Abstract
The acute respiratory distress syndrome (ARDS) is a form of severe hypoxemic respiratory failure characterized by inflammatory injury to the alveolar capillary barrier with extravasation of protein-rich edema fluid into the airspace. Although many modalities have been investigated to treat ARDS for the past several decades, supportive therapies still remain the mainstay of treatment. Here, we briefly review the definition, epidemiology and pathophysiology of ARDS. Next, we present emerging aspects of ARDS pathophysiology that encompass modulators of the innate immune response, damage signals, and aberrant proteolysis that may serve as a foundation of future potential therapeutic targets.
1. Introduction
Definition, epidemiology, and etiology of the acute respiratory distress syndrome
The acute respiratory distress syndrome (ARDS) is a form of hypoxemic respiratory failure characterized by severe impairment in gas exchange and lung mechanics with a high case fatality rate. It is defined by acute hypoxemia (the ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) ≤ 300mmHg on positive end-expiratory pressure (PEEP) ≥5 cm H2O) with bilateral infiltration on chest imaging which cannot be fully explained by cardiac failure or fluid overload (1). Acute lung injury (ALI), a term that has been widely used in experimental lung injury models, is categorized as a mild form of the human disorder, ARDS, per the recent Berlin definition (1). The incidence of this clinical syndrome has been on the rise, now reported up to 86.2 per 100,000 person-years (2), which totals about 200,000 cases yearly in the United States. The hospital mortality is high at 38.5% (2), and has not significantly improved for the past several decades. The most common risk factor is severe sepsis with either a pulmonary or non-pulmonary source, explaining 79% of the cases (2). Other risk factors include aspiration, toxic inhalation, lung contusion, acute pancreatitis, trauma, transfusion, burn injury and cardiopulmonary bypass surgery (3).
Existing treatments
Numerous treatment measures aiming to modulate inflammation or its physiological consequences have been investigated for the treatment of ARDS patients. However, current anti-inflammatory therapies (corticosteroids (4), neutrophil elastase inhibitor (5), granulocyte-macrophage colony stimulating factor (6), statins (7), and omega-3 fatty acid (8)) and therapies targeted at improving lung mechanics (surfactant (9), inhaled β agonists (10), and nitric oxide (11)) failed to show a mortality benefit. Only supportive therapies that minimize pressure-induced lung injury (barotrauma) during mechanical ventilation, such as lung protective ventilation (12) with the use of neuromuscular blockers (13) or prone positioning (14), have shown mortality improvement and thus these treatments remain the mainstay of care.
Fundamentals of pathophysiology (inflammation and immunosuppression)
The innate immune response plays a profound role in the pathophysiology of ARDS. Multiple immunologic processes involving neutrophils, macrophages, and dendritic cells partake in mediating tissue injury. Inflammatory insults, either locally from the lungs or systemically from extra-pulmonary sites, affect bronchial epithelium, alveolar macrophages, and vascular endothelium, causing accumulation of protein-rich edema fluid into the alveoli and, subsequently, hypoxemia due to impaired gas exchange. Alveolar macrophages play a central role in orchestrating inflammation as well as the resolution of ARDS (15). Once alveolar macrophages are stimulated, they recruit neutrophils and circulating macrophages to the pulmonary sites of injury. These cells partake in the elaboration of a diverse array of bioactive mediators including proteases, reactive oxygen species, eicosanoids, phospholipids, and cytokines that perpetuate inflammatory responses. One profound effect of these mediators is to damage or induce distal cell death, specifically alveolar type 2 epithelial cells. These cells serve vital functions by synthesizing and secreting pulmonary surfactant, which is an indispensable material that lines the inner lung surface to lower alveolar surface tension. Type 2 cells also actively partake in ion transport to control lung fluid. Together, these inflammatory events lead to histological changes typical of an acute exudative phase that results in significant impairment in lung mechanics and gas exchange (Fig 1) (16). During the initial inflammatory and/or resolution phases of ARDS, alveolar macrophages also coordinate in a paracrine manner to interact with other cells including epithelial cells (17), lymphocytes (18), and mesenchymal stem cells (19) that can result in augmentation of the inflammatory response or accentuation of tissue injury. Prolonged M1 (classically activated macrophages) or M2 (alternatively activated macrophages) phenotypes appear to be associated with non-healing chronic ARDS (20).
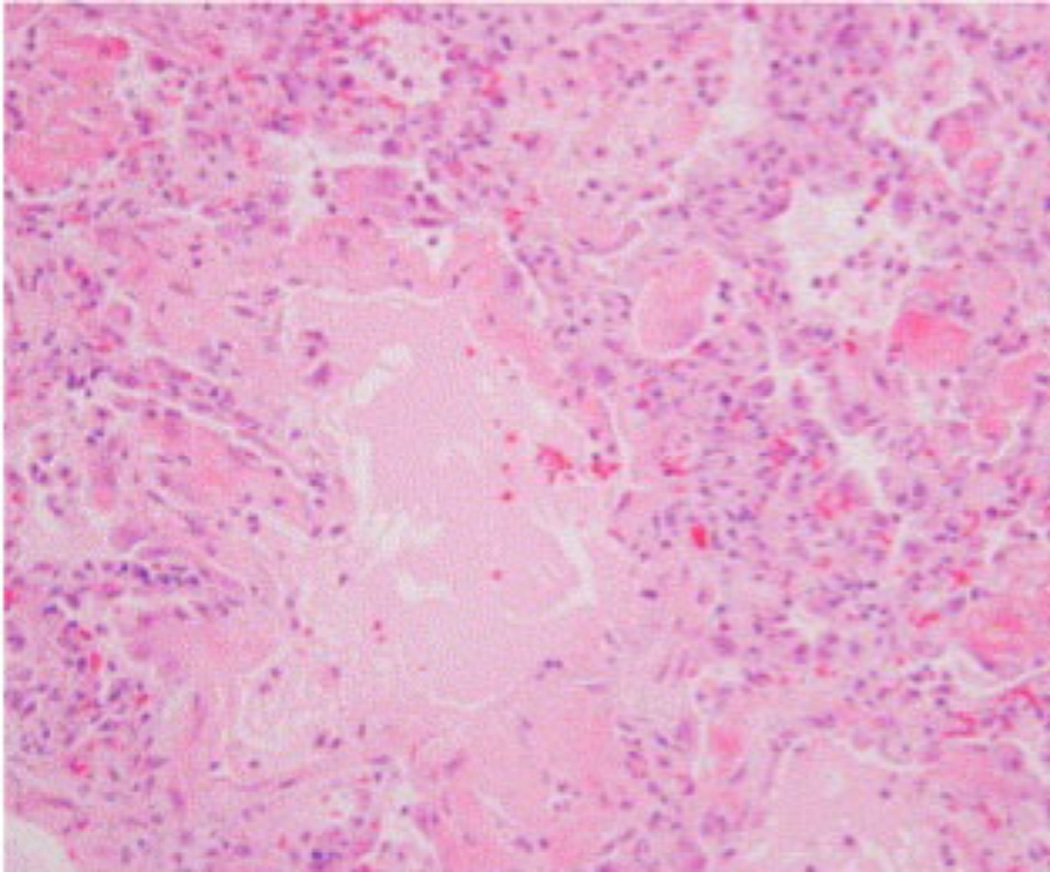
Figure 1.
The acute exudative phase of ARDS. Shown is a low-magnification hematoxylin and eosin stain micrograph showing alveolar inflammatory infiltration and filling of air sacs with protein-rich fluid (16).
ARDS is a systemic inflammatory disease with bidirectional involvement between the lungs and other organ systems, rather than a local pulmonary process. Inflammatory cytokines such as IL-1β, TNF-α, IL-6, and IL-8 are elevated both in bronchoalveolar lavage (BAL) fluid and circulating plasma in ARDS subjects (21). Interestingly, systemic immunosuppression is also observed in prolonged non-resolving ARDS patients even though pulmonary inflammation is persistent simultaneously. A human observational study showed that peripheral blood samples from 23 subjects with ARDS secondary to trauma or surgery had decreased cytokine release after lipopolysaccharide exposure (22). Also, autoantibodies are rapidly produced during ARDS (23). While ARDS is characterized by lung inflammation, it is worth noting that many risk factors for ARDS can themselves induce organ-specific inflammation. For example, traumatic brain injury (24), sepsis (25), and burn injury (26) cause robust inflammation in lungs, but also systemic immunosuppression. Further studies are necessary to clarify whether ARDS contributes to this differential inflammation independent of these risk factors.
2. Emerging aspects of ARDS pathophysiology
Pattern recognition receptors: Toll-like receptors and NOD-like receptors
Pattern recognition receptors (PRRs) are critical to surveillance in innate immunity, detecting components of foreign pathogens referred to as pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) are one of the transmembrane PRR proteins and are highly conserved molecules throughout vertebrates (27). So far, 10 functional TLRs have been identified in humans (28). They recognize non-endogenous PAMPs and trigger a rapid response to cause pro-inflammatory signaling. Recently, some TLRs are found to recognize endogenous danger (or damage)-associated molecular patterns (DAMPs) as well. Unlike TLRs, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are cytosolic PRRs which respond to the various PAMPs and DAMPs to trigger pro-inflammatory responses.
Development and resolution of ARDS seems to be related to TLR signaling pathways. Hyaluronan, an extracellular matrix glycosaminoglycan produced after tissue injury, initiates the inflammatory response in ARDS through engagement of TLR2 and TLR4, and at the same time, promotes recovery from ARDS (29). TLR4 was described as playing a pivotal role inducing ARDS in various murine models (30). TLR3 mediates hyperoxia-induced ARDS (31), and TLR2 mediates hemorrhage induced ARDS (32).
Recent studies have shown that NLRs are responsible for sterile inflammation in acute lung injury. One of them, NLRP (NLR family, pyrin domain containing) is an important component of the inflammasome, a large multi-protein complex. This complex is activated by pore-forming toxins or hypoxic cellular injury when conditions are primed with microbial ligands or endogenous cytokines (33). Specifically, the NLRP3 inflammasome is comprised of three components: NLRP3 (NLR family, pyrin domain containing protein-3), ASC (apoptosis-associated speck-like protein containing a CARD), and pro-caspase1. Once assembled, inflammasomes cleave pro-IL-1β and pro-IL-18 generating active IL-1β and IL-18. The NLRP3 inflammasome and its interaction with extracellular histones (34) was found to be required for the development of hypoxemia in a murine ARDS model (35). Also, inflammasome-regulated cytokines are associated with worse outcomes in ARDS subjects (36).
Mitochondrial DAMPs
Further compounding the inflammation from sepsis-induced ARDS is the release of mitochondrial components into the circulation from cellular damage. These mitochondrial-derived products include mitochondrial DNA, formyl peptides, and cardiolipin which serve as DAMPs to other cells (37, 38), triggering sterile inflammation and a clinical phenotype, the systematic inflammatory response syndrome (39). Both traumatic and operative injuries (40), which are risk factors for ARDS, release mitochondrial DAMPs into the circulation and activate polymorphonuclear neutrophils (PMNs) as a pro-inflammatory response. Mitochondrial DAMPs are present in blood transfusion products, suggesting a possible link with transfusion-related ARDS (41). Increased permeability of endothelial cells, which is a critical event causing hypoxemia in ARDS, is triggered by fragmented mitochondria (mitochondrial DAMP) both in PMN-dependent and PMN-independent fashions (42). Elevated mitochondrial DNA levels in plasma are also associated with higher mortality in patients with or without ARDS in the surgical and trauma (43), as well as the medical (44) intensive care unit. Finally, a mitochondrial-targeted inhibitor has been shown to mitigate the apoptosis of mouse lung endothelial cells after irradiation (45). These mitochondrial DAMPs may also be the cause of the differential inflammation observed in trauma, sepsis, and ARDS (46), but further studies are necessary to confirm the mechanistic basis for these findings.
Ubiquitin biology in lung injury
Ubiquitin is a small regulatory molecule found universally in most tissues in eukaryotic organisms. Ubiquitination is a post-translational modification process where ubiquitin is attached to a substrate protein, usually serving as the signal for their degradation via the proteasome or lysosome. Ubiquitination is carried out in three main steps performed by one or two ubiquitin-activating enzymes (E1s), several ubiquitin-conjugating enzymes (E2s), and hundreds of ubiquitin ligases (E3s). In the ubiquitination cascade, the ability of E3s to target a specific substrate for its degradation provides an elegant mechanism for protein disposal in cells but also opens up attractive opportunities for relatively selective therapeutic intervention.
ARDS is characterized by activation of the ubiquitin proteasome system (47), increased expression of ubiquitin within alveolar (type II) epithelia (48), and release of ubiquitin proteasome components into lung fluid (49). Ubiquitin components (E3 ligases) are also activated by endotoxin (50). An endotoxin responsive ubiquitin E3 ligase component, termed Fbxo3, is elevated in subjects with sepsis. It profoundly stimulates cytokine release when expressed in human peripheral blood mononuclear cells by mediating the degradation of another E3 ligase subunit, Fbxl2. Fbxl2 acts as an anti-inflammatory protein, inhibiting tumor necrosis factor receptor-associated factors (TRAFs) (51). Ubiquitination has been also reported to play an important role in regulating the Na, K-ATPase and the epithelial Na+ channel (ENaC) function during ARDS (47). Specifically, the E3 ubiquitin ligase Cblb negatively regulates TLR4 signaling to prevent hyper-activation of nuclear factor (NF)-κB in a sepsis-induced ARDS murine model (52). The disruption of the alveolar-capillary barrier is one of the key pathophysiologic events causing lung edema and hypoxemia in ARDS. The Na, K-ATPase is located in the basolateral surface of alveolar type 2 epithelial cells (53), contributing to lung liquid clearance. During hypoxia, the Na, K-ATPase is internalized and degraded by endocytosis via ubiquitination, resulting in alveolar epithelial barrier dysfunction and consequently decreased alveolar fluid clearance (54, 55). ENaC is responsible for salt and fluid absorption in lung epithelia, and its cellular abundance is regulated by the E3 ligase Nedd4-2 (56). Interestingly, hypoxia inhibits the expression of ENaC subunit at the apical membrane of murine alveolar epithelial cells, which may be through Nedd4-2-mediated ubiquitination (57). Furthermore, Nedd4-2 knockout mice develop sterile lung inflammation with some similarities to an ARDS phenotype (58). The emerging role of these protein degradation factors in ARDS raises opportunity for identification of unique therapeutic targets.
Neutrophil biology/Neutrophil extracellular traps (NETs)
Neutrophil influx into the lungs in response to activated alveolar macrophages is associated with the severity of ARDS, and may directly influence the development of this disorder (59). Several chemokines, including IL-8 (CXCL8), seem to play a central role in regulating neutrophil recruitment and consequent tissue damage, and altered alveolar-capillary permeability in both human and animal studies (60). The neutrophil extracellular traps (NETs) are produced by neutrophils and released to the extracellular space to trap pathogens such as bacteria, fungi, viruses and protozoa (61), which is a process called netosis. In a murine ARDS model, NETs are formed in lung tissue directly inducing the cell death of lung epithelia and endothelia (62). NETs are found in a variety of ARDS models including both infection-related injury (influenza (63), bacterial endotoxin (64), and fungi (65)) and sterile lung injury (transfusion-related ARDS (66)). The lower levels of surfactant proteins A and D, commonly observed in ARDS, appear to be responsible for excessive NETs in ARDS as surfactant proteins are involved in clearing NET-nucleic acid (64).
3. New potential therapeutic targets in ARDS
Targeting the ubiquitin proteasome system
Proteasome inhibitors such as bortezomib (67) and carfilzomib (68) were recently approved for the treatment of multiple myeloma by the U.S. Food and Drug Administration (FDA). Several new proteasome inhibitors are now in development as anti-cancer therapies. There is mounting evidence that inhibiting the proteasome may induce anti-inflammatory effects (69). Hypoxia-inducible factor 1 (HIF-1α), a transcription factor that controls expression of numerous genes, is targeted for ubiquitin proteasomal degradation. HIF-1α appears to be protective from ARDS as pharmacologic stabilization of HIF-1α lessens ALI severity in preclinical models (70). Inhibiting the pro-inflammatory protein, Fbxo3, effectively lessens the severity of viral pneumonia, septic shock, cytokine-driven systemic inflammation and ARDS in preclinical models, underscoring potential for targeting of the ubiquitin proteasome system in ARDS (51, 71).
Inflammasome: modification of the up- and down-stream pathways
As reviewed earlier, inflammasomes play a critical role in developing sterile inflammation during ARDS. Several agents to modify the NLRP3 inflammasome signaling have been studied. Antioxidants (72) and glyburide (73) block upstream signaling of the NLRP3 inflammasome in vitro. P2X7R antagonists (74) also inhibit upstream pathways before inflammasomes are assembled, but have not been tested for lung inflammation. On the other hand, a caspase-1 inhibitor decreases the release of IL-1β and IL-18 in rat endotoxemia (75), targeting a down-stream pathway to inhibit the products of inflammasome activation. Anti-IL-1 therapy is another approach to limit inflammasome activation. A monoclonal antibody against human IL-1β (Canakinumab) is licensed to treat cryopyrin-associated periodic syndromes (CAPS) (76), a rare genetic disease caused by autosomal-dominant mutations of the NLRP3 gene. Recombinant IL-1Ra (Anakinra) and IL-1 Trap (Rilonacept) are approved to treat rheumatoid arthritis and CAPS, respectively (77). However, the purported role of these agents as anti-inflammatory therapy for ARDS has yet to be evaluated in preclinical settings. New chemical entities directly targeting the inflammasome (NLRs) have not yet been identified.
Modulating inflammation
Numerous anti-inflammatory agents have failed to show any mortality benefit in ARDS subjects. Currently untested alternatives for the treatment and prevention of ARDS in human randomized controlled trials (RCT) are inhaled corticosteroids, angiotensin converting enzyme (ACE) inhibitors, and peroxisome proliferator receptor (PPAR) agonists. Animal data suggests that nebulized corticosteroids improve dynamic lung compliance and oxygenation, and decrease lung inflammation in sepsis-induced ARDS models (78). Angiotensin II induces NF-κB gene expression (79), hence, blocking the renin-angiotensin axis may be beneficial to ARDS patients based on animal data (80) and an observational human study (81). On the other hand, PPARs and their respective ligands negatively control pro-inflammatory gene expression (82). Their agonists reduce inflammation and vascular leakage in animal ARDS experimental models (83). However, human RCTs are necessary to examine the effect and efficiency of all these modalities.
Cell-based therapy (stem cell)
An exciting area of investigation is assessing cell-based therapy for ARDS with stem cells because these cells have the potential to differentiate into alveolar epithelial or lung endothelial cells and directly replenish the alveolar capillary barrier during cellular injury (84). Mesenchymal stem cells (MSC) are rapidly advancing to the clinical settings as they have practical advantages: they are easy to isolate and propagate, and do not generate ethical issues compared to embryonic stem cells. Preclinical studies show that MSC reduces lung inflammation and mortality in a murine ARDS model (85). This beneficial effect of MSC therapy was reproduced in ex vivo perfused human lungs (86), which seems to be mainly due to the release of keratinocyte growth factor by MSC. There was no toxicity in a recently published phase I clinical trial (87). Another phase I trial has started enrolling subjects to receive MSC (START; NCT01775774) (88).
4. Conclusions
In summary, the highly complex signaling and cellular networks that mediate tissue injury in ARDS present significant challenges in devising novel therapies for this disorder (Fig 2). However, this pathobiologic model provides a mechanistic platform offering unique opportunities to identify new targets for intervention. The pathobiology of ARDS involves cellular, biochemical, and organelle-based mediators with activation of components within the innate immune response that incites significant pulmonary inflammation. The identification of unique druggable targets within the ubiquitin cascade, pattern recognition receptors, and protein or cell based strategies hold promise in a new frontier based on the mechanistic biology of this critical illness.
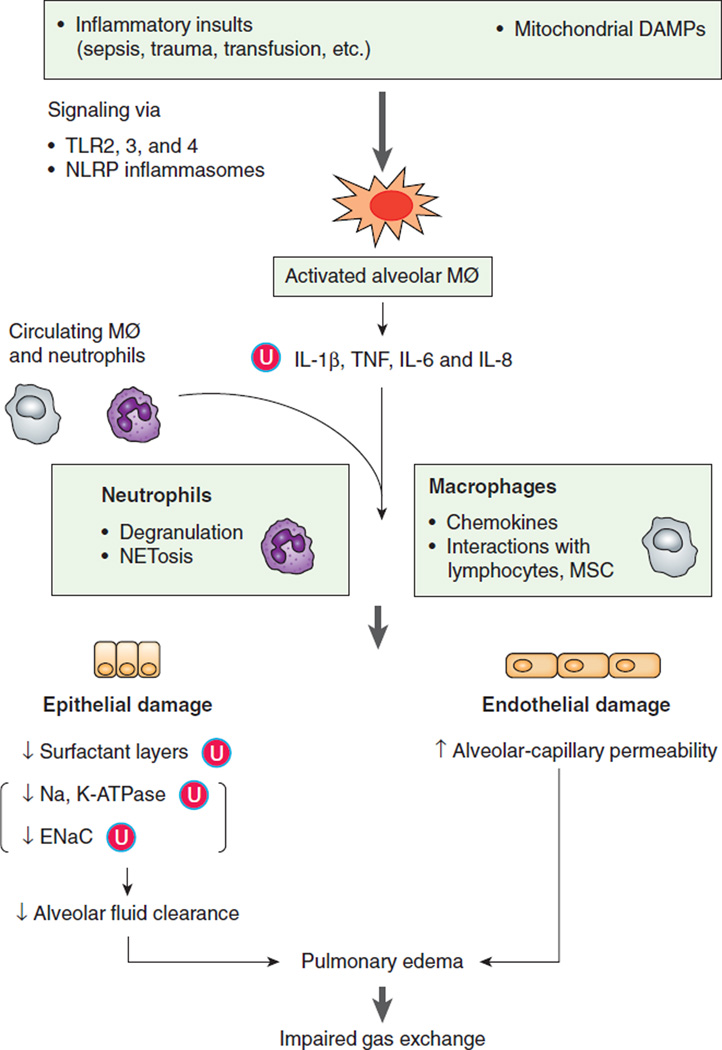
Figure 2. Pathophysiology of the acute respiratory distress syndrome.
Initial inflammatory insults including mitochondrial DAMPs activate alveolar macrophages via TLRs and NLRs signaling pathways. Activated alveolar macrophages release proinflammatory cytokines and recruit circulating macrophages and neutrophils to injured sites. Excessive neutrophils and persistently activated macrophages cause extensive damage to lung epithelia and endothelia resulting in an impaired alveolar-capillary barrier. Disruption of this barrier allows protein-rich fluid to enter the alveoli causing fluid accumulation in alveolar spaces (pulmonary edema) interfering with gas exchange. Ubiquitination (Ⓤ) plays an important role in modulating the abundance of key proteins in ARDS resulting in secretion of cytokines, lower levels of surfactant proteins, and decreased function of ion channels (Na, K-ATPase and ENaC). ARDS is associated with surfactant depletion leading to increased NETosis, a process that alters lung cell viability. Mitochondrial DAMPs can directly increase microvascular permeability independent to leukocytes.
Mitochondrial DAMPs, mitochondrial-derived products released by cellular damage, which serve as danger-associated molecular patterns; TLRs, Toll-like receptors; NLRP, nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family, pyrin domain containing; Alveolar MØ, alveolar macrophage; NETs, neutrophil extracellular traps; MSC, mesenchymal stem cell; ENaC, epithelial Na+ channel; Ⓤ, ubiquitination.
Acknowledgments
This material is based upon work supported, in part, by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. This work was supported by a Merit Review Award from the US Department of Veterans Affairs and National Institutes of Health R01 grants HL096376, HL097376, HL098174, HL081784, 1UH2HL123502, and P01HL114453 (to R.K.M.).
Footnotes
The contents presented do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA : the journal of the American Medical Association. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 4.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. Bmj. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata K, Doi A, Ohji G, Oka H, Oba Y, Takimoto K, Igarashi W, Gremillion DH, Shimada T. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Internal medicine. 2010;49:2423–2432. doi: 10.2169/internalmedicine.49.4010. [DOI] [PubMed] [Google Scholar]
- 6.Paine R, 3rd, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, Thannickal VJ, Burnham EL, Brown MB, Hyzy RC. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Critical care medicine. 2012;40:90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Heart L, Blood Institute ACTN, Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, Rock P, Douglas IS, deBoisblanc BP, Hough CL, Hite RD, Thompson BT. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. The New England journal of medicine. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P, Investigators NNARDSNo, Network NACT. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA : the journal of the American Medical Association. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, Lemaire F, Long W, Zaccardelli DS, Pattishall EN. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. The New England journal of medicine. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 10.National Heart L, Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT N. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. American journal of respiratory and critical care medicine. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K, Jr, Hyers TM, Papadakos P Inhaled Nitric Oxide in ARDS Study Group. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Critical care medicine. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 13.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A, Investigators AS. Neuromuscular blockers in early acute respiratory distress syndrome. The New England journal of medicine. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 14.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, Group PS. Prone positioning in severe acute respiratory distress syndrome. The New England journal of medicine. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. American journal of physiology. Lung cellular and molecular physiology. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro CY. ARDS and diffuse alveolar damage: a pathologist's perspective. Seminars in thoracic and cardiovascular surgery. 2006;18:13–19. doi: 10.1053/j.semtcvs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. American journal of respiratory and critical care medicine. 2001;163:1384–1388. doi: 10.1164/ajrccm.163.6.2006131. [DOI] [PubMed] [Google Scholar]
- 18.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. The Journal of clinical investigation. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental hematology. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schutte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. American journal of physiology. Lung cellular and molecular physiology. 2000;279:L25–L35. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- 21.Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 22.Buttenschoen K, Kornmann M, Berger D, Leder G, Beger HG, Vasilescu C. Endotoxemia and endotoxin tolerance in patients with ARDS. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2008;393:473–478. doi: 10.1007/s00423-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 23.Burbelo PD, Seam N, Groot S, Ching KH, Han BL, Meduri GU, Iadarola MJ, Suffredini AF. Rapid induction of autoantibodies during ARDS and septic shock. Journal of translational medicine. 2010;8:97. doi: 10.1186/1479-5876-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeij JD, Aslami H, Fluiter K, Roelofs JJ, van den Bergh WM, Juffermans NP, Schultz MJ, Van der Sluijs K, van de Beek D, van Westerloo DJ. Traumatic brain injury in rats induces lung injury and systemic immune suppression. Journal of neurotrauma. 2013;30:2073–2079. doi: 10.1089/neu.2013.3060. [DOI] [PubMed] [Google Scholar]
- 25.Lechner AJ, Tredway TL, Brink DS, Klein CA, Matuschak GM. Differential systemic and intrapulmonary TNF-alpha production in Candida sepsis during immunosuppression. The American journal of physiology. 1992;263:L526–L535. doi: 10.1152/ajplung.1992.263.5.L526. [DOI] [PubMed] [Google Scholar]
- 26.Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. Journal of leukocyte biology. 2008;84:607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 29.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature medicine. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 30.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infection and immunity. 2005;73:1754–1763. doi: 10.1128/IAI.73.3.1754-1763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray LA, Knight DA, McAlonan L, Argentieri R, Joshi A, Shaheen F, Cunningham M, Alexopolou L, Flavell RA, Sarisky RT, Hogaboam CM. Deleterious role of TLR3 during hyperoxia-induced acute lung injury. American journal of respiratory and critical care medicine. 2008;178:1227–1237. doi: 10.1164/rccm.200807-1020OC. [DOI] [PubMed] [Google Scholar]
- 32.Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, Reznikov L, McIntyre RC. Hemorrhage-induced acute lung injury is TLR-4 dependent. American journal of physiology. Regulatory, integrative and comparative physiology. 2004;287:R592–R599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 33.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nature immunology. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. Journal of immunology. 2014;192:5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, Arditi M, Shimada K. The NLRP3 Inflammasome Is Required for the Development of Hypoxemia in LPS/Mechanical Ventilation Acute Lung Injury. American journal of respiratory cell and molecular biology. 2014;50:270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. American journal of respiratory and critical care medicine. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, Waltenbaugh AK, O'Donnell CP, Henderson FC, Etscheidt CA, McCoy DM, Agassandian M, Hayes-Rowan EC, Coon TA, Butler PL, Gakhar L, Mathur SN, Sieren JC, Tyurina YY, Kagan VE, McLennan G, Mallampalli RK. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nature medicine. 2010;16:1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 40.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. Journal of orthopaedic trauma. 2010;24:534–538. doi: 10.1097/BOT.0b013e3181ec4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YL, King MB, Gonzalez RP, Brevard SB, Frotan MA, Gillespie MN, Simmons JD. Blood transfusion products contain mitochondrial DNA damage-associated molecular patterns: a potential effector of transfusion-related acute lung injury. The Journal of surgical research. 2014 doi: 10.1016/j.jss.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, Itagaki K. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PloS one. 2013;8:e59989. doi: 10.1371/journal.pone.0059989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Annals of surgery. 2013;258:591–596. doi: 10.1097/SLA.0b013e3182a4ea46. discussion 596-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AM. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS medicine. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577. discussion e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, Maeda A, Feng W, Wasserloos K, Belikova NA, Tyurin VA, Wang H, Fletcher J, Wang, Vlasova Y, II, Klein-Seetharaman J, Stoyanovsky DA, Bayir H, Pitt BR, Epperly MW, Greenberger JS, Kagan VE. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nature communications. 2011;2:497. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao C, Itagaki K, Gupta A, Odom S, Sandler N, Hauser CJ. Mitochondrial damage-associated molecular patterns released by abdominal trauma suppress pulmonary immune responses. The journal of trauma and acute care surgery. 2014;76:1222–1227. doi: 10.1097/TA.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vadasz I, Weiss CH, Sznajder JI. Ubiquitination and proteolysis in acute lung injury. Chest. 2012;141:763–771. doi: 10.1378/chest.11-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada T, Uehara K, Kawanishi R, Mizutani T, Sunagawa K, Araya J, Kawabata Y. Immunohistochemical detection of ubiquitin-positive intracytoplasmic eosinophilic inclusion bodies in diffuse alveolar damage. Histopathology. 2006;48:846–854. doi: 10.1111/j.1365-2559.2006.02445.x. [DOI] [PubMed] [Google Scholar]
- 49.Majetschak M, Sorell LT, Patricelli T, Seitz DH, Knoferl MW. Detection and possible role of proteasomes in the bronchoalveolar space of the injured lung. Physiological research / Academia Scientiarum Bohemoslovaca. 2009;58:363–372. doi: 10.33549/physiolres.931526. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y, Nguyen TT, Bui KC, Demello DE, Smith JB. A novel inflammation-induced ubiquitin E3 ligase in alveolar type II cells. Biochemical and biophysical research communications. 2005;333:253–263. doi: 10.1016/j.bbrc.2005.05.102. [DOI] [PubMed] [Google Scholar]
- 51.Chen BB, Coon TA, Glasser JR, McVerry BJ, Zhao J, Zhao Y, Zou C, Ellis B, Sciurba FC, Zhang Y, Mallampalli RK. A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nature immunology. 2013;14:470–479. doi: 10.1038/ni.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, Frey RS, Vogel S, Minshall R, Christman JW, Tiruppathi C, Malik AB. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nature medicine. 2007;13:920–926. doi: 10.1038/nm1607. [DOI] [PubMed] [Google Scholar]
- 53.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circulation research. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 54.Coppi MV, Guidotti G. Ubiquitination of Na,K-ATPase alpha1 and alpha2 subunits. FEBS letters. 1997;405:281–284. doi: 10.1016/s0014-5793(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 55.Dada LA, Welch LC, Zhou G, Ben-Saadon R, Ciechanover A, Sznajder JI. Phosphorylation and ubiquitination are necessary for Na,K-ATPase endocytosis during hypoxia. Cellular signalling. 2007;19:1893–1898. doi: 10.1016/j.cellsig.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. The EMBO journal. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 57.Gille T, Randrianarison-Pellan N, Goolaerts A, Dard N, Uzunhan Y, Ferrary E, Hummler E, Clerici C, Planes C. Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway. American journal of respiratory cell and molecular biology. 2014;50:526–537. doi: 10.1165/rcmb.2012-0518OC. [DOI] [PubMed] [Google Scholar]
- 58.Boase NA, Rychkov GY, Townley SL, Dinudom A, Candi E, Voss AK, Tsoutsman T, Semsarian C, Melino G, Koentgen F, Cook DI, Kumar S. Respiratory distress and perinatal lethality in Nedd4-2-deficient mice. Nature communications. 2011;2:287. doi: 10.1038/ncomms1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? American journal of physiology. Lung cellular and molecular physiology. 2014;306:L217–L230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto T, Kajikawa O, Martin TR, Sharar SR, Harlan JM, Winn RK. The role of leukocyte emigration and IL-8 on the development of lipopolysaccharide-induced lung injury in rabbits. Journal of immunology. 1998;161:5704–5709. [PubMed] [Google Scholar]
- 61.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta biochimica Polonica. 2013;60:277–284. [PubMed] [Google Scholar]
- 62.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PloS one. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. The American journal of pathology. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. Journal of immunology. 2011;187:1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 65.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cellular microbiology. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 66.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, Aster RH, Wagner DD. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, Zweegman S, Vellenga E, Broyl A, Blau IW, Weisel KC, Wittebol S, Bos GM, Stevens-Kroef M, Scheid C, Pfreundschuh M, Hose D, Jauch A, van der Velde H, Raymakers R, Schaafsma MR, Kersten MJ, van Marwijk-Kooy M, Duehrsen U, Lindemann W, Wijermans PW, Lokhorst HM, Goldschmidt HM. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 68.Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V, Musto P, Boccadifuoco L, Offidani M, Omede P, Gentilini F, Ciccone G, Benevolo G, Genuardi M, Montefusco V, Oliva S, Caravita T, Tacchetti P, Boccadoro M, Sonneveld P, Palumbo A. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124:63–69. doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 69.Orlicky S, Tang X, Neduva V, Elowe N, Brown ED, Sicheri F, Tyers M. An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. Nature biotechnology. 2010;28:733–737. doi: 10.1038/nbt.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eckle T, Brodsky K, Bonney M, Packard T, Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M, Eltzschig HK. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol. 2013;11:e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mallampalli RK, Coon TA, Glasser JR, Wang C, Dunn SR, Weathington NM, Zhao J, Zou C, Zhao Y, Chen BB. Targeting F box protein Fbxo3 to control cytokine-driven inflammation. Journal of immunology. 2013;191:5247–5255. doi: 10.4049/jimmunol.1300456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. Journal of immunology. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert opinion on investigational drugs. 2011;20:897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boost KA, Hoegl S, Hofstetter C, Flondor M, Stegewerth K, Platacis I, Pfeilschifter J, Muhl H, Zwissler B. Targeting caspase-1 by inhalation-therapy: effects of Ac-YVAD-CHO on IL-1 beta, IL-18 and downstream proinflammatory parameters as detected in rat endotoxaemia. Intensive care medicine. 2007;33:863–871. doi: 10.1007/s00134-007-0588-0. [DOI] [PubMed] [Google Scholar]
- 76.Feist E, Burmester GR. Canakinumab for treatment of cryopyrin-associated periodic syndrome. Expert opinion on biological therapy. 2010;10:1631–1636. doi: 10.1517/14712598.2010.530653. [DOI] [PubMed] [Google Scholar]
- 77.Molto A, Olive A. Anti-IL-1 molecules: new comers and new indications. Joint, bone, spine : revue du rhumatisme. 2010;77:102–107. doi: 10.1016/j.jbspin.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Walther S, Jansson I, Berg S, Lennquist S. Pulmonary granulocyte accumulation is reduced by nebulized corticosteroid in septic pigs. Acta anaesthesiologica Scandinavica. 1992;36:651–655. doi: 10.1111/j.1399-6576.1992.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 79.Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Current opinion in investigational drugs. 2002;3:569–577. [PubMed] [Google Scholar]
- 80.Chen C, Zhang Z, Li Z, Zhang F, Peng M, Chen Y, Wang Y. Losartan attenuates microvascular permeability in mechanical ventilator-induced lung injury in diabetic mice. Molecular biology reports. 2014;41:809–814. doi: 10.1007/s11033-013-2920-9. [DOI] [PubMed] [Google Scholar]
- 81.Yao S, Feng D, Wu Q, Li K, Wang L. Losartan attenuates ventilator-induced lung injury. The Journal of surgical research. 2008;145:25–32. doi: 10.1016/j.jss.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 82.Paola RD, Cuzzocrea S. Peroxisome proliferator-activated receptors and acute lung injury. PPAR research. 2007;2007:63745. doi: 10.1155/2007/63745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu D, Zeng BX, Zhang SH, Wang YL, Zeng L, Geng ZL, Zhang SF. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Critical care medicine. 2005;33:2309–2316. doi: 10.1097/01.ccm.0000183161.81503.7d. [DOI] [PubMed] [Google Scholar]
- 84.Maron-Gutierrez T, Laffey JG, Pelosi P, Rocco PR. Cell-based therapies for the acute respiratory distress syndrome. Current opinion in critical care. 2014;20:122–131. doi: 10.1097/MCC.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 85.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. Journal of immunology. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 86.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory research. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu K, Wilson J, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Calfee C, Lee J-W, Kangelaris K, Gotts J, Rogers A, Levitt J, Wiener-Kronish J, Delucchi K, Leavitt A, McKenna D, Thompson B, Matthay M. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Annals of intensive care. 2014;4:22. doi: 10.1186/s13613-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]