Abstract
The hypothesis that immunity and inflammation participate in the pathogenesis of vascular diseases has now gained widespread recognition and stimulated work around the globe. Broadening knowledge has extended the recognition of the role of immune and inflammatory mechanisms to all of the layers of the artery, to all levels of the arterial tree, and implicated virtually all arms, cellular “players,” and effector molecules and pathways involved in these crucial host defenses, that turn against us in disease. We provide here a guide to a compendium series of papers that aimed to look forward, and broaden the traditional focus of immunopathogenesis of arterial disease, with the goal of integrating the “players” and the “layers” involved. While the field has advanced remarkably, much remains to be done, and this commentary also aims to highlight some of the gaps that future research should strive to close regarding the participation of inflammation and immunity in arterial diseases.
Keywords: vasculitis, lymph nodes, lymphocyte, aneurysm, atherosclerosis
It is no longer news that atherosclerosis is more than a mere cholesterol storage disease. Far from a passive accumulation of cholesterol debris on the artery wall, the atheroma teams with cellular and biochemical activity and communication networks modulated by multiple risk factors. Among these intersecting networks, those that involve inflammation and immunity have emerged as a central hub for integrating the multiple pathways that drive atherogenesis and the complications of this disease. 1 Recent reviews have recounted the early days of discovery of immune reactions in the atheromata and the participation of cytokines in inflammatory signaling and immunomodulation at work in the plaque. 2,3 Many excellent compilations have covered this well-trodden turf. 4-7 Indeed, these recent reviews contain catalogues of the multitude of “players” – cells and mediators implicated in immunopathogenesis of vascular diseases.
Yet, contemporary laboratory approaches have enabled a continuing expansion and integration of knowledge regarding the details of how these individual “players” participate in the cellular and molecular systems or networks at work within vessels. The series of papers assembled in this collection aim to look forward, and broaden the traditional focus of immunopathogenesis of arterial disease beyond atherosclerosis to encompass pathological processes in the entire arterial tree. We also have strived to reach beyond the intima, and incorporate exciting emerging work relating to the roles of the other layers of the artery wall in immune and inflammatory aspects of a variety of arterial diseases. Hence, our goal of integrating the “players” and the “layers” involved into a broader concept of the pathogenesis of arterial diseases.
Inflammation and immunity: a common pathway in the pathogenesis of diseases that affect all levels of the arterial tree
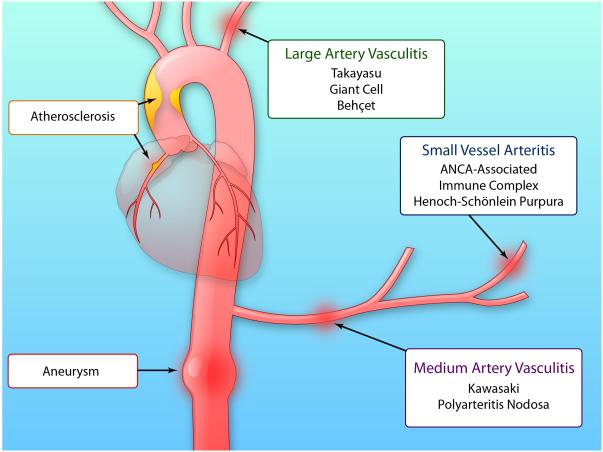
Indeed, inflammation and immunity participate not only in atherosclerosis but also in a number of other clinically important diseases that affect the arterial tree at various levels (Figure 1.) Aneurysmal disease, commonly affecting the large elastic arteries such as the aorta, previously considered a “degenerative” condition, has become understood as an active disease process. The pathways of inflammation and immunity contribute to the pathogenesis of this extreme form of arterial remodeling. At the other end of the arterial tree, a number of vasculitic processes affect the smaller muscular arteries and arterioles. Long recognized as inflammatory diseases, the arteridites involve many of the same pathways now invoked in the immunopathogenesis of diseases of the larger arteries. Thus, inflammatory and immune mechanisms influence diseases that affect arteries of all sizes.
Figure 1. The immune and inflammatory diseases affect all levels of the arterial tree and smaller vessels.
Atherosclerosis typically affects the large and medium arteries, as exemplified in this diagram by the plaque in the right coronary artery. Aneurysmal disease can affect the thoracic or infrarenal abdominal aorta as depicted in this drawing. Large and medium artery vasculitides include Takayasu and giant cell arteritis. Medium sized artery vasculitides include Kawasaki disease and polyarteritis nodosa. The small vessel vasculitides include those associated with anti-neutrophil cytoplasmic antibodies (ANCA) that recognize antigens of polymorphonuclear leukocyte such as proteinase-3 or myeloperoxidase. The ANCA-associated vasculitides include microscopic polyangiitis, granulomatosis with polyangiitis previously known as Wegener’s granulomatosis, and Churg-Strauss syndrome. Henoch-Schönlein purpura also affects smaller vessels. Immune complex vasculitides also typically affect smaller vessels. Some vasculitides affect arteries of various sizes including Behçet’s disease, relapsing polychondritis, and Cogen’s arteritis. (Illustration Credit: Ben Smith)
Inflammation and immune processes involve all layers of the arterial wall
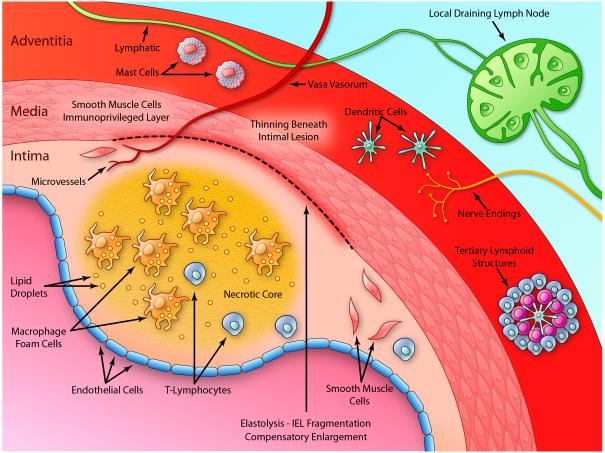
Initially, exploration of the immune and inflammatory aspects of atherogenesis focused on the intima, the site where atheromata take root. As our probing has deepened, we have come to recognize that influences arising from all three layers of arteries can influence the pathogenesis of this disease (Figure 2.) The intima itself harbors the “usual suspects” such as macrophages and T cells implicated in innate and adaptive immunity. An abundant microvasculature penetrates all the way to the intimal lesions during atherogenesis, likely arising from deeper layers of the artery and providing a communication network for both humoral and cellular elements. Innate immune cells likely help drive plaque angiogenesis. 8,9
Figure 2. The immune and inflammatory responses involve all layers of arteries.
The diagram depicts an atherosclerotic plaque as a prototypical inflammatory lesion in arteries. The intima, the usual focus in atherosclerosis research, covered by the endothelial monolayer, generally includes a fibrous cap overlying a central, lipid-rich “necrotic core”. The intima contains multiple cellular “players” including smooth muscle cells, macrophages, and T lymphocytes, among other cells involved in the innate and adaptive immune responses as described in the text. The intima may also contain a rich microvasculature that provides a portal for the entry and potential egress of leukocytes involved in immune and inflammatory responses. The internal elastic lamina (IEL) separates the intima from the media. Underneath atherosclerotic plaques, the IEL often undergoes fragmentation as depicted by the break in the dark line separating the intima from the media. The tunica media contains primarily smooth muscle cells and extracellular matrix. Indoleamine dioxygenase (IDO) produced by smooth muscle cells contributes to the immunoprivilege of the intima as described in the text. The media typically thins underlying atherosclerotic plaques. The media can grow outward during atherogenesis accommodating the growth of the plaque in an abluminal direction, a form of geometrical remodeling known as compensatory enlargement. The external elastic lamina separates the media from the adventitia. Previously often overlooked, the adventitia hosts a hotbed of immune and inflammatory activity. It furnishes the vasa vasorum that give rise to the plaque microvasculature. Lymphatic vessels provide channels for trafficking of immune cells and drain to local lymph nodes, a site of generation of immune responses to antigens implicated in arterial diseases. Organized tertiary lymphoid structures also localize in the adventitia. Dendritic cells patrol this and other regions of the plaque sampling antigens and presenting them to T lymphocytes, a vascular site of the afferent limb of adaptive immunity. Mast cells in the adventitia may also participate in arterial diseases. T cells, B cells, and plasma cells also inhabit this outermost layer of the artery wall. Nerve endings in the aventitia can secrete mediators that modulate vascular function. (Illustration Credit: Ben Smith).
The arterial tunica media has received much less attention than the intima with respect to the immune and inflammatory processes that contribute to arterial disease. Yet, much of the geometrical remodeling that characterizes diseases of the large elastic and medium-size muscular arteries must involve the media. We now recognize that inflammatory processes contribute decisively to extracellular matrix degradation (notably elastolysis) during aneurysm formation. The expansive remodeling or compensatory enlargement that occurs during the life history of evolving atheromata may indeed replicate some of the alterations in extracellular matrix metabolism that contribute, in the extreme, to aneurysm formation. 10 Thus, immune and inflammatory processes likely modulate aspects of the functions of the media, often ignored, that participate in a variety of arterial diseases. Signs of active immune and inflammatory processes appear less prominent in the medial layer of the artery, at least until the latter stages of aneurysm formation.
Recent work has disclosed mechanisms that explain this relative quiescence of the tunica media. Arterial smooth muscle cells stimulated by gamma interferon, the signature cytokine of Th1 lymphocytes, can induce indoleamine dioxygenase conferring “immunoprivilege” on this arterial layer, as explained in the Tellides and Pober contribution to this series. 11,12 While the intima hosts ongoing combat among the protagonists of the innate and adaptive immunity, an active inhibitory process appears to limit adaptive immune responses in the media. The immunoprivilege of the media may protect arterial integrity by helping to limit phlogistic inflammatory and immune responses to the intima. 13 A failure of this medial immunoprivilege could contribute to the pathogenesis of aneurysms and other diseases that affect elastic arteries. Thus, the media, far from a passive bystander, may serve as a bulwark against untrammeled spread of intimal inflammation, due to active immunosuppressant properties.
The outermost layer of the artery wall, the adventitia, has only recently received the attention it deserves. Oft ignored, like the dark side of the moon, many investigators stripped the adventitia off of specimens before study. Yet, we appreciate increasingly how the adventitia can serve as a “staging ground” for some of the immune and inflammatory struggles in the intima. 14 The rich tufts of microvessels found in many atheromatous lesions probably arise from adventitial sources. Progenitor cells and myofibroblasts inhabit the adventitia, providing a source of cells that participate in the vascular response to injury and repair. 15,16 The adventitia contains mast cells, a leukocyte subtype increasingly implicated in the pathogenesis of arterial diseases. Autonomic and nociceptive nerves travel in and around the adventitia where they may influence the neurohumoral milieu that bathes the vessel, including the different types of immune cells that normally populate this layer of the artery wall. Recent work in mice has disclosed ordered structures known as tertiary lymphoid organs in the adventitia beneath advanced atherosclerotic plaques. These observations have engendered considerable interest in the potential functions of these tertiary lymphoid structures in instigating and modulating adaptive immune responses, as discussed in the contribution of Habenicht and colleagues in this series.17
Much effort in contemporary vascular biology research has focused on the mechanisms that recruit cells of the immune and inflammatory responses to arterial lesions. We know a great deal about the adhesion molecules and chemokines that govern the entry of various inflammatory cell types into the artery wall. What happens to these cells once they have accumulated in the artery has become increasingly controversial. While monocytes and dendritic cells may traffic out of arteries as well as in, the fate of macrophages, the most numerous cell type in the atheromatous intimal lesion has become a “ hot topic.” Do these cells perish in the artery wall, is their clearance (efferocytosis) impaired under some circumstances, or do they actually egress from the artery wall, perhaps into lymph channels in the adventitia? The contribution of Gwendolyn Randolph to this series considers these various possibilities. 18 White cell trafficking in large vessel disease has focused primarily on the mononuclear leukocytes. In the vasculitides, particularly those of the smaller vessels and microvessels and especially acute and subacute forms, prominently involve granulocytes, as recounted in the forthcoming review by Sackstein.19
In sum, this series of articles on the immunopathogenesis of arterial disease attempts to broaden the traditional focus on the intima to encompass all layers of the artery wall and all sizes of vessels, areas of emerging science and likely pathophysiological importance.
The expanding repertoire of inflammatory cells and their subsets implicated in the immune pathogenesis arterial disease
In the early days of investigation of inflammation and immunity in atherosclerosis, the availability of reagents that selectively recognized T lymphocytes and macrophages permitted their rigorous identification and stimulated exploration of their functions. In the ensuing years, characterization of cells involved in inflammation has become a major theme of immunology research. As soon as immunologists have subdivided a cell type, vascular biologists have rushed to explore the implications of cellular heterogeneity for arterial diseases. Thus, the list of cellular “players” implicated in the immunopathogenesis of arterial diseases has expanded continuously. The cast of characters no longer just includes T or B lymphocytes or CD4 and CD8 T cells, but Th1 and Th2 T cells, regulatory T cells, Th17 cells and so forth. B cells likewise, come in several “flavors” as discussed in the contribution of Binder and colleagues to this series.20 Cells of the innate immune response have also undergone categorization into functional subtypes. We now recognize proinflammatory and less inflammatory subsets of monocytes and of macrophages, although the monocytic lineage appears to allow more plasticity between subtypes than the highly specialized T cell lineages. Dendritic cells also have multiple manifestations, and distinctions between macrophages and dendritic cells have engendered controversy and suggest a continuum more than cut and dried dichotomous categories. The contribution of Randolph in this series delves into some of the depths of this controversial area.18
Out of the study of the perplexing proliferation of subtypes of cells that mediate immune pathogenesis of arterial disease has come more than just “alphabet soup.” A general principle has emerged from the rush to identify various cell types and their functions in arterial diseases. An ongoing tug-of-war between pro-an anti-inflammatory cell types and mediators, between cell types and mediators that promote inflammation and those that mediate its resolution, between cell proliferation and cell death, characterizes many of the inflammatory diseases that affect arteries. In both chronic arterial diseases such as atherosclerosis, and in the more acute inflammatory diseases such as certain vasculitides, the net manifestation of the disease process depends on a delicately regulated balance between these competing arms of the immune and inflammatory responses. The individual contributions to this series highlight many of these internal struggles underway in the lesions of arterial disease.
Frontiers and future questions relating to the immune of pathogenesis of arterial diseases
The individual contributions to this series of articles highlight cutting-edge controversies and unresolved questions relating to the particular subject matter discussed. In general, two major themes present challenges for future work in this realm.
The first issue has to do with the hegemony of the mouse. Most of the studies recounted in this series have involved experiments using mice. This species offers immense advantages for experimental work. Genetic and other strategies for gain of function and loss of function investigations have enabled enormous strides in understanding the functional consequences of various cell types and mediators in arterial diseases. Yet, we must recognize the limitations of our laboratory experiments on mice. 7,21 The inbred strains that we use and the pathogen-free environments in which we raise our mice permit well controlled experiments, but do not reflect the rich heterogeneity of human populations, and their daily commerce with microbes of all sorts, both pathogenic and commensal. Moreover, our laboratory experiments often use mouse preparations that exaggerate disease processes so that we can accomplish experiments in practical time spans and with limited numbers of animals. In the case of atherosclerosis, we study mice over weeks to months, while our human patients develop this disease over decades. The levels of dyslipidemia achieved in our common mouse experiments represent a caricature rather than a faithful replica of the human situation. Often functional attributes of cell subpopulations in inbred mouse strains present a more clear cut dichotomy than the human counterparts, which more resemble shades of gray than stark black and white contrasts in functional pallets. While these limitations by no means cast doubt on the utility of mouse experiments to dissect mechanisms, we must remain humble about the ability to extrapolate the results glibly to clinical disease. As a minimum, we must always attempt to validate findings made in mice and other experimental systems in human biobanks, tissue samples, patient studies etc. before making conclusions about their relevance to human medicine.
A second issue raised by advances in understanding the immunopathogenesis of arterial diseases relates to harnessing this knowledge for therapy to prevent or treat the clinical conditions. Manipulation of the adaptive immune response has proven enormously productive in certain areas of clinical medicine. For example, the use of calcineurin inhibitors as immunosuppressive agents permitted the widespread adoption of organ transplantation. Treatment with antibodies that target T or B cells, or immune checkpoint molecules has shown promise in the treatment of certain cancers. Yet, for chronic smoldering immune/inflammatory diseases such as atherosclerosis, systemic, long-term use of such draconian measures for benign diseases may entail unwanted effects over the time span of treatment one would often desire. For this reason, the continued exploration of the functions of particular cellular subsets and the proliferation of cytokine and chemokine targets, as elaborated in the individual contributions in this series, provides hope that more targeted strategies directed at this growing menu of cells and mediators will permit the development of therapies that mitigate the disease process without wholesale interference with host defenses or tumor surveillance. An interesting example would be the development of vaccines against atherosclerosis. 22 The recognition that natural antibodies derived from B1 cells produce antibodies that can eliminate modified LDL particles may offer promising opportunities in this work, as discussed by Binder et al. to this series. 20 A number of attempts at clinical translation adopting this strategy have begun.
With respect to innate immunity, the plethora of mediators and cell types likewise present an expanding panel of potential targets for therapy. Indeed, several large-scale trials currently in progress are testing the proposition that interference with pathways of innate immunity (e.g. neutralization of interleukin-1 beta or administration of methotrexate) may reduce cardiovascular events in humans. 23,24
Conclusion
From the early discoveries of the involvement of immune cells and cytokines in atherosclerosis, the field of the immunopathogenesis of arterial disease has burgeoned. The complexities outlined in the individual contributions to this series highlight the yin and yang of the immune and inflammatory responses as they apply to diseases that affect all levels of the arterial circulation. This collection of papers also illustrates the need to think beyond the intima and include the other layers of the artery wall. Finally, the very complexity discussed in the articles in this series furnish and expanding a variety of potential therapeutic targets that may allow us to modulate immune and inflammatory aspects of the pathogenesis of arterial disease to treat and ultimately prevent them.
Acknowledgments
Sources of Funding
P.L. was supported by grants from the National Heart, Lung, and Blood Institute (HL080472) and the National Institutes of Health (HL080731). G.K.H. was supported by grants from the Swedish Research Council (6816 and 8703), the Swedish Heart-Lung Foundation, the Foundation for Strategic Research, the Stockholm County Council, and the European Commission.
Non-standard Abbreviations and Acronyms
- IEL
The internal elastic lamina
- IDO
Indoleamine dioxygenase
- ANCA
Anti-neutrophil cytoplasmic antibodies
Footnotes
Disclosures
None
Contributor Information
Peter Libby, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115.
Göran K. Hansson, Department of Medicine and Center for Molecular Medicine, Karolinska University Hospital, Karolinska Institutet, Stockholm 17176, Sweden
References
- 1.Libby P, Hansson GK. Involvement of the immune system in human atherogenesis: Current knowledge and unanswered questions. Lab. Invest. 1991;64:5–15. [PubMed] [Google Scholar]
- 2.Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714–1717. doi: 10.1161/ATVBAHA.108.179713. doi:29/11/1714[pii]10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. History of discovery: inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. doi:ATVBAHA.107.149179[pii]10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 5.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. doi:31/5/969[pii]10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 6.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. doi:63108[pii]10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Hansson GK, Lichtman AH. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brogi E, et al. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and non-atherosclerotic arteries: Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest. 1993;92:2408–2418. doi: 10.1172/JCI116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sluimer JC, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. doi:S0735-1097(08)00263-5[pii]10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 11.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. doi:S1471-4906(11)00154-2[pii]10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tellides G, Pober J. Inflammatory and immune responses in the arterial media. Circulation Research. 2014 doi: 10.1161/CIRCRESAHA.116.301312. [DOI] [PubMed] [Google Scholar]
- 13.Dal Canto AJ, Swanson PE, O’Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest. 2001;107:R15–22. doi: 10.1172/JCI11540. doi:10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. doi:10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, et al. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 16.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr., Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. doi:31/7/1530[pii]10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habenicht Tertiary Lymphoid Organs Contribute to Innate and Adaptive Immune Responses in Advanced Mouse Atherosclerosis. Circulation Research. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 18.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circulation Research. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sackstein R, Libby P. Leukocytes and the pathogenesis of the arteritides. Circulation Research. in press. [Google Scholar]
- 20.Binder CJ. B cells and humoral immunity in atherosclerosis. Circulation Research. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 22.Klingenberg R, et al. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–952. doi: 10.1161/ATVBAHA.109.202671. doi:ATVBAHA.109.202671[pii]10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Everett BM, et al. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]