INTRODUCTION
Osteoarthritis (OA) is a musculo-skeletal disease defined by progressive cartilage degradation leading to increasing joint pain and functional disability [39,50]. Patients suffering from painful knee OA and increasing disability in activities of daily living are prime candidates for total knee arthroplasty (TKA) [8]. TKA is a surgical procedure that resurfaces the damaged articular cartilage surface with metal, aiming to reduce pain and restore function to patients suffering from OA.
The socioeconomic impact of knee OA and subsequent dramatic rise in the annual number of TKA procedures worldwide have led to numerous investigations focused on understanding the pathophysiological, molecular, and biochemical mechanisms that underlie cartilage degradation, associated pain in OA [23,50], and perioperative TKA pain (i.e. persistent knee pain develops in 10-20% of TKA patients) [15]. It is now well-established that dysregulated biosynthesis and degradation of extracellular matrix components (e.g. collagen type II) by chondrocytes secondary to increased production of cytokines and matrix-degenerating enzymes leads to the breakdown of the extracellular matrix and cartilage [50]. Arthritic pain arises via nociceptors located in the affected joint triggered by inflammatory and catabolic mediators [23,37]. A host of pro-inflammatory mediators that contribute to painful OA have been identified including interleukins (e.g., IL-6) and prostaglandins [26,50].
The endocannabinoid system has recently emerged as a promising target for the development of novel analgesics [12,31,48]. For example, activation of cannabinoid receptors by their endogenous ligands, anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), or by synthetic agonists have been shown to reduce nociception in preclinical models of pain including OA [1,8,10,28,33,48]. Furthermore, the structurally related N-acylethanolamines (NAEs), palmitoylethanolamide (PEA) and oleoylethanolamide (OEA), activate the nuclear receptor peroxisome proliferator-activated receptor alpha, thereby producing antinociceptive and anti-inflammatory effects in preclinical pain models including OA [24,34,35]. Interestingly, recent data suggest that in addition to activating cannabinoid receptors, 2-AG is also a major source of arachidonic acid in numerous tissues and is therefore a precursor to pro-inflammatory eicosanoids [41], which could in principle contribute to OA pain.
To date a detailed analysis of endocannabinoid levels in OA patients and their influence upon OA-associated pain has not been reported despite the well-documented role of endocannabinoids in the modulation of pain and the recognized presence of cannabinoid receptors on chondrocytes [14]. A single clinical study has examined endocannabinoid levels in the synovial fluid of OA patients and found elevated AEA and 2-AG levels and reduced content of the anti-inflammatory PEA [45], possibly reflecting a pro-inflammatory and proalgesic environment. However, the association of the endocannabinoid tone with OA pain was not reported. Furthermore, the influence of the endocannabinoid tone upon post-operative pain and opioid usage in a clinical setting has likewise never been reported. Here, we begin to address these outstanding questions by providing the first comprehensive profile of endocannabinoid/NAE levels in serum, synovial fluid, and cerebrospinal fluid (CSF) in patients with painful OA and explore whether endocannabinoid levels correlate with baseline functional pain disability status, acute post-operative pain, and post-operative opioid use following TKA.
METHODS
Study participants
The local institutional review board approved our study protocol and written consent was obtained from each patient. Eligible TKA patients enrolled for this prospective, observational study were all scheduled for elective unilateral TKA under spinal anesthesia and femoral nerve block as per our standard of care clinical protocol. As part of the routine clinical care protocol, TKA patients had refrained from taking non-steroidal anti-inflammatory drugs for 7 days prior to surgery. However, as part of our multi-modal analgesic care protocol, all TKA patients (unless contraindicated) received a COX-2 selective inhibitor (Celecoxib) immediately prior to surgery (typically given at the time of the spinal anesthesia when the patient is sitting up). We excluded patients with 1) medical conditions which precluded use of regional anesthesia, 2) chronic pain with opioid usage over 100mg morphine-equivalents orally daily, 3) patients with documented rheumatoid arthritis, 4) history of abuse of opioids, 5) patients scheduled for bilateral TKA, and 6) patients scheduled for a TKA revision.
Experimental design & data collection
Prior to anesthesia and surgery, the TKA patients underwent assessment of pain disability status using the Pain Disability Questionnaire (PDQ) [3], which is well validated in patient groups with chronic pain or musculoskeletal disorders compared to asymptomatic normal individuals. The PDQ functional subscale consists of nine items, each of which are answered on a scale from 0-10, with 0 representing no disability while 10 representing maximal disability [3,13]. Functional items include the ability to walk, run, bend, lift, and reach objects overhead, personal care, work and travel. Pain was also assessed using the numerical rating scale (NRS. 0=no pain; 10=worst possible pain) pre- and post-operatively. At the time of the spinal anesthesia, blood and CSF were collected from the TKA patients. Synovial fluid was collected immediately prior to TKA surgery by the orthopedic surgeon (JN). The participating TKA patients’ demographic information and post-operative opioid consumption (morphine equivalent dose, mg) were also collected.
Quantification of Endocannabinoids
Serum, synovial fluid, or CSF (0.5 ml) was mixed with 3.5 ml of 2:1:1 CHCl3:MeOH:Tris (50 mM, pH 8) and spiked with 4 ng d4-PEA, d2-OEA, d5-2-AG, and 400 pg d4-AEA. Following centrifugation, the organic layer was removed and extracted again with the same buffer. The resulting organic layer was dried down under argon and resuspended with 120 μl of 2:1 CHCl3:MeOH and 10 μl was injected into a Thermo TSQ Quantum Access Triple Quadropole mass spectrometer in triplicate. Endocannabinoid quantification was performed exactly as described [25].
Analysis of IL-6
Serum samples were prepared from clotted blood that had been drawn from the patients using tubes without anticoagulant (BD Vacutainer®). The serum samples were flash frozen and stored at −80°C until analysis. Freshly drawn CSF was collected in sterile uncoated tubes, subjected to centrifugation at 1800 × g for 15 min at 4°C, and the supernatant was flash frozen in liquid N2 and stored at −80°C. A highly sensitive enzyme-linked immunosorbent assay specific for IL-6 (Quantikine® immunoassay kits, R&D Systems, Inc, MN) was used. Briefly, each sample was diluted with buffer and measured in triplicate in wells coated with monoclonal antibody specific for IL-6. Once unbound materials were rinsed away, a second cytokine-specific antibody conjugated to horseradish peroxidase (HRP) was added. Subsequent to removal of the unbound second antibody, HRP substrate was added to each well, followed by color development reagent. The color intensities were read at 450 nm and background corrected. The cytokine concentrations were calculated from the slopes of linear fits to the cytokine standard. The sensitivity of the control assays were within the manufacturer's specifications.
Statistical analysis
To examine differences in demographic parameters between male and female TKA patients, the mean differences in age, BMI, baseline PDQ functional scores and opioid usage over the first 12- and 24 hrs post-operatively were compared by a two-sided t-test for independent groups. The differences in pain assessed by the average NRS pain scores at 12- and 24 hrs post-operatively were compared using a two-sided Fisher's exact test. Spearman's rank correlation coefficient was used to quantify the correlation between each pair of endocannabinoids, as well between each endocannabinoid, baseline PDQ functional score and IL-6 levels. Correlations between endocannabinoids, age, and BMI were also examined. For each of the variables, observations that fell above 1.5*IQR (Interquartile range: difference between the 1st quartile and 3rd quartile) plus 3rd quartile or below 1st quartile minus 1.5*IQR were considered outliers and were excluded from the analysis. Pairwise comparisons were performed for each metabolite in each compartment. Correction for multiple testing was performed using the Benjamini and Hochberg false discovery rate of 5% [6]. All the statistical analyses were performed using SAS (Version 9.3, Cary NC), R (version 3.0.0). We consider a p-value less than 0.05 as statistically significant.
RESULTS
Subjects
Demographic information of male and female OA patients presenting for TKA surgery (n = 45) selected for endocannabinoid/NAE analysis is presented in Table 1. There were no differences in age, BMI, and pre-operative PDQ scores between male and female TKA patients. Acute postoperative average NRS pain scores assessed over 12- and 24 hrs averaged 4.5 with no observed pain differences between genders. In addition, average opioid consumption over 12- and 24 hrs was similar in male and female patients.
Table 1.
Demographics of subjects selected for the study (Mean ± S.E.).
| Male | Female | P value | |
|---|---|---|---|
| Number of Subjects | 20 | 25 | |
| Age | 67.3 ± 1.33 | 65.04 ± 1.74 | 0.335 |
| BMI | 30.88 ± 1.00 | 33.54 ± 1.00 | 0.071 |
| Diabetes, (%) | 6, (30%) | 7, (28%) | |
| Depression, Bipolar, Anxiety, (%) | 6, (30%) | 7, (28%) | |
| PDQ (functional) | 41.39 ± 4.04 | 40.28 ± 3.75 | 0.842 |
| NRS Pain 12 hrs | 4.46 ± 0.47 | 4.57 ± 0.50 | 0.872 |
| NRS Pain 24 hrs | 4.10 ± 0.45 | 4.31 ± 0.45 | 0.758 |
| Opioid usage, 12 hrs | 13.34 ± 2.71 | 17.88 ± 2.15 | 0.191 |
| Opioid usage, 24 hrs | 27.57 ± 5.61 | 35.73 ± 3.85 | 0.223 |
aBMI = Body Mass Index (kg/m2); PDQ=Pain disability Score; NRS=Numerical rating scale. Opioid usage defined as morphine equivalent dose (mg) over first 12 hrs or 24 hrs post-operatively.
Correlation between serum, CSF, and synovial fluid endocannabinoid levels
Comparison of endocannabinoids in serum, CSF, and synovial fluid revealed similar levels of PEA, OEA, AEA, and 2-AG in male and female subjects (Table 2). Therefore, all subsequent analyses of endocannabinoid levels in serum, CSF and synovial fluid included both genders. It should be noted that fewer synovial fluid samples were analyzed because synovial fluid was not collected from the initial set of patients from whom serum and CSF were obtained (see Table 2).
Table 2.
Comparison between serum, CSF, and synovial fluid pre-operative levels of PEA, OEA, AEA, and 2-AG (ng/ml) in male and female OA patients (mean ± S.E.). For each metabolite the number of samples for each of the two groups is indicated.
| Male | Female | P value | |
|---|---|---|---|
| PEAser | 1.74 ± 0.14 (N=20) | 1.70 ± 0.12 (N=24) | 0.813 |
| OEAser | 2.12 ± 0.24 (N=20) | 2.27 ± 0.21 (N=24) | 0.638 |
| AEAser | 0.22 ± 0.02 (N=20) | 0.26 ± 0.02 (N=24) | 0.139 |
| 2-AGser | 2.98 ± 0.35 (N=20) | 3.20 ± 0.34 (N=25) | 0.659 |
| PEAcsf | 0.16 ± 0.02 (N=18) | 0.15 ± 0.02 (N=21) | 0.729 |
| OEAcsf | 0.03 ± 0.00 (N=19) | 0.04 ± 0.01 (N=20) | 0.725 |
| AEAcsf | 0.01 ± 0.00 (N=19) | 0.01 ± 0.00 (N=22) | 0.819 |
| 2-AGcsf | 0.10 ± 0.01 (N=19) | 0.11 ± 0.02 (N=19) | 0.641 |
| PEAsyn | 0.85 ± 0.07 (N=12) | 0.86 ± 0.06 (N=10) | 0.861 |
| OEAsyn | 1.11 ± 0.13 (N=14) | 1.13 ± 0.10 (N=10) | 0.879 |
| AEAsyn | 0.09 ± 0.01 (N=14) | 0.09 ± 0.01 (N=10) | 0.693 |
| 2-AGsyn | 1.33 ± 0.21 (N=13) | 1.51 ± 0.30 (N=10) | 0.634 |
In the serum, AEA levels were positively correlated with PEA (r = 0.57, p < 0.0001) and OEA (r = 0.67, p < 0.0001) while PEA levels also correlated with OEA (r = 0.80, p < 0.0001) (Table 3). 2-AG levels did not correlate with PEA, OEA, or AEA. PEA and OEA levels were strongly (positively) correlated with each other in CSF as well as synovial fluid (Table 3). Serum and synovial fluid PEA levels were positively correlated (r = 0.62, p < 0.0001). Similar results were observed for serum and synovial fluid 2-AG levels (r = 0.45, p < 0.05). In contrast, no correlation was observed between serum and CSF 2-AG levels suggesting independent regulation of these two 2-AG compartments. Collectively, our data demonstrate significant correlations between endocannabinoids in different tissue compartments of OA patients suggesting synergy between their production and/or peripheral and central access/transport.
Table 3.
Correlation between serum, CSF, and synovial fluid NAE/endocannabinoid levels (Spearman correlation coefficients).
| PEAser | OEAser | AEAser | 2-AGser | PEAcsf | OEAcsf | AEAcsf | 2-AGcsf | PEAsyn | OEAsyn | AEAsyn | 2-AGsyn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEAser | 1 | - | - | - | - | - | - | - | - | - | - | - |
| OEAser | 0.80c | 1 | - | - | - | - | - | - | - | - | - | - |
| AEAser | 0.57c | 0.67c | 1 | - | - | - | - | - | - | - | - | - |
| 2-AGser | −0.14 | −0.06 | 0.22 | 1 | - | - | - | - | - | - | - | - |
| PEAcsf | −0.22 | −0.02 | 0.17 | 0.16 | 1 | - | - | - | - | - | - | - |
| OEAcsf | −0.07 | 0.06 | 0.02 | 0.08 | 0.53b | 1 | - | - | - | - | - | - |
| AEAcsf | 0.54b | 0.49b | 0.36a | −0.10 | −0.36a | −0.13 | 1 | - | - | - | - | - |
| 2-AGcsf | 0.14 | 0.06 | 0.12 | −0.03 | 0.05 | 0.00 | 0.12 | 1 | - | - | - | - |
| PEAsyn | 0.62c | 0.32 | 0.36 | 0.08 | −0.48a | −0.18 | 0.57b | 0.33 | 1 | - | - | - |
| OEAsyn | 0.56b | 0.30 | 0.27 | 0.18 | −0.42 | −0.11 | 0.55b | 0.36 | 0.88c | 1 | - | - |
| AEAsyn | −0.23 | −0.46a | 0.04 | 0.28 | 0.05 | −0.28 | −0.11 | 0.07 | 0.15 | 0.29 | 1 | - |
| 2-AGsyn | −0.08 | −0.10 | −0.02 | 0.45a | −0.17 | −0.10 | 0.00 | 0.05 | 0.13 | 0.33 | 0.20 | 1 |
p < 0.05
p < 0.01
p < 0.0001
Correlations between endocannabinoids, IL-6, and functional disability in OA patients
In addition to endocannabinoids, we also profiled levels of IL-6 in all compartments. There was a positive correlation between serum and synovial fluid IL-6 levels (r = 0.43, p < 0.05). IL-6 and endocannabinoid correlations are summarized in Table 4. In the serum, there were no significant correlations between endocannabinoids and IL-6. However, we observed trends for positive correlations between 2-AG and IL-6 levels in the CSF (r = 0.36, p = 0.054) and synovial fluid (r = 0.39, p = 0.071). A trend for a negative correlation between synovial fluid AEA and IL-6 levels was also observed (r = -0.37, p = 0.085). We also examined correlations between endocannabinoid levels and functional disability associated with OA. As shown in Table 5, only synovial fluid PEA exhibited a positive correlation with functional PDQ scores (r = 0.44, p < 0.05).
Table 4.
Correlation between endocannabinoid and IL-6 levels (Spearman correlation coefficients).
| PEAser | OEAser | AEAser | 2-AGser | PEAcsf | OEAcsf | AEAcsf | 2-AGcsf | PEAsyn | OEAsyn | AEAsyn | 2-AGsyn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6ser | 0.16 | 0.09 | 0.26 | 0.00 | −0.10 | −0.15 | 0.08 | 0.14 | 0.28 | 0.34 | −0.03 | 0.12 |
| IL-6csf | 0.23 | −0.08 | 0.01 | −0.11 | 0.00 | 0.20 | 0.19 | 0.36 | 0.17 | 0.20 | −0.13 | 0.00 |
| IL-6syn | 0.25 | 0.26 | 0.16 | 0.10 | 0.00 | 0.25 | −0.01 | 0.17 | 0.28 | 0.29 | −0.37 | 0.39 |
Table 5.
Correlation between post-operative pain, opioid use, and endocannabinoid levels (Spearman correlation coefficients).
| PDQ | NRS Pain 12 hrs | NRS Pain 24 hrs | Opioid Usage 12 hrs | Opioid Usage 24 hrs | |
|---|---|---|---|---|---|
| PEAser | 0.25 | 0.07 | −0.03 | 0.03 | −0.12 |
| OEAser | 0.20 | 0.04 | −0.05 | 0.09 | −0.02 |
| AEAser | 0.06 | −0.08 | −0.15 | 0.04 | −0.02 |
| 2-AGser | −0.06 | 0.18 | 0.13 | 0.25 | 0.23 |
| PEAcsf | 0.11 | −0.10 | −0.02 | −0.26 | −0.23 |
| OEAcsf | 0.15 | 0.07 | 0.14 | −0.04 | −0.14 |
| AEAcsf | 0.18 | 0.12 | 0.12 | 0.05 | −0.14 |
| 2-AGcsf | 0.13 | 0.27 | 0.33 | 0.13 | 0.00 |
| PEAsyn | 0.44a | 0.17 | −0.04 | 0.11 | −0.13 |
| OEAsyn | 0.26 | 0.18 | −0.03 | 0.15 | −0.18 |
| AEAsyn | 0.08 | 0.03 | 0.07 | −0.13 | −0.06 |
| 2-AGsyn | −0.04 | 0.42a | 0.35 | 0.55b | 0.52a |
p < 0.05
p < 0.01
PDQ=Pain disability Score; NRS=Numerical rating scale. Opioid usage defined as morphine equivalent dose (mg) over first 12 hrs and 24 hrs post-operatively.
2-AG is positively correlated with acute post-operative pain and opioid use following TKA
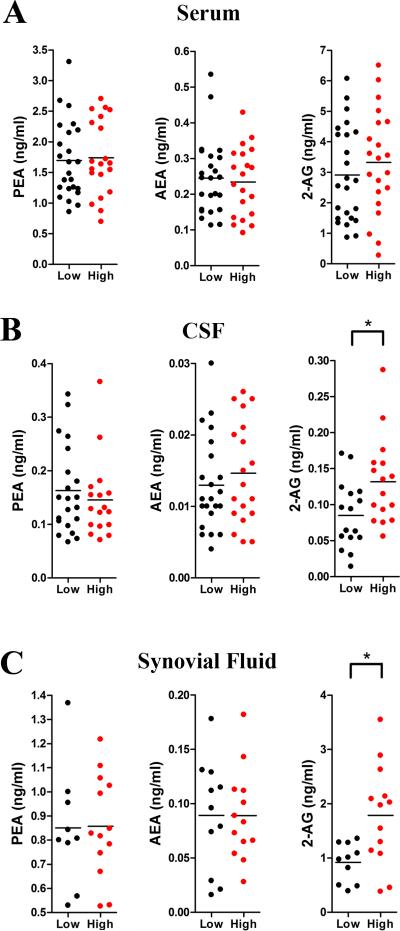
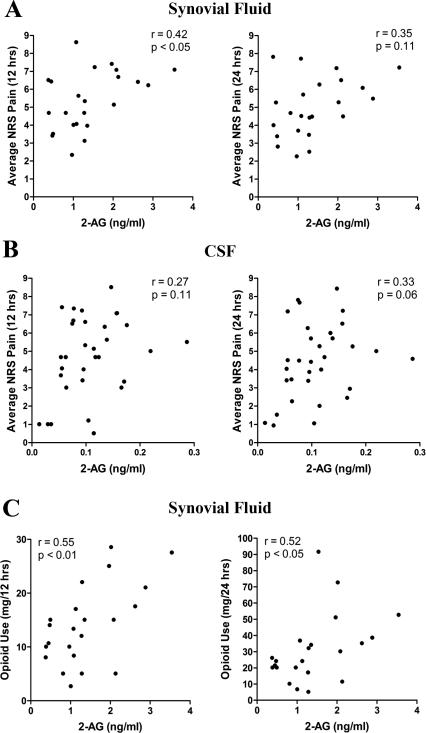
Previous work has demonstrated that PEA levels are reduced while AEA and 2-AG levels are elevated in the synovial fluid of OA patients [45]. Because 2-AG is a precursor to pro-inflammatory and proalgesic eicosanoids [41], we hypothesized that elevated pre-operative 2-AG and reduced PEA levels may predispose patients to develop greater post-operative pain. To test this hypothesis, we subdivided the TKA patients into those who developed low (NRS < 5) and high (NRS ≥ 5) post-operative acute pain at 12 hrs following TKA. In support of our hypothesis, the analysis revealed that patients who reported higher post-operative pain were characterized by elevated pre-operative levels of 2-AG in the CSF and synovial fluid (Figure 1). In contrast, PEA and AEA levels in all compartments as well as serum 2-AG levels did not differ in patients who reported low or high levels of pain. We subsequently examined whether 2-AG levels in the CSF and synovial fluid correlated with post-operative cumulative average pain scores measured at 12 and 24 hrs after surgery. Pain at 12 hrs was positively correlated with synovial fluid 2-AG levels (r = 0.42, p < 0.05) and a trend was also observed for the 24 hr pain measurements (r = 0.35, p > 0.05) (Table 5 and Figure 2A). A positive trend between pain at 24 hrs and CSF 2-AG levels was also observed (r = 0.33, p = 0.06) (Table 5 and Figure 2B).
Figure 1.
2-AG levels are elevated in patients who developed a high level of post-operative pain. PEA, AEA, and 2-AG levels in the (A) serum, (B) CSF, and (C) synovial fluid were examined in patients who developed low (NRS < 5, n = 24) and high (NRS ≥ 5, n = 21) post-operative pain. 2-AG levels were elevated in the CSF and synovial fluid of high pain patients (p < 0.05).
Figure 2.
Correlation between post-operative pain, opioid use, and 2-AG levels. Correlations between 12 and 24 hr NRS pain scores and 2-AG levels in the (A) synovial fluid and (B) CSF. (C) Correlations between synovial fluid 2-AG levels and cumulative post-operative opioid use during the first 12 hrs and 24 hrs after surgery.
Lastly, we explored whether elevated 2-AG levels were associated with post-operative opioid usage. We first confirmed that TKA patients’ post-operative opioid consumption depended on the severity of their reported acute pain (r = 0.48, p < 0.01). In support of our hypothesis, synovial fluid 2-AG levels were positively correlated with opioid use at 12 hrs (r = 0.55, p < 0.01) and at 24 hrs (r = 0.52, p < 0.05) after surgery (Table 5 and Figure 2C).
DISCUSSION
We characterized the endocannabinoid tone in patients undergoing TKA surgery and demonstrated that patients who reported more severe post-operative pain had higher pre-operative levels of 2-AG in CSF and synovial fluid, suggesting that 2-AG may predispose patients to acute pain after TKA surgery. Consequently, elevated pre-operative 2-AG levels in CSF and/or synovial fluid would predict greater intake of opioid analgesics post-operatively, which is corroborated by the positive correlations between synovial fluid 2-AG levels and opioid usage at 12 hrs and 24 hrs post-operatively.
The endocannabinoid system regulates nociception in rodents and humans [4,5,42,49]. Preclinical and clinical data suggest that activation of the endocannabinoid system produces antinociceptive effects in diverse animal models of pain and in neuropathic pain patients [36,44,48]. To date, only one study has examined endocannabinoid levels in OA patients [45] and demonstrated that PEA levels were significantly reduced while AEA and 2-AG levels were markedly elevated in the synovial fluid of OA patients. Here, we examined the influence of the endocannabinoid tone upon OA pain. Our data reveal that only synovial fluid PEA levels correlate with pre-operative functional disability, suggesting that the endocannabinoid tone (i.e., AEA and 2-AG) does not appear to modulate OA-associated disability as measured by PDQ scores. The positive correlation between PEA levels and PDQ scores may reflect the recruitment of this anti-inflammatory lipid [35] in response to chronic OA pain. Indeed, preclinical and clinical evidence suggests that PEA levels fluctuate under inflammatory states [45,47,52].
Although endocannabinoid levels were not examined in serum and synovial fluid from non-OA patients, serum endocannabinoid levels in our study are similar to previous reports of healthy and non-OA patients and did not correlate with pain, suggesting that the serum endocannabinoid tone is not altered by OA [11,16,18,19]. Our negative results in regard to endocannabinoids and chronic OA pain are in agreement with a recent clinical report demonstrating that inhibition of the endocannabinoid hydrolyzing enzyme fatty acid amide hydrolase, and consequent elevation of PEA, OEA, and AEA levels, does not alter pain in OA patients [22]. Furthermore, our results are also in agreement with other studies investigating other chronic pain states. For example, reduced AEA levels were observed in the CSF of chronic migraine patients [46], yet these did not correlate with pain severity. A lack of correlation was also observed for 2-AG [46]. Fibromyalgia patients possess elevated circulating AEA levels, which likewise did not correlate with pain [27]. In contrast, circulating 2-AG levels were negatively correlated with mechanical pain in neuromyelitis optica patients [43]. These data suggest that the endocannabinoid system may be differentially recruited under distinct chronic pain states.
The most interesting results of our study are the observations pertaining to the significant positive correlations between 2-AG levels in the CSF and synovial fluid and acute post-operative pain as well as opioid usage. One possible explanation for this association may be a potential mobilization of the endocannabinoid system in response to acute pain and its subsequent failure to dampen pain. Indeed, in preclinical models of acute post-operative pain, activation of cannabinoid receptors reduces nociception [2,32,38]. However, such a scenario is unlikely given that the CSF was sampled prior to the onset of surgery. Instead, although speculative, we favor a model wherein 2-AG levels are upregulated in OA and contribute to post-operative pain. Indeed, recent work has shown that in addition to serving as a cannabinoid receptor agonist, 2-AG serves as a major source of free arachidonic acid, the precursor to pro-inflammatory eicosanoids in the brain and peritoneal macrophages, and inhibition of 2-AG biosynthesis reduces pain in preclinical inflammatory and neuropathic pain models (Figure 3) [20,41,51]. 2-AG is likewise a substrate for cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) enzymes [17,29,30,40] and the COX-2 metabolite of 2-AG, prostaglandin E2-glycerol ester, induces pain [21]. Importantly, 2-AG levels are undetectable in the synovial fluid of non-inflamed normal patients but are significantly elevated in OA patients [45]. Therefore, elevated biosynthesis of 2-AG in OA and its possible subsequent metabolism during the post-operative period may facilitate the establishment of a pro-inflammatory and proalgesic environment, thereby enhancing postoperative pain. Indeed, pre-operative administration of non-steroidal anti-inflammatory drugs (i.e., COX inhibitors) is associated with reduced pain and opioid consumption during the postoperative period following TKA surgery [9].
Figure 3.
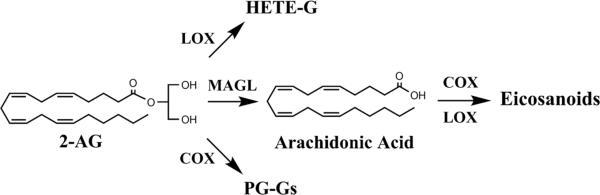
Model of 2-AG metabolism and its possible contribution to post-operative pain. Enzymes that mediate 2-AG metabolism. 2-AG metabolism occurs primarily through hydrolysis by monoacylglycerol lipase (MAGL), yielding arachidonic acid, which is subsequently converted into eicosanoids by COX and LOX enzymes. In addition, 2-AG can be metabolized into prostaglandin glycerol esters (PG-Gs) by COX-2 and hydroperoxyeicosatetraenoic acid glycerol esters (HETE-Gs) by LOX enzymes.
TKA is a painful procedure [7] and results in significant acute post-operative pain that can be partially controlled with analgesics such as opioids. Our data reveal that 2-AG levels in the synovial fluid are positively correlated with post-operative opioid use. Therefore, it is possible that the pre-existing pro-inflammatory environment of the arthritic knee, reflected here by elevated 2-AG levels, may predispose patients to higher post-operative pain, which necessitates elevated opioid consumption.
Collectively, our data are the first to demonstrate a correlation between 2-AG and acute post-operative pain in humans. Because CSF and synovial fluid levels of 2-AG are independently regulated (Table 3), this suggests compartment-specific mobilization of 2-AG pools in OA. Therefore, identification of possible 2-AG metabolites and their contribution towards postoperative pain may facilitate the development of novel opioid sparing analgesics.
ACKNOWLEDGEMENTS
We would like to thank Robert Rieger at the Stony Brook Proteomics Facility for help with mass spectrometry. This work was supported by the Department of Anesthesiology and by National Institutes of Health grants DA035923 and DA035949.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest with respect to this manuscript.
REFERENCES
- 1.Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16(4):411–20. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkaitis MS, Solorzano C, Landry RP, Piomelli D, DeLeo JA, Romero-Sandoval EA. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS One. 2010;5(5):e10891. doi: 10.1371/journal.pone.0010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostis C, Gatchel RJ, Mayer TG. The pain disability questionnaire: a new psychometrically sound measure for chronic musculoskeletal disorders. Spine (Phila Pa 1976) 2004;29(20):2290–302. doi: 10.1097/01.brs.0000142221.88111.0f. discussion 303. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17(10):1228–30. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain. 2013;154(3):361–7. doi: 10.1016/j.pain.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 7.Brander VA, Stulberg SD, Adams AD, Harden RN, Bruehl S, Stanos SP, Houle T. Predicting total knee replacement pain: a prospective, observational study. Clinical orthopaedics and related research. 2003;(416):27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 8.Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, Kendall DA, Lichtman A, Chapman V. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS One. 2013;8(11):e80440. doi: 10.1371/journal.pone.0080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, Rosenberg AG. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA. 2003;290(18):2411–8. doi: 10.1001/jama.290.18.2411. [DOI] [PubMed] [Google Scholar]
- 10.De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23(1):1–15. doi: 10.1016/j.beem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Fichna J, Wood JT, Papanastasiou M, Vadivel SK, Oprocha P, Salaga M, Sobczak M, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Malecka-Panas E, Krajewska WM, Koscielniak P, Makriyannis A, Storr MA. Endocannabinoid and Cannabinoid-Like Fatty Acid Amide Levels Correlate with Pain-Related Symptoms in Patients with IBS-D and IBS-C: A Pilot Study. PLoS One. 2013;8(12):e85073. doi: 10.1371/journal.pone.0085073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides medical journal. 2013;4(4):e0022. doi: 10.5041/RMMJ.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatchel RJ, Mayer TG, Theodore BR. The pain disability questionnaire: Relationship to one-year functional and psychosocial rehabilitation outcomes. Journal of Occupational Rehabilitation. 2006;16(1):75–94. doi: 10.1007/s10926-005-9005-0. [DOI] [PubMed] [Google Scholar]
- 14.Gomez R, Conde J, Scotece M, Lopez V, Lago F, Gomez Reino JJ, Gualillo O. Anandamide impairs cell growth and induces apoptosis in chondrocytes. J Orthop Res. 2014 doi: 10.1002/jor.22660. [DOI] [PubMed] [Google Scholar]
- 15.Grosu I, Lavand'homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013 doi: 10.1007/s00167-013-2750-2. [DOI] [PubMed] [Google Scholar]
- 16.Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, Hamuni G, Karabatsiakis A, Atsak P, Vogeser M, Kolassa IT. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One. 2013;8(5):e62741. doi: 10.1371/journal.pone.0062741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, Colbran RJ, Reese J, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci. 2013;16(9):1291–8. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, Hillard CJ, Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38(12):2952–61. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho WS, Hill MN, Miller GE, Gorzalka BB, Hillard CJ. Serum contents of endocannabinoids are correlated with blood pressure in depressed women. Lipids Health Dis. 2012;11:32. doi: 10.1186/1476-511X-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, Cravatt BF. DAGLbeta inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol. 2012;8(12):999–1007. doi: 10.1038/nchembio.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol. 2008;153(7):1538–49. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):1837–46. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ. Osteoarthritis. Best practice & research Clinical rheumatology. 2011;25(6):801–14. doi: 10.1016/j.berh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Impellizzeri D, Esposito E, Di Paola R, Ahmad A, Campolo M, Peli A, Morittu VM, Britti D, Cuzzocrea S. Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice. Arthritis Res Ther. 2013;15(6):R192. doi: 10.1186/ar4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaczocha M, Rebecchi MJ, Ralph BP, Teng YH, Berger WT, Galbavy W, Elmes MW, Glaser ST, Wang L, Rizzo RC, Deutsch DG, Ojima I. Inhibition of Fatty Acid binding proteins elevates brain anandamide levels and produces analgesia. PLoS One. 2014;9(4):e94200. doi: 10.1371/journal.pone.0094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature reviews Rheumatology. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann I, Schelling G, Eisner C, Richter HP, Krauseneck T, Vogeser M, Hauer D, Campolongo P, Chouker A, Beyer A, Thiel M. Anandamide and neutrophil function in patients with fibromyalgia. Psychoneuroendocrinology. 2008;33(5):676–85. doi: 10.1016/j.psyneuen.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain. 2010;11(12):1420–8. doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, Marnett LJ. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277(26):23278–86. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 30.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275(43):33744–9. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 31.La Porta C, Bura SA, Negrete R, Maldonado R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur J Neurosci. 2014;39(3):485–500. doi: 10.1111/ejn.12468. [DOI] [PubMed] [Google Scholar]
- 32.LaBuda CJ, Koblish M, Little PJ. Cannabinoid CB2 receptor agonist activity in the hindpaw incision model of postoperative pain. Eur J Pharmacol. 2005;527(1-3):172–4. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Lambert DM, Fowler CJ. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J Med Chem. 2005;48(16):5059–87. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 34.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67(1):15–9. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 35.LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319(3):1051–61. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 36.Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–44. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mapp PI. Innervation of the synovium. Ann Rheum Dis. 1995;54(5):398–403. doi: 10.1136/ard.54.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins DF, Mazzardo-Martins L, Cidral-Filho FJ, Gadotti VM, Santos AR. Peripheral and spinal activation of cannabinoid receptors by joint mobilization alleviates postoperative pain in mice. Neuroscience. 2013;255:110–21. doi: 10.1016/j.neuroscience.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 39.Millennium WHOSGotBoMCatSotN The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser. 2003;919:i–x. 1–218. back cover. [PubMed] [Google Scholar]
- 40.Moody JS, Kozak KR, Ji C, Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry. 2001;40(4):861–6. doi: 10.1021/bi002303b. [DOI] [PubMed] [Google Scholar]
- 41.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–13. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29(10):1964–78. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellkofer HL, Havla J, Hauer D, Schelling G, Azad SC, Kuempfel T, Magerl W, Huge V. The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica. PLoS One. 2013;8(8):e71500. doi: 10.1371/journal.pone.0071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci. 2014;17(2):164–74. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10(2):R43. doi: 10.1186/ar2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, Calabresi P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32(6):1384–90. doi: 10.1038/sj.npp.1301246. [DOI] [PubMed] [Google Scholar]
- 47.Sasso O, Moreno-Sanz G, Martucci C, Realini N, Dionisi M, Mengatto L, Duranti A, Tarozzo G, Tarzia G, Mor M, Bertorelli R, Reggiani A, Piomelli D. Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models. Pain. 2013;154(3):350–60. doi: 10.1016/j.pain.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009;11(1):39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suplita RL, 2nd, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49(8):1201–9. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–44. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 51.Wilkerson J, Ghosh S, Hsu KL, Cravatt B, Lichtman A. Diacylglycerol Lipase Beta: New evidence for inflammatory and neuropathic pain relief in mice. International Cannabinoid Research Society; Burlington, Vermont: 2014. [Google Scholar]
- 52.Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, Piomelli D. Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Mol Pharmacol. 2011;79(4):786–92. doi: 10.1124/mol.110.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]