;278:1663–9. [PubMed] [Google Scholar]
- 51.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. European heart journal. 2009;30:834–9. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. The New England journal of medicine. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 53.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–96. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 54.Aron D, Conlin PR, Hobbs C, Vigersky RA, Pogach L. Individualizing glycemic targets in type 2 diabetes mellitus. Ann Intern Med. 2011;155(5):340–1. doi: 10.7326/0003-4819-155-5-201109060-00025. [DOI] [PubMed] [Google Scholar]
- 55.Strain WD, Lukashevich V, Kothny W, Hoellinger M-J, Paldánius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382(9890):409–16. doi: 10.1016/S0140-6736(13)60995-2. [DOI] [PubMed] [Google Scholar]
- 56.Price HC, Thorne KI, Dukát A, Kellett J. European physicians overestimate life expectancy and the likely impact of interventions in individuals with Type 2 diabetes. Diabet Med. 2009;26(4):453–5. doi: 10.1111/j.1464-5491.2009.02702.x. [DOI] [PubMed] [Google Scholar]
- 57.Loxterkamp D. Humanism in the time of metrics--an essay by David Loxterkamp. BMJ. 2013;347:f5539. doi: 10.1136/bmj.f5539. [DOI] [PubMed] [Google Scholar]
- 58.Hayward RA. All-or-nothing treatment targets make bad performance measures. The American journal of managed care. 2007;13:126–8. [PubMed] [Google Scholar]
- 59.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Annals of internal medicine. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 60.Eddy DM. Clinical decision making: from theory to practice. Designing a practice policy. Standards, guidelines, and options. JAMA: the journal of the American Medical Association. 1990;263:3077, 3081, 3084. doi: 10.1001/jama.263.22.3077. [DOI] [PubMed] [Google Scholar]
- 61.Management of Diabetes Mellitus Update Working Group. VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus. Version 4.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010. [Google Scholar]
- 62.Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle H-J. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)61500-7. [DOI] [PubMed] [Google Scholar]
- 63.Food and Drug Administration. Guidance for Industry: Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes [Internet] 2008 Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071627.pdf.
- 64.Food and Drug Administration. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting [Internet] 2012 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM340145.pdf.
- 65.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013;369(14):1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 66.White WB, Cannon CP, Heller SR, et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med. 2013;369(14):1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 67.Legare F, Turcotte S, Stacey D, Ratte S, Kryworuchko J, Graham ID. Patients’ perceptions of sharing in decisions: a systematic review of interventions to enhance shared decision making in routine clinical practice. The patient. 2012;5:1–19. doi: 10.2165/11592180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Brown GC, Brown MM, Sharma S, Brown H, Gozum M, Denton P. Quality of life associated with diabetes mellitus in an adult population. Journal of diabetes and its complications. 2000;14:18–24. doi: 10.1016/s1056-8727(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 69.Tsevat J, Goldman L, Soukup JR, et al. Stability of time-tradeoff utilities in survivors of myocardial infarction. Medical decision making: an international journal of the Society for Medical Decision Making. 1993;13:161–5. doi: 10.1177/0272989X9301300210. [DOI] [PubMed] [Google Scholar]
- 70.Mathias SD, Bates MM, Pasta DJ, Cisternas MG, Feeny D, Patrick DL. Use of the Health Utilities Index with stroke patients and their caregivers. Stroke; a journal of cerebral circulation. 1997;28:1888–94. doi: 10.1161/01.str.28.10.1888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.