Abstract
Background
Presence of coronary artery calcium (CAC), carotid plaque, and increased carotid intima media thickness (IMT) may indicate elevated cardiovascular disease (CVD) risk; however, no large studies have compared them directly. This study compares predictive utilities of CAC presence, carotid artery plaque presence, and high IMT for incident CVD events.
Methods and Results
Participants were from the Multi-Ethnic Study of Atherosclerosis. Predictive values of carotid plaque, IMT and CAC presence were compared using Cox proportional hazards models, c-statistics, and net reclassification indices. The 6,779 participants were mean (standard deviation) 62.2 (10.2) years old; 49.9% had CAC, 46.7% had carotid plaque. The mean left and right IMT were 0.754 (0.210) mm and 0.751 (0.187) mm, respectively. After 9.5 years (mean), 538 CVD events, 388 coronary heart disease (CHD) events, and 196 stroke/transient ischemic attacks (TIA) were observed. CAC presence was a stronger predictor of incident CVD and CHD than carotid ultrasound measures. Mean IMT ≥75th percentile (for age, sex and race) alone did not predict events. Compared to traditional risk factors, c-statistics for CVD (c=0.756) and CHD (c=0.752) increased most by adding of CAC presence (CVD 0.776, CHD, 0.784; p<0.001) followed by carotid plaque presence (CVD c=0.760, CHD 0.757; p<0.05). Compared to risk factors (c=0.782), carotid plaque presence (c=0.787, p=0.045) but not CAC (c=0.785, p=0.438) improved prediction of stroke/TIA.
Conclusions
In adults without CVD, CAC presence improves prediction of CVD and CHD more than carotid plaque presence or high IMT. CAC and carotid ultrasound parameters performed similarly for stroke/TIA event prediction.
Keywords: atherosclerosis, cardiovascular disease, carotid artery, imaging, risk factor
Evaluation of carotid intima-media thickness (IMT), carotid artery plaque and detection and quantification of coronary artery calcium (CAC) are among the best studied imaging modalities for screening of atherosclerosis (1–4).
Multi-Ethnic Study of Atherosclerosis (MESA) investigators previously described superiority of CAC over IMT for coronary heart disease (CHD) and CVD risk prediction and superiority of IMT over CAC for prediction of stroke, but these analyses were limited by a short duration of follow-up with few CVD events (1,2). The predictive utilities of CAC and IMT were compared in the Cardiovascular Health Study; however, participants were older and follow-up was short (5). Although the predictive utilities of IMT, carotid plaque presence, and CAC presence have been described in several cohorts and meta-analyses (1,5–9), they have not been compared directly in a single cohort with extended follow-up and a large number of cardiovascular disease (CVD) events.
MESA is a large, ethnically diverse cohort of individuals without clinically evident CVD at study baseline in whom participants had carotid ultrasound and CT scans for carotid IMT measurement, carotid plaque presence, and CAC presence. With a mean of 9.5 years of follow-up and over 500 CVD events, this analysis directly compares IMT, carotid plaque presence, and CAC presence for predicting CVD events.
Methods
Study Participants and Design
The MESA is a large prospective, cohort study of the prevalence, causes, and progression of subclinical CVD. MESA is a population-based sample of 6,814 men and women aged 45 to 84 years who were free of known CVD at baseline, recruited from 6 United States communities. Study objectives and design have been published previously (10). All participants gave informed consent. It was approved by the institutional review boards of the field and reading centers.
This analysis was pre-specified and included all MESA participants with Exam 1 CAC evaluation and follow-up data (N=6,799) who also had Exam 1 CCA IMT measurements (N=3,098) and carotid plaque assessment (N=3,310) re-read by the University of Wisconsin Ultrasound Reading Center. These 3,310 participants were a subset of the original MESA cohort and had Exam 1 IMT measurements re-read because they subsequently had Exam 5 ultrasound studies (mean 9.5 years later), though Exam 5 data were not used in this report. Of the 3,310 participants with carotid ultrasounds, 266 were missing left CCA IMT and 212 were missing right CCA IMT. In this analysis, 418 (12.6%) participants with ultrasound evaluations were missing 1 or more IMT segments. Poor image quality only accounted for ~3% of missing IMT data. Exam 5 ultrasounds were conducted for MESA participants who participated in Exam 5, including those who had CVD events prior to Exam 5. Follow-up data are described in detail below and in the supplementary material (Supplementary Tables I–VIII).
This analysis was performed using the University of Wisconsin CCA IMT readings because (i) the associations between CCA IMT measures and most traditional risk factors (age, systolic blood pressure, body-mass index, sex, African-American race, and use of antihypertensive medications) were stronger than in the original IMT set, (ii) reproducibility of IMT measurements was stronger than for the original readings (correlations 0.96–0.99 versus 0.84–0.86) (11), and (iii) the University of Wisconsin readings provided standardized plaque scoring in accordance with clinical consensus recommendations (4,12). Since re-reads only were available for a subset of MESA participants, multiple imputation was used to account for missing data from the entire MESA cohort (N=6,779), which included the original IMT readings. Demographic, medical history, and laboratory data were obtained from July, 2000 to August, 2002.
Carotid Ultrasonography
At Exam 1, B-mode ultrasound was used to image the near and far walls of the right and left distal CCA, carotid bulb, and proximal internal carotid (ICA) using a Logiq 700 ultrasound system (General Electric Medical Systems, 13 MHz transducer). The carotid bifurcations and internal carotid arteries were interrogated thoroughly from both longitudinal and transverse approaches to identify the thickest regions. Images were stored on super-VHS videotape and digitized at a high resolution and frame rates using a Medical Digital Recording (MDR) device (PACSGEAR, Pleasanton, CA) and converted into DICOM digital records. Mean and maximal IMT of the far wall of distal CCA (distal 1 cm, proximal to the carotid bifurcation point, where the distal CCA diameter remains uniform) and the proximal 1 cm of the ICA were measured in triplicate at the University of Wisconsin using a semi-automated border detection program (Syngo Arterial Health package, Siemens Medical Solutions, Malvern, PA) blinded to subject demographic and medical information. CCA IMT percentiles based on age, sex and race/ethnicity were calculated (Supplementary Table I). Carotid plaque presence was defined as a focal abnormal wall thickness (IMT >1.5 mm) or a focal thickening of >50% of the surrounding IMT (4,12). Presence or absence of plaque acoustic shadowing was recorded. A total plaque score (range 0–12) was calculated to describe carotid plaque burden. One point per plaque was allocated for the near and far walls of each segment (CCA, bulb, ICA) of each carotid artery that was interrogated. Ultrasound reproducibility is described in detail in Supplemental Text (IMT Reproducibility).
Coronary Artery Calcium Presence and Score
Methods for computed tomographic scanning and interpretation have been reported previously (13). CAC was assessed at all six MESA sites at Exam 1 by using either a cardiac-gated electron-beam computed tomography scanner (Chicago, Los Angeles, and New York Field Centers) or a multi-detector computed tomography system (Baltimore, Forsyth County and St. Paul Field Centers). All scans were over-read by a trained radiologist or cardiologist using an interactive scoring system (7). CAC was categorized as present or absent and the Agatston score was reported as a continuous variable with excellent reproducibility (7,14).
Cardiovascular Disease Events
Participants were followed from the baseline examination through October 2012. They were contacted by telephone every 9–12 months to inquire about interim hospital admissions, CVD outpatient diagnoses, and deaths. Events were verified with death certificates and medical records. Two physicians, blinded to study data, independently reviewed and classified CVD events. In cases of disagreement, a mortality and morbidity committee determined the final classification. CVD was defined as CHD (definite or probable myocardial infarction, CHD death, resuscitated cardiac arrest, definite angina, and probable angina – if followed by coronary revascularization), stroke (fatal or non-fatal), or other atherosclerotic CVD death (1). Stroke and transient ischemic attack (TIA) were adjudicated by neurologists. Stroke was defined as a focal neurologic deficit lasting 24 hours or until death with a clinically relevant lesion on brain imaging, and no nonvascular cause was identified. TIA was defined as a focal neurologic deficit lasting 30 seconds to 24 hours, without brain imaging suggesting stroke. In the present analysis, the composite of TIA and stroke are reported since the number of strokes was small. A detailed description of the MESA follow-up methods is available at http://www.mesa-nhlbi.org.
Statistical Analysis
CAC was categorized as present or absent, and continuously as ln(CAC+25) since over 50% of participants had zero CAC. The constant of 25 allows for a more symmetric normal distribution and has been used in prior MESA analyses (15). Carotid plaque was categorized as present or absent and as a carotid plaque score (0–12). Mean and maximum CCA and ICA IMT were described as continuous variables. CCA IMT also was categorized as below or above the highest cohort quartile for age, sex, and race. Internal carotid artery IMT data are presented, for the sake of completeness, in the supplementary material. Carotid plaque presence was our primary carotid plaque variable; carotid plaque score and shadowing were described for the sake of completeness. Cox proportional hazards models were used to assess the effect of multiple covariates on survival and to account for potential confounders.
Multiple imputation was used to account for missing values. A model was developed that included traditional CVD risk factors, demographic information, original (non-core lab) IMT readings in the MESA population, CAC, and the known outcomes of the entire cohort over the follow-up period (16). All ultrasound measurements were taken at baseline for event ascertainment and data were imputed from individuals who died (Supplementary Figure I). The IMT data from the re-reads are missing at random and are highly correlated with the original IMT measurements included in the imputation model. Under these circumstances, multiple imputation performs well even with over 90% missingness (17). The same imputation analysis was performed to account for missing carotid plaque data with one difference. Since the original carotid ultrasound readings did not provide plaque assessment in accordance with clinical consensus recommendations (4,12), those readings could not be imputed; however, all other participant data was included as in the carotid IMT models. Estimates for the Cox models were averaged across 100 datasets. The strength of association for carotid plaque presence, IMT, and CAC and combinations of the independent variables based on the relative size of their hazard ratios and the corresponding chi-square test or Z test of the hazard ratios are reported, as in previous MESA publications (1). Area under the receiver-operating characteristic curves (AUC) using Harrell’s c-statistics were estimated from 5 imputed datasets. Net reclassification improvement (NRI) analyses with bootstrapped standard errors were used to assess the improvement in risk stratification generated by carotid plaque presence, CCA IMT, and CAC presence for each outcome based on 10-year risk of 0–6%, 6–20%, >20% and were estimated from 10 imputed datasets (18). Rubin’s rules for combining the standard errors were used for all models (16). Sensitivity analyses comparing complete case analysis to the imputed data are shown in Table 1.
Table 1.
Risk Factor Adjusted Cox Regression Models for Predicting Incident Events*
| With Multiple Imputation** | Without Multiple Imputation** | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| CVD | ||||
| CAC presence | 3.12 (2.44 – 3.99) |
<0.001 | 3.09 (2.03 – 4.69) |
<0.001 |
| Carotid plaque presence | 1.61 (1.17 – 2.21) |
0.003 | 1.54 (1.09 – 2.17) |
0.015 |
| Mean CCA IMT ≥75th percentile | 1.20 (0.94 – 1.52) |
0.141 | 1.58 (1.11– 2.23) |
0.011 |
| Carotid plaque/CIMT75 | 2.06 (1.46 – 2.91) |
<0.001 | 1.70 (1.11 – 2.59) |
0.014 |
| CHD | ||||
| CAC presence | 4.48 (3.24 – 6.17) |
<0.001 | 3.75 (2.28 – 6.17) |
<0.001 |
| Carotid plaque presence | 1.76 (1.23 – 2.52) |
0.002 | 1.61 (1.10 – 2.36) |
0.015 |
| Mean CCA IMT ≥75th percentile | 1.29 (0.98 – 1.68) |
0.065 | 1.61 (1.10 – 2.37) |
0.015 |
| Carotid plaque/CIMT75 | 2.33 (1.56 – 3.47) |
<0.001 | 1.97 (1.21 – 3.22) |
0.007 |
| Stroke/TIA | ||||
| CAC presence | 1.54 (1.09 – 2.18) |
0.015 | 1.82 (0.97 – 3.42) |
0.061 |
| Carotid plaque presence | 1.40 (1.35 – 1.45) |
<0.001 | 1.34 (0.75 – 2.39) |
0.317 |
| Mean CCA IMT ≥75th percentile | 1.01 (0.70 – 1.47) |
0.944 | 1.31 (0.70 – 2.44) |
0.399 |
| Carotid plaque/CIMT75 | 1.86 (1.10 – 3.13) |
0.020 | 1.60 (0.79 – 3.23) |
0.189 |
Multivariable models adjusted for age, gender, race/ethnicity, education, income, heart rate, body-mass index, smoking, total cholesterol, high-density lipoprotein cholesterol, lipid-lowering medication use, diabetes mellitus status, systolic blood pressure and anti-hypertensive medication use.
Multiple imputation sample size = 6,779; Without multiple imputation sample size = 3,310 (carotid plaque and CAC); 2,892 for mean CCA IMT ≥ 75th percentile and carotid plaque or carotid plaque or mean CCA IMT ≥ 75th percentile
Abbreviations as in Table 1: CI = confidence interval; carotid plaque/CIMT75 = composite of any carotid plaque presence or CCA IMT ≥75th percentile
Results
Participant Characteristics
Baseline characteristics are described in Table 2. Of those who underwent baseline carotid ultrasound evaluation (N= 3,310), at least one carotid plaque was found in 1,544 (46.7%) participants with a mean (standard deviation) total plaque score of 2.4 (1.6) (Table 3). CAC was present in 1,479 (44.7%) with an average CAC score of 222.6 (417.0) units. The average left mean and maximum CCA IMT were 0.754 (0.210) mm and 0.930 (0.246) mm, respectively. The right mean and maximum CCA IMT were 0.751 (0.187) mm and 0.921 (0.219) mm, respectively. Carotid plaque or left or right mean CCA IMT ≥75th percentile for age, sex and race/ethnicity (carotid plaque/CIMT75) (4) was found in 61.7% of participants with ultrasound data. The breakdown of CVD events is shown in Table 2.
Table 2.
Baseline Characteristics* of MESA Participants, 2000–2002
| All Participants | Participants with Re-Read Carotid Ultrasounds | |
|---|---|---|
| Analytic sample size | 6,779 | 3,310 |
| Age (years), mean (SD) | 62.2 (10.2) | 60.3 (9.4) |
| Male gender, % (n) | 47.2 (3,197) | 47.1 (1,559) |
| Race/ethnicity, % (n) | ||
| White | 38.6 (2,614) | 39.5 (1,306) |
| Chinese | 11.8 (800) | 13.2 (437) |
| Black | 27.7 (1,880) | 25.4 (840) |
| Hispanic | 21.9 (1,485) | 22.0 (727) |
| Education, % (n) | ||
| Less than high school | 18.0 (1,215) | 13.7 (453) |
| High school | 46.7 (3,153) | 46.3 (1,531) |
| More than high school | 35.4 (2,390) | 40.1 (1,326) |
| Body mass index (kg/m2), mean (SD) | 28.3 (5.5) | 28.2 (5.2) |
| Smoking, % (n) | ||
| Never | 50.3 (3,400) | 52.2 (1,729) |
| Former | 36.6 (2,475) | 36.4 (1,204) |
| Current | 13.1 (884) | 11.4 (377) |
| Total cholesterol (mg/dL), mean (SD) | 194.2 (35.7) | 194.1 (35.2) |
| LDL cholesterol (mg/dL), mean (SD) | 117.2 (31.5) | 117.3 (31.0) |
| HDL cholesterol (mg/dL), mean (SD) | 51.0 (14.8) | 51.0 (14.7) |
| Lipid-lowering medication, % (n) | 16.2 (1,096) | 16.3 (539) |
| Systolic blood pressure (mmHg) mean (SD) | 126.6 (21.5) | 124.1 (20.0) |
| Diastolic blood pressure (mmHg), mean (SD) | 71.9 (10.3) | 71.9 (10.0) |
| Antihypertensive medication, % (n) | 37.3 (2,526) | 34.2 (1,133) |
| Diabetes mellitus status, % (n) | ||
| Normal | 73.6 (4,972) | 77.1 (2,552) |
| Impaired fasting glucose | 13.8 (933) | 13.0 (430) |
| Untreated diabetes mellitus | 2.7 (179) | 1.8 (60) |
| Treated diabetes mellitus | 9.9 (671) | 8.1 (268) |
Raw data without multiple imputation.
HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation
Table 3.
Numbers of Cardiovascular Disease Events and Descriptive Data on Carotid Plaque Presence, Coronary Artery Calcium, and Carotid Intima-Media Thickness*
| All Participants | All Participants with Re-Read Carotid Ultrasound | Participants with Re-Read Left CCA IMT measurements | Participants with Re-Read Right CCA IMT measurements | |
|---|---|---|---|---|
| Analytic sample size | 6,779 | 3,310 | 3,044 | 3,098 |
| CVD event, % (n) | 7.9 (538) | 5.0 (166) | 5.0 (151) | 5.0 (156) |
| CVD death | 5.5 (370) | 2.8 (91) | 2.7 (82) | 2.7 (83) |
| CHD event, % (n) | 5.7 (388) | 4.1 (136) | 4.0 (121) | 4.2 (130) |
| Myocardial infarction | 2.5 (171) | 1.6 (54) | 1.5 (46) | 1.6 (50) |
| CHD death | 3.5 (235) | 1.7 (55) | 1.5 (47) | 1.7 (51) |
| Cardiac arrest | 0.4 (24) | 0.1 (2) | 0.1 (2) | 0.03 (1) |
| Definite Angina | 2.6 (175) | 2.8 (92) | 2.8 (84) | 2.9 (89) |
| Probable angina w/PCI | 1.1 (77) | 1.2 (38) | 1.2 (35) | 1.1 (34) |
| Stroke, % (n) | 2.2 (148) | 1.1 (36) | 1.2 (35) | 1.0 (32) |
| Stroke + TIA, % (n) | 2.9 (196) | 1.7 (57) | 1.8 (55) | 1.6 (50) |
| CAC present, % (n) | 49.9 (3,382) | 44.7 (1,479) | 44.2 (1,345) | 44.1 (1,366) |
| CAC score (if present), mean (SD) | 290.8 (545.9) | 222.6 (417.0) | 220.0 (415.4) | 222.7 (422.7) |
| Plaque present, % (n) | – | 46.7 (1,544) | – | – |
| Plaque score (if present), mean (SD) | – | 2.4 (1.6) | – | – |
| Left CCA mean IMT (mm), mean (SD) | 0.743 (0.250)** | – | 0.754 (0.210) | – |
| Left CCA max IMT(mm), mean (SD) | 0.853 (0.279)** | – | 0.930 (0.246) | – |
| Right CCA mean IMT (mm), mean (SD) | 0.734 (0.232)** | – | – | 0.751 (0.187) |
| Right CCA max IMT (mm), mean (SD) | 0.846 (0.262)** | – | – | 0.921 (0.220) |
Raw data without multiple imputation.
Left CCA Mean IMT sample size = 6570; Left CCA max IMT sample size = 6570; Right CCA mean IMT sample size = 6623; Right CCA max IMT sample size = 6623
Abbreviations as in Table 1: CCA = common carotid artery; IMT = intima media thickness; CAC = coronary artery calcium; CVD = cardiovascular disease; CHD = coronary heart disease; PCI = percutaneous coronary intervention; TIA = transient ischemic attack
Cox Regression Models for Predicting CVD Events
Cox regression models for CVD events with multiple imputation are shown in the Figure. After adjustment for traditional risk factors and multiple imputation for missing values, CAC presence was the strongest predictor of CVD events (HR 3.12, 95% CI 2.44–3.99, p<0.001). Carotid plaque presence (HR 1.61, 95% CI 1.17–2.21, p=0.003) also was predictive of CVD events, but carotid plaque/CIMT75 (HR 2.06, 95% CI 1.46–2.91, p<0.001) performed better than carotid plaque alone (Figure, Table 1). CAC presence was a stronger predictor of CHD events (HR 4.48, 95% CI 3.24–6.17, p<0.001) than CVD events. CAC presence, carotid plaque presence, and carotid plaque/CIMT75 independently predicted stroke/TIA, with similar hazard ratios. Carotid IMT ≥75th percentile without plaque did not predict stroke/TIA (HR: 1.01 [0.70 – 1.47]; Figure, Table 1). Analyses of the hazard ratios for stroke alone (without TIA), were similar to stroke/TIA, but were limited by the low number of isolated stroke events. Because the hazard ratios for CVD event prediction using CAC score (1.48, 95% CI 1.20–1.86, p<0.001) and carotid plaque score (1.49, 95% CI 1.39–1.58, p<0.001) were not better than CAC and carotid plaque presence, subsequent analyses focus on CAC and carotid plaque presence. Similar results were seen for the continuous CAC and carotid plaque measures for CHD and stroke/TIA events (data not shown).
Figure. Summary of Risk Factor Adjusted Cox Regression Models for Predicting Incident Events, MESA.
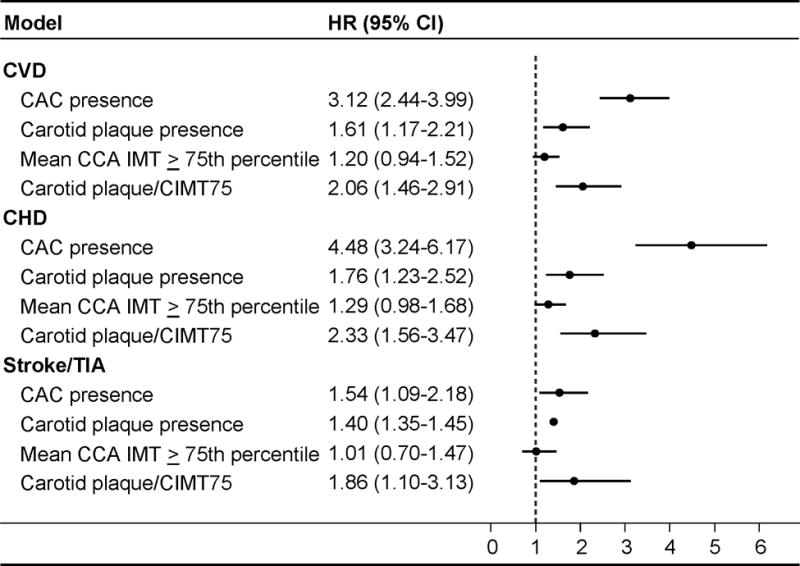
Multivariable models with multiple imputation adjusted for age, gender, race/ethnicity, education, income, heart rate, body mass index, smoking, total cholesterols, high-density lipoprotein cholesterol, lipid-lowering medication use, diabetes mellitus status, systolic blood pressure and anti-hypertensive medication use. Sample size = 6,779. Abbreviations as in Table 1: CI = confidence interval; carotid plaque/CIMT75 = composite of any carotid plaque presence or CCA IMT ≥ 75th percentile
Similar hazard ratio estimates were obtained in models restricted to those participants who were younger (men <50 years old, women <60 years old), and in each race/ethnicity category (Supplementary Tables II–III). When participants with diabetes mellitus (n=850) were excluded from the analyses, similar hazard ratios were obtained (data not shown). Mean, maximum, and combined internal carotid artery IMT did not predict CVD, CHD, or stroke/TIA (all p≥0.05, Supplementary Table IV). Also, the presence of carotid plaque shadowing did not significantly predict CVD (0.92 [0.68–1.24], p=0.6), CHD (0.97 [0.74–1.26], p=0.8), or stroke (0.99 [0.58–1.67], p=1.0) events, but predicted stroke/TIA events (1.65 [1.02–2.65], p=0.04)
Receiver Operator Characteristic Curves and Net Reclassification Index
The area under the receiver operator characteristic curves of the Cox regression models are shown in Table 4. For CVD events, traditional risk factors generated a c-statistic of 0.756. Adding CAC presence to traditional risk factors increased the c-statistic to 0.776 (p<0.001). Adding carotid plaque presence to traditional risk factors improved the c-statistic to 0.760 (p=0.033), but adding CIMT ≥75th percentile to traditional risk factors (c-statistic=0.757) did not increase the AUC compared to traditional risk factors alone (p=0.110). The improvement in c-statistic for carotid plaque/CIMT75 was similar to the AUC for plaque alone (Table 4). The results for CHD were similar. For combined stroke/TIA, traditional risk factors accounted for an AUC of 0.782, but only the addition of carotid plaque led to a statistically significant improvement in the c-statistic (c-statistic=0.787, p=0.045). The NRI analysis results were similar to those AUC results; however, the only statistically significant improvement in NRI, in addition to traditional risk factors, was for CAC predicting CVD and CHD events (Table 4).
Table 4.
Area Under the Curve and Net Reclassification Index for Cox Regression models of Cardiovascular Disease Endpoints
| N = 6,779 | C-statistic | P-value* | Net Reclassification Index (95% CI) |
|---|---|---|---|
| CVD | |||
| Traditional risk factors alone | 0.756 | – | – |
| CAC presence | 0.776 | <0.001 | 0.110 (0.060 – 0.159) |
| Carotid plaque presence | 0.760 | 0.033 | 0.012 (−0.022 – 0.045) |
| Mean CCA IMT ≥ 75th percentile | 0.757 | 0.111 | −0.007 (−0.031 – 0.018) |
| Carotid plaque/CIMT75 | 0.759 | 0.034 | 0.008 (−0.020 – 0.035) |
| CHD | |||
| Traditional risk factors alone | 0.752 | – | – |
| CAC presence | 0.784 | <0.001 | 0.103 (0.052 – 0.155) |
| Carotid plaque presence | 0.757 | 0.043 | 0.006 (−0.026 – 0.037) |
| Mean CCA IMT ≥ 75th percentile | 0.754 | 0.153 | −0.005 (−0.031 – 0.022) |
| Carotid plaque/CIMT75 | 0.756 | 0.055 | 0.012 (−0.016 – 0.039) |
| Stroke/TIA | |||
| Traditional risk factors alone | 0.782 | – | – |
| CAC presence | 0.785 | 0.438 | 0.028 (−0.012 – 0.068) |
| Carotid plaque presence | 0.787 | 0.045 | 0.015 (−0.017 – 0.048) |
| Mean CCA IMT ≥ 75th percentile | 0.783 | 0.160 | 0.000 (−0.003 – 0.034) |
| Carotid plaque/CIMT75 | 0.785 | 0.450 | 0.006 (−0.022 – 0.034) |
Compared to traditional risk factors alone
Abbreviations as in Tables 1.
Discussion
Arterial imaging with computed tomography (for CAC detection and scoring) and carotid ultrasound (for assessment of IMT and plaque presence) has been proposed as a strategy to better identify individuals who may have higher CVD risk than is estimated by traditional risk prediction tools, in order to more efficiently use preventive resources (3,4). This is the first analysis, to our knowledge, that has directly compared carotid plaque presence, IMT, and CAC in a single cohort with extended follow-up and a large number of CVD events. It also is the only study to evaluate these parameters in a multi-ethnic cohort that is highly representative of the US population. Previous studies have directly compared the abilities of CAC and IMT to predict CVD events (1,2). A previous evaluation of 1,330 MESA participants at “intermediate” Framingham CVD risk also demonstrated that CAC was a better predictor of CHD and CVD events compared to IMT. However, since both “high risk” and “low risk” participants were excluded from those analyses, the number of total events was far fewer than in the present study and likely account for the differences between the calculated AUC and NRI data. That study also did not directly compare carotid plaque and IMT (2), though other cohorts have compared their predictive efficacy (6,8,9). Previous studies focused on IMT protocols developed for use in research settings, whereas this study is the first to compare the components of the carotid IMT/plaque examination currently recommended for clinical use (e.g., CCA IMT with carotid plaque screening) (4,12) to CAC presence.
The results of our comprehensive analyses were very consistent. CAC presence was a significantly better predictor of CVD and CHD events compared with any of the ultrasound measurements, including the consensus recommendation (carotid plaque/CIMT75). Carotid plaque was a stronger predictor than IMT. Neither CCA nor internal carotid artery IMT values improved CVD or CHD event prediction more than presence or absence of carotid plaque. Regarding stroke/TIA, the point estimates of the hazard ratios for CAC and carotid plaque presence were similar with overlapping confidence intervals. Addition of carotid plaque presence to traditional risks factors improved prediction with a nominal level of statistical significance, whereas addition of CAC presence did not. Absolute differences in c-statistics and NRIs were small and not likely clinically significant.
In a subset of participants in the Cardiovascular Health Study, CAC and IMT seemed to predict CVD events similarly, but IMT outperformed CAC for stroke prediction (5). These participants were elderly and were not necessarily free of CVD at baseline. Also, their events mostly occurred before the era of aggressive lipid and blood pressure reduction. Two previous reports from MESA showed similar findings regarding total CVD, but did not consider carotid plaque or consensus recommendation approaches to IMT (1,2). They also were based on fewer events and shorter follow-up, with accordingly wider confidence intervals. It is likely that the proximity of the carotid arteries to the brain and the coronary arteries to the heart explains the divergence of prediction between CAC scoring and carotid ultrasound measures. In the current study, CAC clearly was a superior predictor of CVD and CHD events. Our data differ from earlier MESA publications (1) by having longer follow up and more clinically applicable IMT and plaque readings, as well as a wider range of CVD risk (2). Indeed, CAC was a better predictor of stroke/TIA than IMT alone and nearly as good as carotid plaque or the composite of carotid plaque and CIMT≥75th percentile. It is possible that with longer follow up, the effect of risk reducing medications such as statins and antihypertensive medications on IMT, the relative proximity of the arterial disease to the end-organ being assessed becomes less important. With long-term follow up, the ability of CAC to predict CVD events remained strong but the differences between CAC and IMT in ability to predict the type of event diminished. Also, the importance of carotid plaque – a further step along the pathway to clinical CVD – became more evident, likely because carotid plaque presence is less affected by medical therapy than IMT.
Accurate measurements of IMT and detection of carotid plaque are highly dependent on image quality and sonographer skill (4). However, the reproducibility of these measures in this study approached that of CAC scoring. CAC scoring is less user-dependent and a score of zero may be less useful in individuals with high pre-test likelihood of absent CAC such as younger adults, women, and certain ethnic minorities. However, in our subgroup analyses of these individuals, CAC presence and the carotid ultrasound measures did not differ significantly from the primary analysis. Although previous studies have shown that CAC score (3,18) and carotid plaque score (19) can improve prediction of CVD events, in the present study, the predictive ability of either CAC score or plaque score was similar to the presence or absence of CAC or carotid plaque for stroke/TIA and more easily directly compared given the differing scales of the scoring systems (18). Some studies have suggested that characterizing plaque based on echo characteristics may have predictive value; however, the definitions are not uniform and correlations with histological findings of plaque have not been strong (20).
Limitations
This study shares the limitations of all observational research. However, this population is large, well-described, and was followed prospectively with careful imaging, measurement, and event adjudication. The major drawback was the number of missing re-read ultrasound studies. The imputation models included baseline IMT data from all 6,779 (100%) MESA participants, however the original MESA approach did not have a standardized plaque assessment. Thus plaque assessment was imputed from the subset (n=3,310) that survived until Exam 5 and therefore had fewer events. However, since the original reads were included in the imputation model, it will accurately correct implausible baseline readings using the information available from the re-reads, which was a major impetus for choosing an imputation strategy. The imputation model included carotid IMT readings from before all events. This approach was accepted due to the consensus recommendations for clinical use of carotid ultrasound, including carotid plaque detection. It allowed all CVD, CHD, and stroke/TIA events to be included in the analysis and all data from all subjects (Supplementary Tables V–VIII). The proportion of subjects with carotid plaque and CCA IMT >75th percentile was lower than in some but not all previous reports (8,21–23), likely due to differing scan protocols, definitions of plaque, reader variability, and characteristics of the different populations.
Conclusion
In middle-aged adults without pre-existing CVD, CAC presence predicts incident CVD and CHD better than carotid plaque presence, carotid artery IMT, or their composite, over nearly a decade of follow-up. CAC and carotid ultrasound parameters performed similarly for stroke/TIA event prediction. Similar observations were found in subgroup analyses of younger participants and ethnic minorities.
Supplementary Material
Acknowledgments
The authors thank the MESA investigators, staff, and participants for their valuable contributions. Participating MESA investigators/institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, grant ES015915 from the National Institute of Environmental Health Sciences, grant R831697 from the US Environmental Protection Agency, and by grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources and by a T32 HL 07936 Ruth L. Kirschstein National Research Service Award from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center.
Footnotes
Subject Codes: [8] Epidemiology; [121] Primary prevention; [30] CT and MRI; [33] Other diagnostic testing; [135] Risk Factors
Disclosures
JH Stein: Wisconsin Alumni Research Foundation
References
- 1.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr, Stein JH, Tracy CM, Vogel RA, Wesley DJ. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 4.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Naydeck BL, Ives DG, Boudreau RM, Sutton-Tyrrell K, O’Leary DH, Kuller LH. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–192. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O’Leary DH. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Polak JF, Pencina MJ, O’Leary DH, D’Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42:3017–3021. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez HR, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the advisory board of the 3rd and 4th watching the risk symposium 13th and 15th European stroke conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 13.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 16.Little RJA, Rubin DB. Statistical analysis with missing data. New York: J. Wiley & Sons; 2012. [Google Scholar]
- 17.Desai CS, Ning H, Kang J, Folsom AR, Polak JF, Sibley CT, Tracy R, Lloyd-Jones DM. Competing cardiovascular outcomes associated with subclinical atherosclerosis (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;111:1541–1546. doi: 10.1016/j.amjcard.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Roman MJ, Kizer JR, Best LG, Lee ET, Howard BV, Shara NM, Devereux RB. Vascular biomarkers in the prediction of clinical cardiovascular disease: the Strong Heart Study. Hypertension. 2012;59:29–35. doi: 10.1161/HYPERTENSIONAHA.111.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyman RA, Mays ME, McBride PE, Stein JH. Ultrasound-detected carotid plaque as a predictor of cardiovascular events. Vasc Med. 2006;11:123–130. doi: 10.1191/1358863x06vm666ra. [DOI] [PubMed] [Google Scholar]
- 21.Kuo F, Gardener H, Dong C, Cabral D, Della-Morte D, Blanton SH, Elkind MS, Sacco RL, Rundek T. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke. 2012;43:1755–1760. doi: 10.1161/STROKEAHA.112.651059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SK, Choi YJ, Huh BW, Kim CS, Park SW, Lee EJ, Cho YW, Huh KB. Ratio of waist-to-calf circumference and carotid atherosclerosis in Korean patients with type 2 diabetes. Diabetes Care. 2011;34:2067–2071. doi: 10.2337/dc11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie W, Liang L, Zhao L, Shi P, Yang Y, Xie G, Huo Y, Wu Y. Combination of carotid intima-media thickness and plaque for better predicting risk of ischaemic cardiovascular events. Heart. 2011;97:1326–1331. doi: 10.1136/hrt.2011.223032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


