Abstract
Mitochondrial fission and fusion proteins are highly expressed in myocardium. However, mitochondrial fission and fusion are rare, and mitochondrial networks are absent, in adult cardiomyocytes, obviating a need for morphometric mitochondrial remodeling. The critical role of mitochondrial dynamics factors in hearts therefore remains to be determined. In this issue of Circulation Research Ikeda et al describe a central function for the mitochondrial fission protein, Dynamin-related protein 1 (Drp-1), in macroautophagy and mitochondrial autophagy. Together with two other recent reports that cardiac-specific deletion of Drp1 perturbs mitophagy, these findings point to modulation of targeted mitochondrial elimination as a major quality control function for Drp1, and possible other mitochondrial dynamism factors, in the heart.
Conventional wisdom is that mitochondria continuously undergo cyclic fission and fusion, collectively termed “mitochondrial dynamism”. While observable in cells having filamentous mitochondria, mitochondrial dynamism is less evident in adult cardiac myocytes. Indeed, cardiomyocyte mitochondria appear inherently “fragmented”, i.e. stubby/ovoid rather than elongated/filamentous in shape. In non-myocytes, fragmentation of filamentous mitochondria is observed when the balance between mitochondrial fission and fusion is shifted in favor of fission, as during apoptosis1. Accordingly, inhibition of mitochondrial fission mediated by the pro-fission protein Dynamin-related protein (Drp)-1 limits post-ischemic cardiac injury2, 3, in which programmed cardiomyocyte cell death is believed to contribute to long-term heart dysfunction4. Whereas these findings support a role for Drp1-mediated mitochondrial fission in cardiac injury, the normal homeostatic function of Drp1 in the comparatively static mitochondria of the adult heart has not been evaluated.... until now. Three nearly simultaneous descriptions of cardiac-specific Drp1 gene deletion mice, one of which is published in this edition of Circulation Research 5, have revealed not only that Drp1 is essential for normal cardiac function, but that its primary impact may be on autophagy/mitophagy rather than on structural mitochondrial remodeling.
Drp1 is a member of the dynamin family of GTPases. It is recruited to and, via head-to-tail oligomerization and constriction, mediates the fission of mitochondria6. Thus, deletion of Drp1 prevents mitochondrial fission; hence the title of this editorial: “Gone Fission”. In the 1997 motion picture with the same homonymous name, “Gone Fishin’” (co-written by young J.J. Abrams), Joe Pesci and Danny Glover enter a contest and win a free fishing trip, but multiple catastrophes ensue. Pretty much the same thing can be said of “Gone Fission” provoked by cardiac Drp1 deletion.
In a magnum opus of studies defining the consequences of Drp1 insufficiency in cardiomyocytes, the Sadoshima group5 first examined neonatal rat ventricular cardiomyocytes (NRVC) 96 hours after ~75% suppression of Drp1 by adenoviral expression of Drp1 shRNA. The expected consequence of inhibiting Drp1-mediated mitochondrial fission is mitochondrial elongation (from unopposed fusion) and, although quantitative mitochondrial morphometry was not performed, increased numbers of cells having atypically large mitochondria were observed. It is widely believed that suppressing fission will be beneficial by promoting fusion-mediated complementation and interrupting apoptosis7, 8 (although there are exceptions9, 10). Unexpectedly, Ikeda, et al found the opposite: Suppressing mitochondrial fission impaired mitochondrial health and increased metrics of apoptosis and mitochondrial permeability transition pore (MPTP) opening. These abnormalities occurred at baseline, and were exaggerated in response to the macroautophagic stimulus of nutrient deprivation. The researchers linked mitochondrial abnormalities induced by Drp1 deficiency to increased mitochondrial content and impaired autophagy, including decreased autophagic mitochondrial clearance. The investigators were careful to draw a clear distinction between mitochondrial involvement in macroautophagy and mitophagy mediated by the canonical PINK-Parkin mechanism8, which they did not specifically interrogate.
Ikeda, et al translated their in vitro observations to the in vivo heart using Cre-mediated cardiomyocyte-specific Drp1 gene ablation. They observed no viable cardiac Drp1 mice after combining homozygous floxed Drp1 alleles (Drp1 fl/fl) with standard myh6-Cre (designed to induce late embryonic/early postnatal cardiomyocyte-specific Drp1 gene deletion). Thus, they performed their studies in an adult conditional model: tamoxifen-induced activation of myh6-MER-Cre-Mer X Drp1 fl/fl mice; Drp1 ablation was induced at 15 weeks of age. The number of mice and the time points studied were limited, but conditional cardiac Drp1 knockout (KO) mice developed mitochondrial abnormalities similar to Drp1-suppressed NRVC, i.e. elongated mitochondria, increased mitochondrial content, and mitochondrial dysfunction. The cardiac phenotype was hypertrophy with impaired contractility and diastolic non-compliance. Cardiac Drp1 KO mice all died with evidence of left heart failure between 8 and 13 weeks after Drp1 deletion; the most detailed cardiac phenotyping was performed 4 weeks after gene deletion, which reflects developing abnormalities rather than the terminal effects of Drp1 deficiency. As in the NRCM, autophagy was decreased, and markers of apoptosis and MPTP opening were increased. Ikeda et al conclude that absence of Drp1 impairs autophagy (including autophagic mitochondrial clearance), thus promoting accumulation of abnormal mitochondria, which is ultimately associated with increased cardiomyocyte death from apoptosis and/or necrosis. This work therefore functionally links mitochondrial fission, autophagic mitochondrial quality control, and programmed cell death in the heart.
A second paper described the consequences of cardiac Drp1 ablation in myh6-Cre X Drp1 fl/fl mice11. This is the same genetic approach that Ikeda et al attempted without obtaining viable homozygous Drp1 KO mice5. The Kageyama cardiac Drp1 KO mice were born at a frequency consistent with Mendelian predictions (suggesting no prenatal lethality), but homozygous KO mice then all died between 7 and 9 days thereafter. Perhaps because there were few Drp1 KO mice for study the cardiac phenotype was not characterized in great depth: Drp1 KO mouse hearts were modestly hypocontractile and bradycardic, with diminished P-wave amplitude. Studies of isolated mitochondria were not performed, but mitochondrial respiratory enzyme activity was diminished and neonatal cardiomyocyte respiration was impaired. Multiple assays of cardiomyocyte apoptosis were negative.
Kageyama et al observed ultrastructural mitochondrial enlargement with increased branching and connectivity in Drp1 KO hearts11. Greater numbers of structures resembling mitochondria-containing autophagosomes were observed and mitophagy markers (mitochondrial-associated p62 and ubiquitin) were increased, consistent with accelerated early mitophagy. However, mitochondrial delivery to lysosomes (measured as colocalization of mitochondrial pyruvate dehydrogenase, ubiquitin, and lysosomal Lamp1) appeared impaired. Intriguingly, crossing the cardiac Drp1 KO mice with Parkin KO mice (which manifest few cardiac abnormalities until aging unmasks accelerated senescence12) provoked even greater contractile dysfunction and bradycardia in 3 double KO mice. The authors conclude that Drp1 deficiency promotes mitochondrial connectivity and interrupts mitophagy at the point where mitochondria are delivered to lysosomes.
In a third paper (from our laboratory), Song et al also describe early lethality (~5 weeks) with postnatal myh6-“turbo” Cre-mediated Drp1 deletion, analogous to the Kageyama cardiac Drp1 KO, but did not further study this model due to the potential for confounding compensatory influences13. Instead, Song et al produced conditional, tamoxifen-induced Drp1 deletion in myh6-MER-Cre-MER + Drp1 fl/fl mice, analogous to the Ikeda conditional cardiac Drp1 KO. Drp1 was deleted in 8 week old mice and dilated cardiomyopathy developed that proved lethal 6-8 weeks thereafter. As in the other studies, Drp1 deletion produced mitochondrial enlargement. While myocardial TUNEL staining was positive, other apoptosis markers were not. Instead, focal complement activation, increased cardiomyocyte membrane permeability, and partial phenotypic reversal with MPTP inhibition suggested that myocardial fibrosis was attributable to programmed cardiomyocyte necrosis. Mitochondrial-associated p62 and LC3 were increased and mitophagosomes were abundant, suggesting accelerated mitophagy. Likewise, Cre-mediated Drp1 deletion in cultured murine fibroblasts increased mitophagy (measured as Parkin translocation to mitochondria and mitochondrial-lysosomal co-localization) in a time-and MPTP-dependent manner. Mitochondrial content was modestly decreased by multiple quantitative metrics in both conditional Drp1-deficient fibroblasts and hearts, and studies of isolated mitochondria showed no loss of polarization, impairment of substrate-stimulated respiration, or increase in reactive oxygen species production. Song et al concluded that loss of Drp1 impairs quality control through the normal asymmetric fission mechanism. As overall mitochondrial fitness is progressively challenged, however, cell-wide mitophagy is increasingly induced that ultimately promotes generalized mitochondrial depletion13.
The major cardiac findings from the three studies are compared in Table 1. Each study has strengths and weaknesses: For example, quantitative assays of individual mitochondrial morphometry and more detailed functional studies, especially of isolated mitochondria, might have provided additional insight in the Ikeda study5. For the Kageyama paper11, the influence of postnatal cardiac development on stress related to mitochondrial dynamism14, absence of detailed studies of isolated mitochondria, and the limited number of study animals are limitations. It would also have been preferable to directly assess Parkin involvement in Drp1-modulated mitochondrial autophagy, rather than infer Parkin's role based on crossing with the germ-line Parkin knockout mouse; there is solid evidence that opportunistic compensation in this mouse makes it a “knockout” that is not necessarily a complete or specific loss-of-function model10, 15. Finally, Song et al focused exclusively on Parkin-mediated mitophagy without evaluating alterations in macroautophagy or any altered response to stress that may be provoked by Drp1 deletion13; if the Song hypothesis is correct then mitochondrial loss in Drp1 deficient hearts should provoke a metabolic crisis, and interruption of Parkin-mediated mitophagy should improve it. Neither of these aspects were directly evaluated.
Table 1.
Comparative findings of cardiac-specific Drp1 KO mice.
| Ikeda, et al5 | Kageyama, et al11 | Song, et al13 | ||
|---|---|---|---|---|
| Drp1 KO model | Adult (15 wk) cardiac KO | Postnatal cardiac KO | Postnatal cardiac KO | Adult (8wk) cardiac KO |
| Lethality | 8-13 wks p KO | Age 9-11 days | Age 4-6 wks | 7-8 wks p KO |
| Heart size | Hypertrophy | Normal | Normal | Dilated |
| LV EF (%) | Decreased | Decreased | n.r. | Decreased |
| Histology | Fibrosis | n.r. | Normal | Fibrosis |
| Apoptosis | Yes | No | n.r. | No |
| Necrosis | Yes | n.r. | n.r. | Yes |
| Mitochondria phenotype | ||||
| Mito size | Enlarged/elongated | Branched/connected | n.r. | Enlarged/elongated |
| Mito content | Increased | Increased | n.r. | Progressively decreased |
| Respiration | Decreased (cells) | Decreased (cells) | n.r. | Normal (mito) |
| Respiratory complexes | Selectively decreased | Selectively decreased | n.r. | Selectively decreased |
| Oxidative stress | Increased | Increased | n.r. | Normal |
| Mitochondrial biogenesis | Unchanged | n.r. | n.r. | Decreased |
| MPTP | Increased opening | n.r. | n.r. | Increased opening |
| Autophagy/Mitophagy phenotype | ||||
| Autophagic flux | Decreased | Unchanged | n.r. | n.r. |
| Mitophagy | Decreased | Increased but not completely executed | n.r. | Progressively increased |
Results as characterized in the manuscripts without consideration of experimental methodology or alternate interpretation. “n.r.” means not reported.
What can we learn about the functioning of Drp1 in hearts from these studies? Many questions are raised and the need is clear for additional experimentation, especially to distinguish between respiratory dysfunction at the cellular and organelle levels. However, it seems safe to conclude the following: 1. Drp1 is absolutely essential for normal homeostatic functioning of neonatal and adult hearts. Either postnatal11, 13 or conditional adult5, 13 cardiomyocyte-specific ablation of Drp1 compromised hearts. As noted by Ikeda et al5, this finding warrants caution in therapeutically employing Drp1 inhibitors for prolonged periods of time, although this approach may still prove safe and efficacious for short-term use in acute cardiac ischemia2, 3. 2. Having non-fragmented/hyper-fused mitochondria is not necessarily cardioprotective. Mitochondrial fission is frequently considered to be “bad” and mitochondrial fusion to be “good”8, 16. By inference, fragmented mitochondria are less desirable than hyper-fused mitochondria. Consistent with this notion, cardiomyocyte- specific interruption of mitochondrial fusion (by combined Mfn1 and Mfn2 deletion) produces unusually small, toxic mitochondria and dilated cardiomyopathy13, 14, 17. However, the studies reviewed above prove that mitochondrial elongation by Drp1 deletion can be as detrimental as fragmentation evoked by mitofusin deletion. 3. Drp1 functions in cardiac mitophagy signaling. Although the nature of the dysfunction is unclear, each of the three cardiac Drp1 KO mouse studies uncovered abnormalities in autophagy/mitophagy. The non-conformity of mechanistic inferences may be attributable to direct versus compensatory effects in the conditional and non-conditional Drp1 KO mouse models, or to the different battery of tests applied to the various in vivo and in vitro Drp1 deficiency models. However, it is possible to integrate the findings into a unified paradigm (Figure 1). Drp1-mediated mitochondrial fission is essential to prophylactic mitochondrial quality control through segregation and separation of damaged from healthy components prior to selective mitophagy of the depolarized daughter organelle18. Absent Drp1, asymmetric division, selective triage, and targeted mitophagy of damaged components cannot occur5. For this reason, mitochondrial damage will accumulate over time (and may even be accelerated as organelle fusion promotes cross-contamination) until a threshold for mitophagy is achieved without mitochondrial fission, and mitophagy then removes parent organelles. In the adult heart and cultured fibroblasts, Parkin-mediated mitophagy appears intact13, whereas in the immediate postnatal heart Parkin-independent mechanism may dominate, and mitophagy is incomplete11. Testing this notion will require detailed studies of conditional cardiac Drp1 KO mice as the cardiac phenotype progresses, with and without an intact Parkin mechanism, possibly by combining conditional cardiac ablation of Parkin and Drp1. These are early days and there is much to learn.
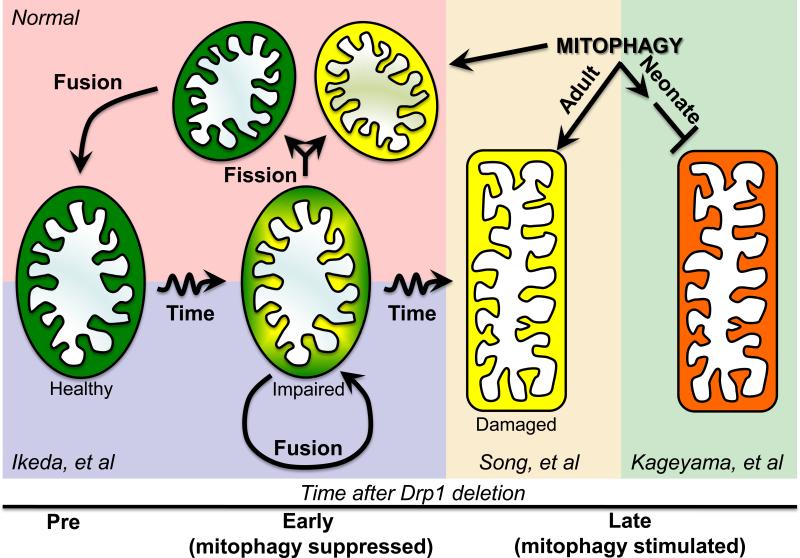
Figure 1. Mechanistic paradigm to explain different cardiac Drp1 KO findings.
The normal role of Drp1-mediated asymmetric fission is shown in upper right (red background), as envisioned by Twig et al18. Impaired/senescent mitochondria undergo asymmetric fission to produce a healthy, fusion competent daughter (green) that is retained, and a depolarized daughter (yellow) that is removed by mitophagy. As described by Ikeda et al5, an early consequence of Drp1 deletion is interruption of asymmetric fission, retaining impaired mitochondria that fuse with similarly impaired partners (blue background). With more time after Drp1 deletion, absence of the normal asymmetric fission quality control pathway ultimately produces widespread activation of mitophagy (yellow background), which Song, et al13 indicate is intact in adult hearts and evokes mitochondrial loss. In neonatal hearts, Kageyama, et al11 suggest that prototypical Parkin-mediated mitophagy does not play a major role in mitochondrial removal after asymmetric fission. Drp1 deletion in this context stimulates mitophagy that is interrupted before lysosomal incorporation (green background).
Acknowledgements
Supported in part by NIH HL59888.
Footnotes
The author declares no conflicts.
References
- 1.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 2.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 3.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res. 1997;81:137–144. doi: 10.1161/01.res.81.2.137. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects the Heart Against Energy Stress. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.303356. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 6.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833:1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell. 2008;29:409–410. doi: 10.1016/j.molcel.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan RS, Konstantinidis K, Wei AC, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW., 2nd Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res. 2014;114:257–265. doi: 10.1161/CIRCRESAHA.114.302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawsn VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014 doi: 10.15252/embj.201488658. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubli DA, Quinsay MN, Gustafsson AB. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun Integr Biol. 2013;6:e24511. doi: 10.4161/cib.24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd Mitochondrial fusion and fission factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.12.011. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem Biol. 2010;17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]