Abstract
Purpose
The early detection of lung cancer in heavy smokers by low-dose CT (LDCT) can reduce the mortality. However, LDCT screening increases the number of indeterminate solitary pulmonary nodules (SPNs) in asymptomatic individuals, leading to overdiagnosis. Making a definitive preoperative diagnosis of malignant SPNs has been a clinical challenge. We have demonstrated that sputum miRNAs could provide potential biomarkers for lung cancer. Here we aimed to develop sputum miRNA biomarkers for diagnosis of malignant SPNs.
Experimental Design
Using quantitative reverse transcriptase PCR, we evaluated expressions of 13 sputum miRNAs, previously identified sputum miRNA signatures of lung cancer, in a training set of 122 patients with either malignant (n=60) or benign SPNs (n=62) to define a panel of biomarkers. We then validated the biomarker panel in an internal testing set of 136 patients with either malignant (n=67) or benign SPNs (n=69), and an external testing cohort of 155 patients with either malignant (n=76) or benign SPNs (n=79).
Results
In the training set, a panel of three miRNA biomarkers (miRs-21, 31, and 210) was developed, producing 82.93% sensitivity and 87.84% specificity for identifying malignant SPNs. The sensitivity and specificity of the biomarkers in the two independent testing cohorts were 82.09% and 88.41%, 80.52% and 86.08%, respectively, confirming the diagnostic value.
Conclusions
Sputum miRNA biomarkers may improve LDCT screening for lung cancer in heavy smokers by preoperatively diagnosing malignant SPNs. Nevertheless, a prospective study in a large population to validate the biomarkers is needed.
Keywords: MicroRNA, sputum, CT, solitary pulmonary nodules, diagnosis
Introduction
Non-small cell lung cancer (NSCLC) is the major type of lung cancer, which is the number one cancer killer for men and women. NSCLC mainly consists of two histological categories: adenocarcinoma (AC) and squamous cell carcinoma (SCC). Cigarette smoking is the most common cause of the disease. A NCI-National Lung Screening Trail (NLST) showed that the early detection of lung cancer by low-dose computed tomography (LDCT) in heavy smokers followed by appropriate treatments significantly reduced the mortality (1). Therefore, many national medical societies recently recommend lung cancer screening in heavy smokers by LDCT (2). However, LDCT increases the number of indeterminate solitary pulmonary nodules (SPNs) in asymptomatic individuals, whereas only a small fraction of SPNs may be lung tumors (2). Therefore, lung cancer screening in heavy smokers with LDCT could lead to a substantial amount of overdiagnosis (2). Radiology-based noninvasive and biopsy-based invasive techniques are used for managements of the indeterminate SPNs (3). However, the noninvasive approaches may cause unnecessary procedures, radiation exposure, anxiety, and cost. Furthermore, biopsies have risks of pneumothorax, hemorrhage, and false negative results. The development of noninvasive biomarkers that can preoperatively identify malignant SPNs, and hence reduce the overdiagnosis of CT scan is urgently needed (2).
Sputum is a noninvasively and easily accessible body fluid that contains exfoliated bronchial epithelial cells (4). Sputum cytology can identify morphological abnormalities of bronchial epitheliums of lung cancer patients (5). Yet it has a poor sensitivity for diagnosis of lung cancer (5, 6). Molecular study of sputum could detect the cells containing lung tumor-associated molecular aberrations, thus providing a noninvasive approach for diagnosis of lung cancer (5). Numerous sputum molecular markers have been identified. However, none has been acceptable for clinical utility in diagnosis of lung cancer (5).
Dysregulation of microRNAs (miRNAs) plays crucial roles in tumorigenesis (7, 8). Specific over- or under-expressions of miRNAs have been found to associate with particular tumor types, and thus open up a new field for molecular diagnosis of cancer (8) (9, 10). We have, for the first time, demonstrated that endogenous miRNAs are resistant to freeze-thaw action and stably exist in sputum (9). Using microarray-based platforms to profile expression signatures of 818 human mature miRNAs on NSCLC and the paired normal lung tissues, we identified a set of 12 miRNAs (miRs-21, 31, 126, 139, 182, 200b, 205, 210, 375, 429, 486 and 708) that displayed dysregulation in NSCLC (11–13). We further showed that 10 of the 12 miRNAs (miRs-21, 31, 126, 182, 200b, 205, 210, 375, 486 and 708) whose abnormal expressions in sputum were related with lung cancer (11, 12). Furthermore, Roa et al. directly defined sputum miRNA profiling of lung cancer, and found that expressions of five sputum miRNAs (miRs-21, 143, 155, 210, and 372) were related with the disease (14). So far, there are 13 sputum miRNAs (miRs-21, 31, 126, 143, 155, 182, 200b, 205, 210, 372, 375, 486, and 708) showing promise as biomarkers of NSCLC. Moreover, the previous studies indicated that the 13 miRNAs could be reproducibly and specifically measured in sputum by using quantitative reverse transcription-PCR (qRT-PCR), providing rationale of developing sputum miRNA biomarkers for preoperative diagnosis of malignant SPNs.
Based on the earlier findings, we aimed to identify and characterize sputum miRNAs that could be used for identifying lung cancer in CT-discovered SPNs. We first evaluated expressions of the 13 sputum miRNAs in a training set of 122 patients with either malignant or benign SPNs to define a panel of biomarkers. We then validated the biomarker panel in an internal testing set of 136 patients with either malignant or benign SPNs, and an external testing cohort of 155 patients with either malignant or benign SPNs.
Materials and Methods
Patient cohorts
The study protocols were approved by the Institutional Review Boards (IRBs) of the University of Maryland Medical Center (UMMC) and the Baltimore VA Medical Center (BVAMC). All subjects were selected and consented based on presence of SPNs on chest CT scan when they visited the SPN clinics in the two medical centers. Final clinical diagnoses were confirmed with histopathologic examinations of specimens obtained by CT-guided transthoracic needle biopsy, transbronchial biopsy, videotape-assisted thoracoscopic surgery, or surgical resection. Of the 258 subjects recruited from UMMC, 127 had malignant SPNs and were diagnosed with early stage NSCLC (stage I or II), and 131 had benign SPNs. The 131 subjects with benign SPNs were diagnosed with granulomatous inflammation (n = 75), nonspecific inflammatory changes (n = 33), or lung infections (n = 23). The 258 cases were randomly spit into a training set and an internal testing set. The training set consisted of 60 individuals with malignant SPNs and 62 individuals with benign SPNs (Table 1). The testing set comprised of 67 subjects with malignant SPNs and 69 individuals with benign SPNs (Table 2). Of the 155 patients recruited from BVAMC, 76 had malignant SPNs (stage I and II NSCLC) and 79 had benign SPNs. The set of cases and controls was used as an external and independent testing cohort (Table 3). All participants with benign SPNs remained cancer-free for a minimum two-year follow-up. The demographic and clinical variables, including information about nodules size range, of the three cohorts are shown in Tables 1–3.
Table 1.
The demographic and clinical variables of a training set of patients with malignant SPNs and patients with benign SPNs
| 60 Patients with malignant SPNs (stage I and II NSCLC)
|
62 Patients with benign SPNs
|
|
|---|---|---|
| Age, Median (SD) | 67.3 (8.5) | 66.4 (SD 8.5) |
| Sex | ||
| Female | 22 (36.7%) | 23 (37.1%) |
| Male | 38 (63.3%) | 39 (62.9%) |
| Race | ||
| African American | 17 (28.3%) | 19 (30.7%) |
| White | 43 (71.7%) | 43 (69.3%) |
| Nodule size (cm) | ||
| Median (SD) | 2.3 (1.5) | 1.7 (1.6) |
| Range | 0.6–4.3 | 0.2–3.8 |
| Pack-years, Median (SD) | 37.3 (SD 26.5) | 32.6 (SD 31.5) |
| Stages | ||
| stage I | 39 (65.0%) | |
| stage II | 21 (35.0%) | |
| Histological types of NSCLC | ||
| AC | 27 (45.0%) | |
| SCC | 29 (48.3%) | |
| Others | 4 (6.7%) | |
Abbreviations: SPNs, solitary pulmonary nodules; SD, standard deviation; NSCLC, non-small-cell lung cancer; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Table 2.
The demographic and clinical variables of an internal testing set of patients with either malignant or benign SPNs
| 67 Patients with malignant SPNs (stage I and II NSCLC)
|
69 Patients with benign SPNs
|
|
|---|---|---|
| Age, Median (SD) | 66.4 (7.9) | 64.9 (SD 8.5) |
| Sex | ||
| Female | 24 (35.8%) | 23 (33.3%) |
| Male | 43 (64.2%) | 46 (66.7%) |
| Race | ||
| African American | 19 (28.4%) | 20 (28.9%) |
| White | 48 (71.6%) | 49 (70.1%) |
| Nodule size (cm) | ||
| Median (SD) | 2.5 (1.6) | 1.8 (1.7) |
| Range | 0.5–4.5 | 0.1–3.9 |
| Pack-years, Median (SD) | 36.9 (SD 25.7) | 34.6 (SD 32.5) |
| Stages | ||
| stage I | 45 (67.2%) | |
| stage II | 22 (32.8%) | |
| Histological types of NSCLC | ||
| AC | 30 (44.8%) | |
| SCC | 31 (46.3%) | |
| Others | 6 (8.9%) | |
Abbreviations: SPNs, solitary pulmonary nodules; SD, standard deviation; NSCLC, non-small-cell lung cancer; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Table 3.
The demographic and clinical variables of an external testing set of patients with either malignant or benign SPNs
| 76 Patients with malignant SPNs (stage I and II NSCLC)
|
79 Patients with benign SPNs
|
|
|---|---|---|
| Age, Median (SD) | 68.7 (9.2) | 67.9 (SD 8.3) |
| Sex | ||
| Female | 27 (35.5%) | 26 (32.9%) |
| Male | 49 (64.5%) | 53 (67.1%) |
| Race | ||
| African American | 22 (28.9%) | 23 (29.1%) |
| White | 54 (71.1%) | 56 (70.9%) |
| Nodule size (cm) | ||
| Median (SD) | 2.3 (1.7) | 1.9 (1.5) |
| Range | 0.4–4.5 | 0.2–4.0 |
| Pack-years, Median (SD) | 39.3 (SD 26.4) | 32.7 (SD 31.3) |
| Stages | ||
| stage I | 51 (67.1%) | |
| stage II | 25 (32.9%) | |
| Histological types of NSCLC | ||
| AC | 34 (44.7%) | |
| SCC | 35 (46.1%) | |
| Others | 7 (9.2%) | |
Abbreviations: SPNs, solitary pulmonary nodules; SD, standard deviation; NSCLC, non-small-cell lung cancer; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Sputum collection, preparation, and sputum cytological study
The subjects were instructed to spontaneously cough sputum as previously described (6, 9, 11–25), before receiving any treatment (e.g., surgery, preoperative adjuvant chemotherapy, and radiotherapy). 30% of the participants (mainly former smokers and non-smokers) were not able to spontaneously cough sputum, thus underwent sputum induction using a Lung Flute (Medical Acoustics, Buffalo, NY)-based technique as described in our previous work (19). Sputum was collected in a sterile cup, and centrifuged at 1,000×g for 15 min. Cytospin slides were prepared and underwent Papanicolaou staining for evaluating whether the specimens were representative of deep bronchial cells. All sputum samples were of lower respiratory origin as indicated by the presence of macrophages and bronchial epithelial cells. Cytologic diagnosis was then performed on the cytospin slides from sputum using the classification of Saccomanno (4). Positive cytology included both carcinoma in situ and invasive carcinoma (15, 16). The cell pellet from each sample was resuspended in Sputolysin (Calbiochem, San Diego, CA) for 15 minutes at 37°C. The cell pellets were then washed in phosphate buffered saline (Sigma-Aldrich, St. Louis, MO) and stored at −80°C until being tested.
The analysis of miRNAs in sputum by qRT-PCR
RNA was extracted from cell pellets of sputum as previously described (9, 11–13, 18, 19). The purity and concentration of RNA were determined by OD260/280 readings using a dual beam UV spectrophotometer (Eppendorf AG, Hamburg, Germany). RNA integrity was determined by capillary electrophoresis using the RNA 6000 Nano Lab-on-a-Chip kit and the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). The expression levels of the 13 sputum miRNAs (miRs-21, 31, 126, 143, 155, 182, 200b, 205, 210, 372, 375, 486, and 708) were determined by using qRT-PCR with Taqman miRNA assays (Applied Biosystems, Foster City, CA) as previously described (9, 11–13, 18, 19). Two internal control genes, U6 and miR-16, were also analyzed in parallel by qRT-PCR in the specimens. Relative expression of a targeted miRNA in a given sample was computed using the equation 2−ΔCt, where ΔCt = Ct (targeted miRNA) − Ct (internal control gene). Ct values were defined as the fractional cycle number, in which, the fluorescence crossed the fixed threshold. All assays were performed in triplicates. Furthermore, two interplate controls and one no-template control were carried along in each experiment. The no template control for RT was RNease free water instead of RNA sample input, and no template control for PCR was RNease free water instead of RT products input.
Statistical analysis
Based on one-sample with binomially distributed outcomes, we required 45 patients with lung cancer and 45 subjects with benign SPNs in a training set at 5% significant level with 80% power to discover a panel of biomarkers. To estimate sample size of a testing set for the validation of the biomarkers, we used utilize Area Under the receiver-operator characteristic (ROC) curve (AUC) analysis. The AUC of H0 (the null hypothesis) was set at 0.5. H1 represented the alternative hypothesis. To have a high reproducibility with adequate precision, we required 60 subjects per group in the testing set. With this sample size, we would have 90% power to detect an AUC of 0.75 at the 2% significance level. Furthermore, we used Pearson’s correlation analysis to evaluate the association between miRNA expressions and demographic and clinical characteristics of the patients with either malignant or benign SPNs. The clinicopathologic results were used as the reference standards to determine the diagnostic value of each miRNA biomarker. We used ROC curve and AUC analyses to decide sensitivity, specificity, and corresponding cut-off value of each miRNA. Sensitivity and specificity indicated the accuracy of biomarkers. In addition, positive predictive value (PPV) and negative predictive value (NPV) were also calculated as previously described (26), which indicated the probability of disease. We further used Logistic regression (13) to develop composite panels of biomarkers, and further identify an optimal panel that could distinguish malignant from benign SPNs with the highest sensitivity and specificity. All analyses, including correlation coefficient, Wilcoxon test, logistic regression, ANOVA, and t test, were performed using log transformed data.
Results
Developing a panel of sputum miRNA biomarkers for diagnosis of malignant SPNs in a training cohort of specimens
All targeted 13 miRNAs had ≤32 Ct values in each sputum sample of the training set, and therefore were reliably detectable in the specimens by using qTR-PCR assay. No product was synthesized in the negative control samples. Of the two evaluated internal control genes (miR-16 and U6), miR-16 displayed a Ct value of 26 (mean ± SD, 26 ± 1.3) in all the 122 sputum samples. U6 had ≤32 Ct values in 95.1% (116/122), however, 36 or higher Ct values in 4.9% (6/122) of the sputum samples. The finding suggested that U6 might not be reliably detectable in some of the tested specimens. Therefore, in this present study, we used miR-16 as an internal control to normalize the data of the 13 targeted miRNAs. As shown in Table 4, the 13 miRNAs displayed a significantly different level between patients with lung cancer and individuals with benign diseases (all P <0.05). Furthermore, the individual miRNAs exhibited AUC values of 0.64–0.85 in distinguishing malignant from benign SPNs (Table 4). We used logistic regression models with constrained parameters as in LASSO to develop a panel of miRNA biomarkers for malignant SPNs. miRs-21, 31, and 210 were selected as the best biomarkers (all P <0.001). The expression level of three sputum miRNAs were significantly higher in patients with lung cancer compared with subjects with benign SPNs (Table 4). The cut-off value for each of the three sputum miRNAs was selected at the point of the highest Youden Index. The cut-off for miR-21, 31, and 210 were 30.38, 1.62, and 36.56, respectively. Combined use of the three miRNAs produced 0.92 AUC (Table 4) (Figure 1). Furthermore, Pearson correlation analysis indicated that the estimated correlations among expression levels of the three miRNAs in sputum were low (All P> 0.05), implying that the diagnostic vales of the miRNAs were complementary to each other. Subsequently, the use of the three miRNAs in combination generated 82.93% sensitivity and 87.84% specificity. Sputum cytology has 43.33% sensitivity and 90.32% specificity. Therefore, the sputum biomarkers had a higher sensitivity (82.93%) compared with sputum cytology (43.33%), while maintaining a similar specificity. The three miRNAs did not display statistical differences of sensitivity and specificity between stages (stage I vs. stage II) (P>0.05). The changes of the three genes were associated with size of SPNs (p < 0.05). The expression of miR-21 in sputum was more closely associated with AC (P<0.05), whereas miR-210 was related to SCC (P<0.05). However, overall, the panel of three biomarkers didn’t exhibit special association with a histological type of the NSCLC cases, and the age, gender and ethnicity of the participants (All p > 0.05). The expression level of miR-31 was associated with smoking history of lung cancer patients at the edge of significance (P=0.05).
Table 4.
The expression difference of sputum miRNAs between patients with either malignant or benign SPNs, the corresponding sensitivity and specificity in distingushing malignant from benign SPNs
| miRNAs | Mean (SEM) of miRNA level * in patients with malignant SPNs | Mean (SEM) of miRNA level * in patients with benign SPNs | P-value | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| miR-205 | 85.66 (19.16) | 72.72 (64.82) | 0.0267 | 0.635 (0.552 to 0.719) | 59.74 | 53.93 |
| miR-708 | 1.01 (0.17) | 4.86 (1.37) | 0.0048 | 0.656 (0.561 to 0.750) | 64.71 | 62.50 |
| miR-375 | 698.81 (185.83) | 81.26 (23.80) | 0.0026 | 0.666 (0.594 to 0.756) | 66.23 | 62.22 |
| miR-200b | 879.82 (228.67) | 49.10 (3.11) | 0.0013 | 0.679 (0.589 to 0.768) | 65.22 | 61.19 |
| miR-182 | 43.95 (12.76) | 2.16 (0.31) | 0.0018 | 0.684 (0.611 to 0.778) | 64.94 | 59.76 |
| miR-155 | 23.76(4.59) | 4.54 (0.59) | < 0.0001 | 0.697 (0.616 to 0.779) | 62.67 | 62.96 |
| miR-372 | 136.49 (32.55) | 17.58 (3.56) | 0.0006 | 0.707 (0.628 to 0.786) | 63.64 | 60.98 |
| miR-143 | 9.46 (1.87) | 1.86 (0.27) | 0.0002 | 0.723 (0.644 to 0.803) | 63.38 | 61.73 |
| miR-486-5p | 29.46 (4.18) | 241.62 (49.80) | 0.0001 | 0.748 (0.674 to 0.821) | 74.03 | 66.67 |
| miR-126 | 3.83 (1.38) | 78.88 (23.62) | 0.0030 | 0.777 (0.704 to 0.851) | 77.63 | 75.00 |
| miR-31 | 2.73 (0.45) | 0.47 (0.07) | < 0.0001 | 0.789 (0.719 to 0.849) | 60.23 | 82.67 |
| miR-21 | 53.60 (6.43) | 7.56 (1.39) | < 0.0001 | 0.819 (0.753 to 0.874) | 78.16 | 71.08 |
| miR-210 | 67.28 (6.07) | 5.79 (0.42) | < 0.0001 | 0.853 (0.792 to 0.901) | 75.27 | 85.88 |
| Combined three miRNAs† | < 0.0001 | 0.919 (0.864 to 0.956) | 82.93 | 87.84 |
Abbreviations: SEM, the standard error of the mean; AUC, the area under receiver operating characteristic curve; CI, confidence interval.
The relative expression of each miRNA was calculated by using 2−ΔΔ threshold cycle (Ct), in which, ΔCT were CT values normalized to miR-16 control gene.
The three miRNAs: miRs-21, 31, and 210.
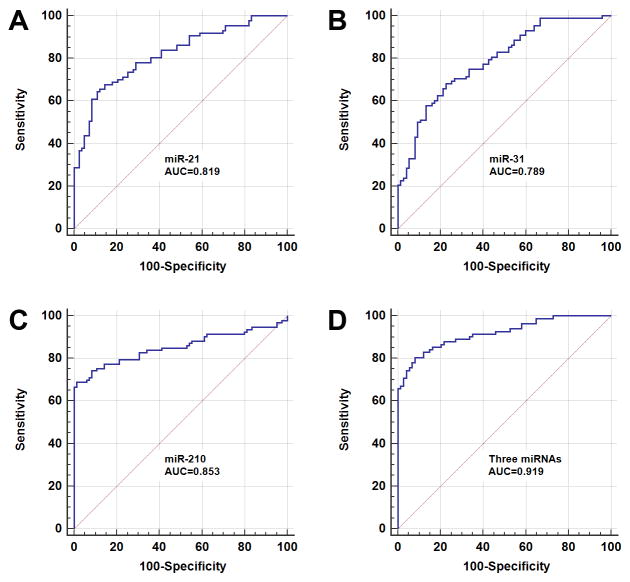
Figure 1.
Receiver-operator characteristic (ROC) curve analysis of three sputum miRNAs (miR-21, 31, and 210) in a training set of 122 patients with either malignant (n=60) or benign SPNs (n=62). The area under the ROC curve (AUC) for each miRNA conveys its accuracy for discriminating malignant from benign SPNs. The individual miRNAs produces 0.789–0.853 AUC values (A–C). Combined analysis of the three miRNAs creates AUC value of 0.919 (D), which is significantly higher than that of a single miRNA used alone (All P<0.05).
Validating the panel of sputum miRNA biomarkers in an internal testing and an external testing cohorts of specimens
The panel of sputum miRNA biomarkers was validated in a testing cohort (Table 2) for the diagnostic value in a blinded fashion by using the optimal thresholds established in the above training set. The panel of the three miRNAs had 82.09% sensitivity and 88.41% specificity, yielding 87.30% PPV and 83.56% NPV in differentiating malignant from benign SPNs (Table 5). The three miRNAs were further tested in an independent testing set of sputum samples (Table 3) collected from a different medical center. The panel of the sputum biomarkers could discern lung cancer from benign diseases with 80.52% sensitivity, 86.08% specificity, 84.93% PPV, and 81.93% NPV (Table 5). Taken together, the results created from the extensive validation confirmed the potential of the miRNAs as sputum biomarkers for the early detection of NSCLC among CT-found SPNs.
Table 5.
The diagnostic values of a panel of three sputum miRNA (miRs-21, 31, and 210) biomarkers in an internal testing set and an external testing set of specimens
| An internal testing set of 122 patients with either malignant (n=67) or benign SPNs (n=69) | An external testing set consisting of 155 subjects with either malignant (n=76) or benign SPNs (n=79) | |
|---|---|---|
| Sensitivity | 82.09% | 80.52% |
| Specificity | 88.41% | 86.08% |
| PPV (95% CI) | 87.30% (76.89 to 93.42) | 84.93% (74.99 to 91.37) |
| NPV (95% CI) | 83.56% (73.43 to 90.34) | 81.93% (72.30 to 88.73) |
PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval.
Discussion
In the present study, we develop a panel of three sputum miRNA biomarkers (miRs-21, 31 and 210) that can discriminate early stage NSCLCs from benign SPNs with 82.93% sensitivity and 87.84% specificity. The biomarker panel has a significant higher sensitivity (82.93% vs. 65.20%) compared with our previously developed two miRNA biomarkers that mainly distinguished NSCLC patients from cancer-free smokers (13). Furthermore, the biomarker panel has a higher sensitivity (82.93% vs. 43.33%) compared with sputum cytology. The validations of the biomarkers in two different testing sets with large sample sizes confirm their performance for diagnosis of malignant SPNs, producing more than 84% PPV and 81% NPV. The higher PPV (84%) of the biomarkers as compared with only 2% PPV of LDCT indicates that the biomarkers would result in much less overdiagnosis. The positive cases detected by the biomarkers in CT-found SPNs are malignant SPNs, and should need instant surgical treatment. Furthermore, the negative cases discovered by the biomarkers in CT-found SPNs are benign growths, and will not be followed up for two years using harmful and expensive approaches. Therefore, the future application of the biomarkers may dramatically decrease CT scan-related overdiagnosis, lead to more personalized therapy by sparing individuals with benign growths from radiation exposure and unnecessary surgical resections or biopsies.
Some limitations may exist in the present study. 1, the sputum samples used in this study were obtained from the individuals with SPNs that were found by contrast-enhanced CT rather than LDCT. The individuals might not be representative of the subjects in LDCT screening setting. Therefore, a larger scale validation study for the biomarkers across multiple centers with a population screened by LDCT is required. It would also be interesting to know if there is any different expression level of the sputum miRNAs between patients with benignant SPNs and healthy subjects. 2, the panel of three miRNAs biomarkers was selected from only 13 sputum miRNA biomarker candidates. Other important miRNAs might not be included in this study. Therefore, the diagnostic efficiency (82.93% sensitivity and 87.84% specificity) is still not sufficient to be used in clinical settings. Applying whole genome next-generation sequencing to globally analyse primary lung tumour tissues, we recently identified 68 miRNA signatures of stage I NSCLC (27). The comprehensively identified miRNA signatures would provide new biomarker candidates for lung cancer. Our ongoing efforts are to identify additional miRNA biomarkers from the new signatures that can improve the overall accuracy of the sputum test.
In sum, we report the development of a panel of sputum miRNAs that may provide potential biomarkers for a definitive preoperative diagnosis of SPNs primarily found by CT scan. However, carrying out a multicenter clinical trial in a large population to prospectively and vigorously validate the biomarkers is required before they can be translated into routine clinical practice.
Statement of clinical relevance.
The early detection of lung cancer in heavy smokers by low-dose CT (LDCT) can reduce the mortality. However, LDCT produces a substantial number of indeterminate solitary pulmonary nodules (SPNs), leading to a high level of overdiagnosis. Having a definitive preoperative diagnosis of malignant SPNs is a clinical challenge. Using a training set of cases and controls, we developed a panel of three miRNA biomarkers (miRs-21, 31, and 210) that could diagnose early stage lung cancer among SPNs with 82.93% sensitivity and 87.84% specificity. We then confirmed the diagnostic performance of the biomarkers in two independent testing cohorts. The results indicate that sputum miRNA biomarkers may have potential utility in risk-stratifying indeterminate SPNs, and improving LDCT screening for lung cancer in heavy smokers.
Acknowledgments
Grant support: This work was supported in part by NCI R01CA161837, VA merit Award I01 CX000512, and LUNGevity/Upstage Foundation Early Detection Award (F. J.).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JS, JS, JL, RZ, and XX conducted the experiments and participated in data interpretation. MAG, HZ, HP, and SAS participated in coordination and acquisition of data. LC and HF participated in data analysis. FJ participated in study design, coordination, and data analysis and interpretation, and prepared the manuscript. All authors read and approved the final manuscript.
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemagi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–74. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 4.Saccomanno G, Saunders RP, Archer VE, Auerbach O, Kuschner M, Beckler PA. Cancer of the lung: the cytology of sputum prior to the development of carcinoma. Acta Cytol. 1965;9:413–23. [PubMed] [Google Scholar]
- 5.Hubers AJ, Prinsen CF, Sozzi G, Witte BI, Thunnissen E. Molecular sputum analysis for the diagnosis of lung cancer. Br J Cancer. 2013;6:530–7. doi: 10.1038/bjc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu Q, Todd NW, Li R, Peng H, Liu Z, Yfantis HG, et al. Magnetic enrichment of bronchial epithelial cells from sputum for lung cancer diagnosis. Cancer. 2008;114:275–83. doi: 10.1002/cncr.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–8. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–6. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing L, Todd NW, Yu L, Fang H, Jiang F. Early detection of squamous cell lung cancer in sputum by a panel of microRNA markers. Mod Pathol. 2010;23:1157–64. doi: 10.1038/modpathol.2010.111. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Todd NW, Xing L, Xie Y, Zhang H, Liu Z, et al. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int J Cancer. 2010;127:2870–8. doi: 10.1002/ijc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Liao J, Guarnera MA, Fang H, Cai L, Stass SA, et al. Analysis of MicroRNAs in sputum to improve computed tomography for lung cancer diagnosis. J Thorac Oncol. 2014;9:33–40. doi: 10.1097/JTO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roa WH, Kim JO, Razzak R, Du H, Guo L, Singh R, et al. Sputum microRNA profiling: a novel approach for the early detection of non-small cell lung cancer. Clin Invest Med. 2012;35:271. doi: 10.25011/cim.v35i5.18700. [DOI] [PubMed] [Google Scholar]
- 15.Romeo MS, Sokolova IA, Morrison LE, Zeng C, Baron AE, Hirsch FR, et al. Chromosomal abnormalities in non-small cell lung carcinomas and in bronchial epithelia of high-risk smokers detected by multi-target interphase fluorescence in situ hybridization. J Mol Diagn. 2003;5:103–12. doi: 10.1016/s1525-1578(10)60459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varella-Garcia M, Kittelson J, Schulte AP, Vu KO, Wolf HJ, Zeng C, et al. Multi-target interphase fluorescence in situ hybridization assay increases sensitivity of sputum cytology as a predictor of lung cancer. Cancer Detect Prev. 2004;28:244–51. doi: 10.1016/j.cdp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Yu L, Shen J, Mannoor K, Guarnera M, Jiang F. Identification of ENO1 As a Potential Sputum Biomarker for Early-Stage Lung Cancer by Shotgun Proteomics. Clin Lung Cancer. 2014;14:110–7. doi: 10.1016/j.cllc.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Ma J, Guarnera MA, Fang H, Cai L, Jiang F. Digital PCR quantification of miRNAs in sputum for diagnosis of lung cancer. J Cancer Res Clin Oncol. 2014;140:145–50. doi: 10.1007/s00432-013-1555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anjuman N, Li N, Guarnera M, Stass SA, Jiang F. Evaluation of lung flute in sputum samples for molecular analysis of lung cancer. Clin Transl Med. 2013;2:15–20. doi: 10.1186/2001-1326-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang F, Todd NW, Li R, Zhang H, Fang H, Stass SA. A panel of sputum-based genomic marker for early detection of lung cancer. Cancer Prev Res (Phila) 2010;3:1571–8. doi: 10.1158/1940-6207.CAPR-10-0128. [DOI] [PubMed] [Google Scholar]
- 21.Jiang F, Todd NW, Qiu Q, Liu Z, Katz RL, Stass SA. Combined genetic analysis of sputum and computed tomography for noninvasive diagnosis of non-small-cell lung cancer. Lung Cancer. 2009;66:58–63. doi: 10.1016/j.lungcan.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz RL, Zaidi TM, Fernandez RL, Zhang J, He W, Acosta C, et al. Automated detection of genetic abnormalities combined with cytology in sputum is a sensitive predictor of lung cancer. Mod Pathol. 2008;21:950–60. doi: 10.1038/modpathol.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Todd NW, Qiu Q, Fan T, Zhao RY, Rodgers WH, et al. Genetic deletions in sputum as diagnostic markers for early detection of stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:482–7. doi: 10.1158/1078-0432.CCR-06-1593. [DOI] [PubMed] [Google Scholar]
- 24.Machida EO, Brock MV, Hooker CM, Nakayama J, Ishida A, Amano J, et al. Hypermethylation of ASC/TMS1 is a sputum marker for late-stage lung cancer. Cancer Res. 2006;66:6210–8. doi: 10.1158/0008-5472.CAN-05-4447. [DOI] [PubMed] [Google Scholar]
- 25.Belinsky SA, Liechty KC, Gentry FD, Wolf HJ, Rogers J, Vu K, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–44. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- 26.Hennessey PT, Sanford T, Choudhary A, Mydlarz WW, Brown D, Adai AT, et al. Serum microRNA biomarkers for detection of non-small cell lung cancer. PLoS One. 2012;7:32307. doi: 10.1371/journal.pone.0032307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Mannoor K, Gao L, Tan A, Guarnera MA, Zhan M, et al. Characterization of microRNA transcriptome in lung cancer by next-generation deep sequencing. Mol Oncol. 2014;8:1208–19. doi: 10.1016/j.molonc.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]