Abstract
Depression is associated with receipt of higher doses of prescription opioids. It is not known if the reverse association exists in that an increased opioid dose is associated with increased depression. Questionnaires were administered to 355 patients with chronic low back pain (CLBP) at baseline, 1 year and 2 year follow-up. Depression, pain, anxiety, health related quality of life (HRQL) and social support/stress were obtained by survey. Opioid type and dose and comorbid conditions were derived from chart abstraction. Random intercept, generalized linear mixed models were computed to estimate the association between change in opioid morphine equivalent dose (MED) thresholds (0, 1–50, >50 mg) and probability of depression over time. Second, we computed the association between change in depression and odds of increasing MED over time. After adjusting for covariates, an increase to >50mg MED from non-use increased a participant’s probability of depression over time (OR=2.65; 95%CI: 1.17–5.98). An increase to 1–50 mg MED did not increase an individual’s probability of depression over time (OR=1.08; 95%CI: 0.65–1.79). In unadjusted analysis, developing depression was associated with a 2.13 (95%CI: 1.36–3.36) increased odds of a higher MED. This association decreased after adjusting for all covariates (OR=1.65; 95%CI: 0.97–2.81). Post-hoc analysis revealed depression was significantly associated with a 10.1 mg MED increase in fully adjusted models. Change to higher MED leads to increase risk of depression and developing depression increases likelihood of higher MED. We speculate that treating depression or lowering MED may mitigate a bi-directional association and ultimately improve pain management.
Keywords: opioids, depression, cohort, epidemiology
INTRODUCTION
For decades, research literature has supported a correlation between pain and depression.[10] Persons with depression report greater sensitivity to painful stimuli, report more severe pain scores and are vulnerable to catastrophizing in response to pain.[6; 10; 13] Numerous reports have established that chronic non-cancer pain patients with depression, compared to those without, are more likely to receive opioids,[21] use opioids for longer periods of time,[3; 16] use higher daily morphine equivalent doses (MED),[12] and misuse and or abuse prescription opioids.[7; 17] Longitudinal data suggests depression is a risk factor for opioid use. Sullivan et al.[21] report that subjects in a community cohort who had a psychiatric illness, including depression, compared to subjects free of a diagnosis at baseline, were twice as likely to be opioid users three years later. Whether opioid use leads to depression is less well understood. At this time, we are aware of only one study designed to determine if the reverse pattern of association exists, that is, do patients who use opioids in larger amounts or for longer duration have an increased risk of new onset depression. After controlling for bias by indication in a retrospective cohort design, increasing duration of opioid use was associated with increasing risk of depression in analysis of data from Veterans Administration (VA) medical records.[18]
A better understanding of the temporal relationship between opioids and depression and the dose of opioids that places patients at risk for depression may inform prescribing and pain management and improve outcomes for chronic, non-cancer, pain patients. In addition to improving pain management, elucidating the nature of the opioid-depression association has public health implications. In 2010, hydrocodone (with acetaminophen),[1] was the most prescribed medication in the United States, and the rate of prescribing opioids increased dramatically in the past 30 years and was not accompanied by parallel increases in painful conditions.[5] Because the prevalence of opioid use is so large, the opioid – depression association is likely a serious, yet poorly understood, public health problem. In particular, if opioids lead to depression and increased severity of depression is associated with more opioid use, it is critical to understand what MED places users at risk to begin identifying where to intervene to break the opioid-depression association.
To determine if patients who increase MED are at risk for increased depression and if patients with increased depression experience increased MED we analyzed data obtained from a cohort of treatment seeking, primary care patients with chronic low back pain, from whom three waves of data were collected prospectively over a 2 year period. Our first objective was to determine if increases to a higher MED (0 mg, 1–50 mg, >50 mg) over time increased individual probability of depression over time. Our second objective was to determine if developing depression over time increased individual probability of higher MED over time. For both objectives, we computed associations before and after adjusting for pertinent covariates, including pain and health related quality of life(HRQL).
METHODS
Subjects
Patients were eligible for the study if they had a diagnosis of non-cancer chronic low back pain on their problem list, and they were regular users of family medicine clinics, defined as 2 or more visits in the past 24 months.
Procedure
Medical students recruited subjects during routine outpatient visits from nine practices of the Residency Research Network of Texas (RRNeT). The RRNeT is a collaboration between AGME-accredited family medicine residency programs in Texas (http://iims.uthscsa.edu/RRNeT/home) and serves as a productive resource for primary care research. [4; 23–26] Students invited potential subjects to participate in a study to examine how pain and health change over time, and how pain medicines are used to manage changes in low back pain. Baseline (Wave 1) was assessed in 2008 and 2009 with follow-up data collection performed at 12 months (Wave 2) and 24 months (Wave 3) after enrollment. At baseline, informed consent was obtained, and participants completed a patient survey, addressing pain, health and function; then the medical students completed chart abstractions addressing diagnoses, comorbidities and prescriptions. At Waves 2 and 3, students gathered survey data in person if patients had a scheduled appointment, or by telephone if patients did not have an appointment at the time of their scheduled follow-up assessments. Details of data collection and subject recruitment have been previously reported.[26] Among 362 patients enrolled at baseline, 337 participated in Wave 2 and 199 in Wave 3. Only 7 subjects were missing data on Baseline measures, resulting in 355 eligible subjects at baseline, 330 at Wave 2 and 194 at Wave 3. We investigated potential non-response bias by computing the distribution of covariates (i.e. demographics, social support/stress and pain duration) across wave and observed no significant difference in the distribution of these variables from baseline, wave 2 and wave 3.
As described below, missing data is accounted for in the analytic design. The IRBs of all participating institutions approved the study procedures and consent form.
Measures
Opioid use
Specific opioid medications and dose were abstracted from medical charts at baseline and each follow-up wave. If an opioid prescription was managed by a provider other than the primary care clinic, this information was in the patient chart because study clinic providers obtained the patient’s current medication list. Chart abstraction obtained the current average daily MED for the patient’s current opioid prescription based on the prescribed type and amount of the following 9 opioids: codeine fentanyl, hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, and propoxyphene. MED was modeled as none, low (1–50 mg/day) and high dose (>50 mg/day) based on previous studies of the association between depression and MED which used the 50 mg and similar thresholds.[2; 18; 26]
Depression
The PHQ-2[9] was administered at each wave to measure depression over the past 30 days. A score of 3 or more indicates probable depression with sensitivity =82.9% and specificity=90.0%. The PHQ-2 has been shown to accurately detect worsening, improving and unchanged depression in outpatients.[11] For all analysis, depression was modeled as a binary variable (yes vs. no).
Covariates were selected based on the theoretical and empirical evidence that each is correlated with opioid use and depression. Covariates included characteristics of pain, social support/stress, health related quality of life (HRQL), physical comorbidities, obesity and anxiety. Pain characteristics included the pain level on an average day in the 30 days prior to survey. Pain level was measured on a 10 point likert scale with 0 equal to none and 10 equal to severe pain. Duration of pain in years was assessed with subjects’ self-report. Participants addressed social support/stress by reporting the number of persons who are sources of stress and sources of support (possible range: 0 to 10). HRQL was measured by the RAND, SF-36 subscales for physical functioning, role physical and general health as well as the SF-36 question for pain interference.[14] Comorbidities were obtained from chart abstraction of the patient’s problem list containing up to 20 conditions. Obesity was derived from chart abstracted height and weight. Last, anxiety was considered present if subjects reported feeling anxious on several or more days in the past 30 days or having a panic attack in the past two weeks. Sociodemographic variables included age, race, gender, disability status, and education.
Analytic Approach
Sociodemographic variables, pain duration and social support/stress were modeled as time independent covariates from baseline status. MED, pain severity, PHQ-2 scores, HRQL, comorbidities, anxiety and BMI were measured at all three waves and were treated as time dependent covariates. The longitudinal nature of the data and analyses only excluded missing waves of data per participant. For example, if a subject did not participate at Wave 3, his or her Wave 1 and Wave 2 data were still utilized in analyses. Thus, all 355 original participants contributed data to the analysis, with 21 (5.9%) contributing one wave of data, 144 (40.6%) contributing two waves, and 190 (53.5%) contributing all three waves of data. Covariate differences by depression status were assessed separately for baseline, Wave 2 and Wave 3. Differences were examined via a chi-square test or a 2-sample independent t-test.
Longitudinal analysis conditioned the probability of depression at each wave on covariates and MED. Random intercept, generalized linear mixed model analyses (Proc Glimmix, Adaptive Quadrature Method, SAS v9.3) examined changes in probability of depression based on changes in opioid use over time, controlling for the effects of different covariate groupings. Model fit and quality were assessed using Akaike's Information Criterion(AIC) and Pseudo R-squared estimates. Pseudo R-squared estimates were derived using methods described by Snijders and Bosker.[19]
Unadjusted models measured the association between changes in MED and changes in depression over time. Models were expanded by adding 1) pain characteristics, 2) HRQL measures, 3) comorbidities, obesity and anxiety, 4) social support, social stress and 5) demographics. All models included a ‘time’ variable, modeled as time since baseline in years (0, 1, or 2) which controls for each participant’s natural course of depression over the study period.
For objective 2, analyses were repeated, except that change in depression was used to predict change in MED over time. Objective 2 analyses utilized mixed ordinal logistic regression models because of the ordinal nature of the MED variable (none, 1–50 mg, >50mg) and the proportional odds assumption was met. Results are interpreted as an individual’s odds of a higher opioid dose versus all lower categories of doses based on changes in probability of depression over time. All analyses were computed using SAS v. 9.3.[8]
RESULTS
As shown in Table 1, respondents were mostly female (72.4%), older than 46 years (75.2%), mostly of minority race (57.5%) and the majority had a high school education or greater (78.6%). The distribution of socio-demographic variables did not significantly change across baseline, Wave 2 and Wave 3.
Table 1.
Cohort characteristics and sample size for baseline, Wave 2 and Wave 3 follow-up.
| Variable | Baseline (n=355) |
Wave 2 (n=330) |
Wave 3 (n=194) |
p-value |
|---|---|---|---|---|
| Age | ||||
| 18 to 45 | 24.8% | 23.9% | 24.2% | 0.96 |
| 46 to 59 | 44.2% | 44.2% | 46.9% | |
| 60 and over | 31.0% | 31.8% | 28.9% | |
| Race | ||||
| White, non-Hispanic | 42.5% | 42.1% | 45.9% | 0.68 |
| Other | 57.5% | 57.9% | 54.1% | |
| Gender | ||||
| Male | 27.6% | 27.3% | 30.9% | 0.63 |
| Female | 72.4% | 72.7% | 69.1% | |
| Education | ||||
| < High school | 21.4% | 21.2% | 19.1% | 0.79 |
| ≥ High school | 78.6% | 78.8% | 80.9% | |
| Applying for/On disability | 49.3% | 47.9% | 58.8% | 0.93 |
The association between depression and patient characteristics at baseline, Wave 2 and Wave 3 is shown in Table 2. Baseline depression was significantly associated with baseline MED. Subjects with depression were significantly (p<0.05) more likely to be on 1–50 mg and >50 mg MED per day and less likely to be non-opioid users. At baseline, subjects with depression, compared to those without, had lower educational achievement(p<0.01), and were significantly more likely to be in the ‘applying for or on disability group’ (p<0.001). Subjects with depression reported more stressful relationships and fewer socially supportive relationships (p<0.01), higher pain severity (p<0.001), more comorbid conditions (p<0.001), worse functioning for each SF-36 subscale, (p<0.001) and were more likely to have anxiety at baseline (p<0.001).
Table 2.
Association between patient characteristics and depression at baseline, Wave 2 and Wave 3 follow-up
| Baseline | Wave 2 | Wave 3 | ||||
|---|---|---|---|---|---|---|
| Variable | Not depressed (n=147) |
Depressed (n=201) |
Not depressed (n=127) |
Depressed (n=104) |
Not depressed (n=75) |
Depressed (n=68) |
| Opioid use | ||||||
| No use | 61.2% | 45.8%* | 53.7% | 40.6% | 59.5% | 37.9%* |
| 50 mg or lower | 32.7% | 45.8% | 39.0% | 44.5% | 23.0% | 36.4% |
| > 50 mg | 6.1% | 8.5% | 7.3% | 14.9% | 17.6% | 25.8% |
| Sociodemographics | ||||||
| Age: | ||||||
| 18 to 45 | 25.8% | 24.4% | 22.8% | 19.2%* | 28.0% | 23.5% |
| 46 to 59 | 38.1% | 49.7% | 35.4% | 53.9% | 34.7% | 47.1% |
| 60 and over | 36.1% | 25.9% | 41.7% | 26.9% | 37.3% | 29.4% |
| Race: | ||||||
| White, non-Hispanic | 47.6% | 39.8% | 53.5% | 35.6%** | 58.7% | 39.7%* |
| Other | 52.4% | 60.2% | 46.5% | 64.4% | 41.3% | 60.3% |
| Gender: | ||||||
| Male | 25.2% | 29.8% | 21.3% | 26.9% | 26.7% | 27.9% |
| Female | 74.8% | 70.2% | 78.7% | 73.1% | 73.3% | 72.1% |
| Education: | ||||||
| < High school | 14.3% | 27.4%** | 14.2% | 27.9%** | 13.3% | 20.6% |
| ≥ High school | 85.7% | 72.6% | 85.8% | 72.1% | 86.7% | 79.4% |
| Applying for/On disability: | 33.3% | 61.2%*** | 36.2% | 55.8%** | 30.7% | 58.8%*** |
| Psychosocial characteristics | ||||||
| # close stressful people (mean± sd) | .09 ± 1.1 | 1.3 ± 1.2** | 1.0 ± 1.2 | 1.2 ± 1.1 | 0.9 ± 1.2 | 1.3 ± 1.2 |
| # close supportive people (mean± sd) | 3.0 ± 1.9 | 2.4 ± 1.7** | 2.9 ± 1.9 | 2.5 ± 1.7 | 3.3 ± 2.0 | 2.6 ± 1.7* |
| Pain Characteristics | ||||||
| Pain severity (mean± sd) | 6.0 ± 2.3 | 7.3 ± 2.0*** | 5.6 ± 2.9 | 7.4 ± 2.1*** | 5.4 ± 2.6 | 6.8 ± 2.3** |
| Pain duration (years; mean± sd) | 13.5 ±13.4 | 13.7 ±13.3 | 13.4 ± 12.6 | 15.9 ± 14.9 | 15.3 ± 16.1 | 15.4 ± 12.2 |
| Health Related Quality of Life2 | ||||||
| SF - Pain interference (mean± sd) | 44.3 ± 26.8 | 22.5 ± 22.3*** | 46.6 ± 22.6 | 25.2 ± 22.4*** | 49.3 ± 28.1 | 25.4 ± 22.2*** |
| SF- Physical functioning (mean± sd) | 43.4 ± 28.7 | 25.0 ± 23.0*** | 47.6 ± 29.4 | 23.5 ± 22.1*** | 49.7 ± 31.4 | 31.8 ± 26.8*** |
| SF - Role physical (mean± sd) | 19.7 ± 31.4 | 6.5 ± 17.2*** | 22.8 ± 33.7 | 4.6 ± 14.4*** | 23.1 ± 36.5 | 8.5 ± 21.0*** |
| SF - General health (mean± sd) | 53.0 ± 23.7 | 32.4 ± 21.4*** | 48.9 ± 23.8 | 30.8 ± 22.0*** | 50.9 ± 24.9 | 35.3 ± 20.9*** |
| Comorbidities | ||||||
| # comorbidities (mean± sd)3 | 2.4 ± 1.7 | 3.1 ± 1.8*** | 3.3 ± 2.1 | 3.6 ± 2.1 | 2.8 ± 1.8 | 4.0 ± 2.1** |
| Anxiety – yes | 59.9% | 96.0%*** | 58.3% | 84.6%*** | 38.7% | 89.7%*** |
| Obese/overweight | 79.9% | 82.7% | 84.1% | 89.5% | 78.5% | 85.3% |
p<0.05,
p<0.01,
p<0.001
1) morphine equivalent dose, 2) RAND-SF36 subscales, higher scores indicate better functioning, 3) comorbid conditions from patient chart, 4 ) of the 330 patients that completed wave 2, there were 234 patients who completed the survey and 322 who had chart data; out of the 194 patients in wave 3, 144 patients completed the survey and 191 patients had chart data.
Subjects with depression at Wave 2 remained more likely to be high dose opioid users in Wave 2. In Wave 2, 14.9% of the depressed subjects used >50mg MED compared to 7.3% of the non-depressed; however, this association was not statistically significant. The associations between subjects with depression at Wave 2 and their pain level, SF-36 subscale scores and anxiety at Wave 2 were significant and followed a pattern similar to the one observed at baseline. Although not significant at baseline, age and race were significantly associated with depression in Wave 2. Subjects with depression at Wave 2 were more 46–59 years of age (p<0.05) and non-white (p<0.01). At Wave 2, the number of comorbid conditions increased overall and was no longer significantly greater among depressed subjects.
Subjects with depression at Wave 3 were also more likely(p<0.05) to be high dose (>50mg MED) opioid users and less likely to be non-users. The covariates significantly associated with depression at Wave 3 were the same as those at baseline. As in previous waves, greater pain severity (p<0.01), lower SF-36 subscale scores (p<0.001) and anxiety (p<0.001) at Wave 3 remained significantly associated with Wave 3 depression. Subjects with depression in Wave 3 were still more likely to be non-whites and seeking or applying for disability. In Wave 3, depression was no longer significantly associated with age and education. Last, the mean number of comorbid conditions in depressed patients was significantly (p<0.01) larger than in non-depressed Wave 3 respondents.
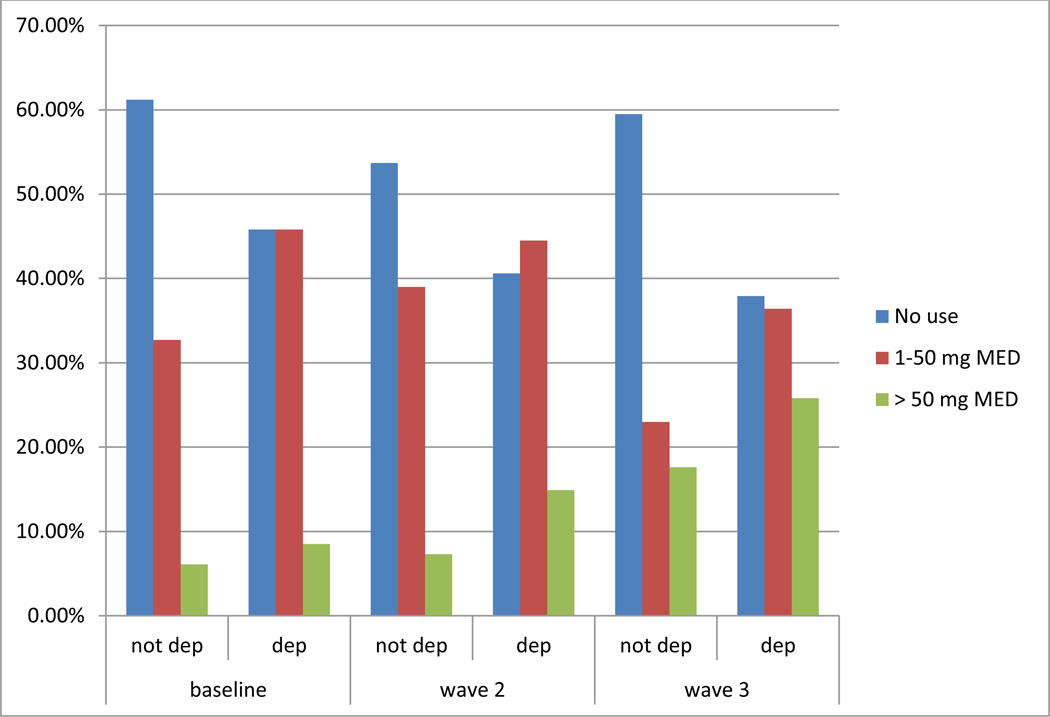
To better illustrate the longitudinal association of MED and depression, the first three rows of table 2 were plotted in Figure 1. As shown, there is a clear increase from wave 1 to wave 3 in the proportion of patients with depression receiving 50 mg MED per day.
Figure 1.
Association between morphine equivalent dose (MED) and depression (dep) over 3 waves of data collection with chronic low back pain patients in primary care
The results of generalized linear mixed models predicting changes in depression from changes in opioid use are shown in Table 3. In Model 1, the unadjusted probability of having depression was significantly greater when a subject was on >50mg MED as opposed to when he or she was taking no opioids (OR=3.32; 95% CI: 1.43–7.69). Similarly, relative to when a subject was not using opioids, if that subject increased his or her dose to 1–50 mg per day, they had a higher probability of depression, but at a lower magnitude (OR=1.99; 95% CI:1.19–3.31) than if they had increased to >50mg per day.
Table 3.
Mixed logistic regression models measuring the association (Odds Ratio (95%CI)) between change in morphine equivalent opioid dose (MED) and change in depression over time before and after adjusting for covariates.
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
Model 5 OR (95% CI) |
Model 6 OR (95% CI) |
Model 7 OR (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Opioid use | |||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≤ 50 mg | 1.99 (1.19–3.31) |
1.45 (0.87–2.41) |
1.12 (0.67–1.85) |
2.00 (1.24–3.22) |
1.84 (1.11–3.06) |
1.71 (1.03–2.82) |
1.08 (0.65–1.79) |
| > 50 mg | 3.32 (1.43–7.69) |
2.95 (1.30–6.68) |
1.81 (0.81–4.04) |
3.25 (1.47–7.20) |
3.09 (1.35–7.10) |
2.96 (1.30–6.78) |
2.65 (1.17–5.98) |
|
Pain Characteristics | |||||||
| Pain Severity | 1.40 (1.25–1.56) |
1.05 (0.93–1.19) |
|||||
| Pain Duration (yrs) | 1.01 (0.99–1.03) |
1.02 (0.99–1.04) |
|||||
|
Health Related Quality of Life | |||||||
| SF-Pain Interfere | 0.97 (0.96–0.99) |
0.98 (0.96–0.99) |
|||||
| SF-Physical Func | 0.99 (0.98–1.00) |
0.99 (0.98–1.01) |
|||||
| SF-Physical Role | 1.00 (0.99–1.01) |
1.00 (0.99–1.01) |
|||||
| SF-Gen Health | 0.97 (0.96–0.98) |
0.99 (0.98–1.00) |
|||||
|
Comorbidities | |||||||
| # comorbidities | 1.11 (0.99–1.26) |
1.04 (0.92–1.19) |
|||||
| Anxiety: | 12.13 (6.50–22.64) |
8.33 (4.39–15.81) |
|||||
| Obese/over weight | 1.29 (0.68–2.42) |
0.87 (0.45–1.69) |
|||||
|
Psychosocial characteristics | |||||||
| # stressful people | 1.50 (1.17–1.93) |
1.32 (1.05–1.65) |
|||||
| # support people | 0.78 (0.67–0.92) |
0.92 (0.81–1.06) |
|||||
|
Demographic characteristics | |||||||
| Age: | |||||||
| ≥ 60 | 0.80 (0.38–1.70) |
0.72 (0.34–1.53) |
|||||
| 46–59 | 1.46 (0.73–2.90) |
1.10 (0.59–2.05) |
|||||
| 18–45 | 1.00 | 1.00 | |||||
| Race: Non-white | 1.73 (0.97–3.10) |
1.75 (1.03–2.98) |
|||||
| Education: < high school | 3.03 (1.46–6.27) |
1.57 (0.83–2.98) |
|||||
| Gender: female | 0.82 (0.44–1.52) |
0.61 (0.35–1.05) |
|||||
| Disability Status: On/apply | 3.28 (1.82–5.92) |
1.00 (0.59–1.70) |
|||||
|
Model Information | |||||||
| Pseudo R-square | 19.25% | 25.42% | 36.82% | 30.60% | 21.44% | 24.92% | 48.28% |
| AIC | 914.40 | 873.70 | 781.10 | 759.56 | 899.55 | 879.32 | 667.30 |
All models adjusted for years since baseline assessment.
As shown in Model 2, pain severity over the study period, but not duration of pain at baseline, was significantly associated with an increased probability of depression (OR=1.40; 95%CI:1.25–1.56). After adjusting for pain severity and pain duration, the probability of a subject who used ≤ 50 mg MED per day having depression over the study period was no longer statistically significant. However if a subject increased from no use to using >50mg per day he or she had a significantly greater probability of depression (OR=2.95; 95%CI:1.30–6.68).
In Model 3, the probability of depression in a subject who used 1–50 mg MED per day, and the probability of depression in a subject who used >50 mg MED per day, both decreased after adjusting for SF-36 subscales. In Model 3, higher SF-Pain and SF-General Health scores, indicating better HRQL, were significantly associated with a lower probability of depression (OR=0.97;95%CI:0.96–0.99 and OR=0.97; 95%CI: 0.96–0.98, respectively). After adjusting for SF-36 subscales, an increase from no use to 1–50 mg MED and to >50 mg MED per day was no longer significantly associated with increased odds of depression (OR=1.12; 95%CI:0.67–1.85 and OR=1.81; 95%CI:0.81–4.04, respectively). This effect did not remain in the full model 7.
The odds ratio measuring the association between opioid MED and depression was similar for the unadjusted Model 1, Model 4 (adjusting for number of comorbidities, anxiety, and BMI) and Model 5 (adjusting for number of stressful and supportive social relationships). After adjusting for demographic variables in Model 6, the associations between an increase from no use to 1–50 mg MED and to >50 mg MED remained significantly associated with depression (OR=1.71; 95%CI:1.03–2.82 and OR=2.96; 95%CI:1.30–6.78, respectively). Last, after simultaneous adjustment for all covariates in Model 7, pain was no longer significantly associated with depression (OR=1.05;95%CI:0.93–1.19), and relative higher functioning for a subject in SF-Pain remained associated with lower risk of depression (OR=0.98; 95%CI: 0.96–0.99). In Model 7, a subject had a statistically significant increased probability of depression when he or she changed MED from no use to >50 mg MED per day (OR=2.65; 95%CI:1.17–5.98), but not if change was from no use to 1–50 mg MED per day (OR=1.08; 95%CI:0.65–1.79).
The results of the ordinal logistic mixed models are shown in Table 4. In the unadjusted model (Model 1), developing depression was associated with more than a 2-fold increased probability of changing to a higher MED as opposed to a lower MED (OR=2.13; 95% CI: 1.36–3.36). When adjusting for pain characteristics in Model 2, a subject was still at greater risk for increasing to a higher MED as opposed to lower MED when depressed, but the effect was attenuated (OR=1.85; 95% CI: 1.17–2.92). Model 2 also showed that with each one unit increase of pain severity a subject had a 14% increased probability of changing to a higher MED as opposed to lower MED (OR=1.14; 95% CI: 1.03–1.25). The greatest attenuation of the association between change in depression and change to a higher MED was observed after adjusting for health related quality of life (SF-36 subscales) in Model 3, (OR=1.38; 95% CI: 0.86–2.22). When simultaneously adjusting for all covariates in model 7, a subject who changed from non-depressed to depressed had a 65% increased odds of having a higher as opposed to lower MED, although this effect was not significant (OR=1.65; 95% CI: 0.97–2.81; p=.06).
Table 4.
Mixed ordinal logistic regression models measuring the association (Odds Ratio (95%CI)) between change in depression and change in morphine equivalent opioid dose (MED) over time before and after adjusting for covariates.
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
Model 5 OR (95% CI) |
Model 6 OR (95% CI) |
Model 7 OR (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Depression | 2.13 (1.36–3.36) |
1.85 (1.17–2.92) |
1.38 (0.86–2.22) |
2.38 (1.43–3.97) |
2.03 (1.28–3.21) |
1.95 (1.23–3.10) |
1.65 (0.97–2.81) |
|
Pain Characteristics | |||||||
| Pain Severity | 1.14 (1.03–1.25) |
1.03 (0.91–1.18) |
|||||
| Pain Duration (yrs) | 1.00 (0.98–1.02) |
1.00 (0.97–1.02) |
|||||
|
Health Related Quality of Life | |||||||
| SF-Pain Interfere | 0.99 (0.98–1.00) |
0.99 (0.98–1.01) |
|||||
| SF-Physical Func | 0.99 (0.98–1.00) |
0.99 (0.98–1.00) |
|||||
| SF-Physical Role | 0.99 (0.98–1.00) |
0.99 (0.98–1.01) |
|||||
| SF-Gen Health | 1.00 (0.99–1.01) |
1.01 (1.00–1.02) |
|||||
|
Comorbidities | |||||||
| # comorbidities | 1.17 (1.01–1.34) |
1.12 (0.97–1.29) |
|||||
| Anxiety: | 0.76 (0.42–1.39) |
0.75 (0.41–1.38) |
|||||
| Obese/over weight | 0.46 (0.22–0.94) |
0.74 (0.35–1.52) |
|||||
|
Psychosocial characteristics | |||||||
| # stressful people | 1.03 (0.81–1.32) |
1.01 (0.78–1.31) |
|||||
| # support people | 0.86 (0.74–1.01) |
0.88 (0.75–1.04) |
|||||
|
Demographic characteristics | |||||||
| Age: | |||||||
| ≥ 60 | 0.99 (0.46–2.13) |
0.66 (0.27–1.63) |
|||||
| 46–59 | 0.99 (0.49–1.98) |
0.80 (0.38–1.68) |
|||||
| 18–45 | 1.00 | 1.00 | |||||
| Race: Non-white | 0.57 (0.31–1.02) |
0.55 (0.30–1.03) |
|||||
| Education: < high school | 0.58 (0.28–1.18) |
0.59 (0.27–1.26) |
|||||
| Gender: female | 0.69 (0.37–1.28) |
0.61 (0.33–1.16) |
|||||
| Disability Status: On/apply | 3.64 (2.01–6.59) |
2.51 (1.31–4.82) |
|||||
|
Model Information | |||||||
| Pseudo R-square | 42.41% | 42.89% | 45.33% | 44.46% | 42.69% | 44.94% | 48.09% |
| AIC | 1250.03 | 1245.4 | 1205.24 | 1140.85 | 1250.51 | 1232.73 | 1104.16 |
All models adjusted for years since baseline assessment
DISCUSSION
In a cohort of 355 CLBP, primary care patients assessed for pain severity, depression and opioid use over 3 waves of data collection, we observed that increasing to a higher daily MED (>50 mg) also significantly increased individual probability of depression over time. This association remains even after adjusting for repeated measures of pain severity in the month prior to each survey. Better HRQL partly accounted for this association in sub models. But in the full model, after adjusting for all covariates, an increase to >50 mg MED was significantly associated with a participant’s greater probability of depression. This suggests HRQL only influences the MED to depression pathway under select combinations of other covariates. Consistent with our previous study,[18] change to a lower daily MED (1–50 mg MED) did not increase individual risk of depression in a full model. In our previous work[18] low dose <38 mg MED was not associated with increased risk of depression even in long term, greater than 180 day users. The present study replicates our previous findings in a VA patient population. Replication in the present cohort of primary care patients residing in Texas, using different measures of depression and pain, and different analytic approach, provides compelling evidence that high dose opioid use over time is an independent risk factor for developing depression.
Results of objective 2 indicate there is a significant increased odds (OR=2.13; 95%CI:1.36–3.36) of a subject with depression receiving a higher daily MED over the study interval. Adjusting for HRQL greatly attenuated this association (OR=1.38), suggesting the relationship between change from non-depressed to depressed and change to larger MED is partly explained by lower HRQL. However, simultaneous adjustment for all covariates leads us to conclude that there is evidence, albeit marginally non-significant, that developing depression leads to a participant’s increased likelihood of change to a higher MED (OR=1.65;95%CI:0.97–2.81). The present categorization of MED into three ordinal groups reduced statistical power. At baseline, only 6.1% of non-depressed and 8.5% of depressed subjects had >50mg MED. Thus, to increase statistical power we re-evaluated treating MED as a continuous variable in post-hoc analysis. In the post-hoc general linear mixed model regression, we observed that a patient who becomes depressed over time has a corresponding MED increase of 10.1 mg which remained significant after adjusting for all covariates shown in model 7 (p<0.01). Thus depression is significantly associated with patients increasing opioid dose. Although our observation that depression is associated with greater opioid use has been previously reported,[3; 12; 16; 20–22] the present finding is novel in identifying the association between an increase in opioid use and increase in depression.
The present study and our previous research[18] point to the amount of daily morphine exposure, and not just the duration of exposure, as the contributing factor for new onset depression. Additional data collection is needed to determine if patients are at risk due to past depressive episodes or recent depression symptoms. Another plausible pathway to incident depression due to high dose MED is opioid abuse. High dose opioid use is associated with risk of opioid misuse and abuse[12; 15] and prospective data is needed to determine if relationship dysfunction, job loss, family disruption and additional life consequences associated with opioid abuse are in the pathway from high dose MED to new onset depression. Last, determining the covariate combinations for which HRQL partly mediates the opioid to depression association could identify those subjects for whom high dose opioid use leads to depression independent of changes in quality of life and functioning.
Depression may be associated with greater sensitivity to pain and higher MED required to control pain symptoms. Others[26] have offered explanations for why depressed patients receive more opioid prescriptions which include patients using opioids for emotional regulation resulting in using more often than pain symptoms warrant and thereby requesting and receiving larger doses. We speculate that greater pain sensitivity leads to higher MED that in turns precipitates or worsens depression leading to continued or worsened pain sensitivity and patient requests for more opioids. Last, depression may contribute to opioid misuse,[26] and we speculate a bi-directional relationship could be mediated by substance abuse.
Limitations
The present study is limited by geographic region and may not generalize beyond ambulatory care patients, but as described above, our primary results replicated our previous study in a national VA patient cohort. Depression was assessed by self-report using the PHQ-2. This instrument is a good screener to detect probable depression in the past month but is not the gold standard for psychiatric diagnosis and it does not measure lifetime history of depression. Thus we are unable to account for the effect of depression prior to baseline. Dates of depression onset were not collected, thus onset of depression could have been a few months after exposure or up to a year after increasing dose. Longitudinal studies that include diagnostic interviews are warranted to determine which depression symptoms onset first, symptom duration and the characteristics of depression (cognitive vs. somatic) associated with opioid exposure.
Pain severity was reported for CLBP. Results may not generalize to other painful conditions (e.g. fibromyalgia). It is possible that changes in depression and MED occurred between assessments. This may limit our ability to identify how quickly change in MED or depression occurs and prevents assessment of the lag time between change in value of the exposure variable and change in value of the outcome variable. Our categories of MED do not provide information on other levels of high dose use. Therefore we computed a fully adjusted model with MED categories being none, 1–50mg, 51–100 mg and >100 mg. After adjusting for all covariates shown in Table 3, Model 7, increasing to >100 mg was associated with 3.66 (95%CI:1.13–11.89) odds of developing depression which suggests a dose-response relationship. Last, we are not able to determine if the present study has identified a bi-directional relationship or a cyclical one due to insufficient sample size and insufficient waves of follow-up data.
Conclusions
Results support the conclusion that use of opioids at a dose equal or greater than 50 mg MED per day is associated with increasing depression, and worsening depression is associated with increased MED. These associations remain after accounting for the influence of worsening pain severity and HRQL. Providers should consider current opioid dose when pain patients present with depression. Both providers and patients should be aware and discuss the risk of depression when considering opioid medications that equate to more than 50 mg MED per day. Providers should routinely screen for depression in patients receiving more than 50 mg of opioid per day and have frank and open discussions with patients prior to increasing dose beyond 50 mg.
The present study does not establish causation but results do support evidence for both directions of association, morphine dose to depression and depression to morphine dose, and suggest the possibility that a bi-directional relationship does exist. Additional prospective cohort studies may help identify which patients, such as those with a history of major depression, are most vulnerable to developing depression when using prescription opioids.
Summary.
An increase in opioid use was associated with an increase in depression and an increase in depression was associated with an increase in opioid use.
ACKNOWLEDGMENTS
Funding: Health Resources and Services Administration (Award # D54HP16444); Texas Academy of Family Physicians Foundation; the Office of the Medical Dean of the University of Texas Health Science Center at San Antonio; and the National Center for Research Resources (Award # UL 1RR025767). The funder had no role in the design, conduct, analysis and interpretation of the data. The funder had no role in the decision to submit this manuscript for publication.
Footnotes
The authors have no conflicts of interest involving the work under consideration for publication, relevant financial activities outside of the submitted work or other relationships or activities that give the appearance of potential conflicts of interest.
Prior presentations: none
REFERENCES
- 1.IIfH Informatics, editor. The use of medicines in the United States: Review of 2010. 2011. [Google Scholar]
- 2.Braden JB, Russo J, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31(6):564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burge SK, White D, Bajorek E, Bazaldua O. Correlates of Medication Knowledge and Compliance: Findings from RRNeST. Fam Med. 2005;37(10):712–718. [PubMed] [Google Scholar]
- 5.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Fishbain D, Cutler R, Rosomoff H, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Grattan A, Sullivan M, Saunders K, Campbell C, Von Korff M. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10:304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute S. Base SAS 9.3 Procedures Guide. Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- 9.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2. Validity of a two-item depression screener. Medical care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay PG, Wyckoff M. The depression-pain syndrome and its response to antidepressants. Psychosomatics. 1981;22:571–573. doi: 10.1016/S0033-3182(81)73478-9. [DOI] [PubMed] [Google Scholar]
- 11.Lowe B, Kroenke K, Grafe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J Psychosom Res. 2005;58:163–171. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Merrill JO, Von Korff M, Banta-Green CJ, Sullivan MD, Saunders KW, Campbell CI, Weisner C. Prescribed opioid difficulties, depression and opioid dose among chronic opioid therapy patients. Gen Hosp Psychiatry. 2012;34(6):581–587. doi: 10.1016/j.genhosppsych.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moix J, Kovacs FM, Martin A, Plana MN, Royuela A Network. atSBPR. Catrastrophizing, state anxiety, anger, and depressive symptoms do not correlate with disability when variations of trait anxiety are taken into account. A study of chronic low back pain patients treated in Spanish pain units. Pain Medicine. 2011;12:1008–1017. doi: 10.1111/j.1526-4637.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- 14.Otis JD, Keane TM, Kerns RD, Monson C, Scioli E. The development of an integrated treatment for veterans with comorbid chronic pain and posttraumatic stress disorder. Pain medicine (Malden, Mass) 2009;10(7):1300–1311. doi: 10.1111/j.1526-4637.2009.00715.x. [DOI] [PubMed] [Google Scholar]
- 15.Paulozzi LJ, Zhang K, Jones CM, Mack KA. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J Am Board Fam Med. 2014;27:329–338. doi: 10.3122/jabfm.2014.03.130290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, Pahor M, Furberg CD. Assessment and control for confounding by indication in obsrvational studies. J Am Geriatr Soc. 1999;47:749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 17.Rassen JA, Glynn RJ, Brookhart MA, Schneeweiss S. Covariate selection in high-dimensional propensity score analyses of treament effects in small samples. Am J Epidemiol. 2011;173(12):1404–1413. doi: 10.1093/aje/kwr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, Lawler EV, Lustman PJ. Prescription opioid analgesics and risk of depression. Journal of General Internal Medicine. 2014;29:491–499. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snijders TABBR. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage Publications; 1999. [Google Scholar]
- 20.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: Association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166(19):2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan MDEM, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 23.Young RA, Bayles B, Benold T, Hill JH, Kumar KA, Burge SK. Family physicians' perceptions on how they deliver cost-effective care. Fam Med. 2013;45(5):311–318. [PubMed] [Google Scholar]
- 24.Young RA, Bayles B, Hill JH, Kumar KA, Burge S. Family Physicians' Opinions on the Primary Care Documentation, Coding, and Billing System: A Qualitative Study from the Residency Research Netwrok of Texas. Fam Med. 2014;46(5):378–384. [PubMed] [Google Scholar]
- 25.Young RA, Bayles B, Hill JH, Kumar KA, Burge S. Family Physicians' Suggestions to Improve the Documentation, Coding, and Billing System: A Study from the Residency Research Network of Texas. Fam Med. 2014;46(6):470–472. [PubMed] [Google Scholar]
- 26.Young RA, Benold T, Whitham J, Burge SK. Factors influencing work interference in patients with chronic low back pain: A Residency Reasearch Network of Texas (RRNeT) Study. J Am Board Fam Med. 2011;24:503–510. doi: 10.3122/jabfm.2011.05.100298. [DOI] [PubMed] [Google Scholar]