Abstract
Summary Statement
Impaired olfaction, identified in 33% of patients undergoing cardiac surgery, was associated with the adjusted risk for postoperative delirium but not cognitive decline.
Objectives
The prevalence and significance of impaired olfaction is not well characterized in patients undergoing cardiac surgery. Because impaired olfaction has been associated with underlying neurologic disease, impaired olfaction may identify patients who are vulnerable to poor neurological outcomes in the perioperative period. The objective of this study was to determine the prevalence of impaired olfaction among patients presenting for cardiac surgery and the independent association of impaired olfaction with postoperative delirium and cognitive decline.
Design
Nested prospective cohort study
Setting
Academic hospital
Participants
165 patients undergoing coronary artery bypass and/or valve surgery
Measurements
Olfaction was measured using the Brief Smell Identification Test, with impaired olfaction defined as an olfactory score < 5th percentile of normative data. Delirium was assessed using a validated chart-review method. Cognitive performance was assessed using a neuropsychological testing battery at baseline and 4–6 weeks after surgery.
Results
Impaired olfaction was identified in 54 of 165 patients (33%) prior to surgery. Impaired olfaction was associated with increased adjusted risk for postoperative delirium (relative risk [RR] 1.90, 95% CI 1.17–3.09; P=0.009). There was no association between impaired olfaction and change in composite cognitive score in the overall study population.
Conclusion
Impaired olfaction is prevalent in patients undergoing cardiac surgery and is associated with increased adjusted risk for postoperative delirium, but not cognitive decline. Impaired olfaction may identify unrecognized vulnerability for postoperative delirium among patients undergoing cardiac surgery.
Keywords: Cognition Disorders, Delirium, Olfaction Disorders
INTRODUCTON
Impaired olfaction has been reported in 24.5% of participants in a community cohort study, with higher prevalence among older adults.1 Although the most common causes of impaired olfaction are sinus disease, history of upper respiratory infections/rhinitis, and head trauma,2 importantly, impaired olfaction has been shown to be an early marker of neurodegenerative disease.3 Olfactory abnormalities may predict Alzheimer’s disease, mild cognitive impairment, trajectory of cognitive decline, and neuropathologic hallmarks of Alzheimer’s disease, including pathology of the brain’s olfactory centers.4 Thus, assessment of olfaction may provide insights into pathologic conditions in the brain not normally evaluated before surgery, thereby potentially identifying patients with neurologic vulnerability at increased risk of postoperative delirium or cognitive decline.
The prevalence of impaired olfaction prior to cardiac surgery has not been systematically investigated. Further, whether there is an association between olfactory abnormalities and postoperative brain dysfunction, such as cognitive decline or delirium, is not known. We hypothesize that olfaction impairment is prevalent in patients undergoing cardiac surgery, and that it is independently associated with both postoperative cognitive decline and delirium.
METHODS
Patients
This study was a prospective observational study, nested in an ongoing multi-year randomized control trial study evaluating the association between cerebral blood flow autoregulation5 and brain injury after cardiac surgery (registration: www.clinicaltrials.gov NCT 00981474). The study procedures met with the approval of the Johns Hopkins Institutional Review Board (Baltimore, MD) and were performed after receiving individual written informed consents. Patients were enrolled between September 2010 and September 2012. Inclusion criteria were primary or re-operative coronary artery bypass graft (CABG) and/or valve surgery that required cardiopulmonary bypass and a high risk for neurologic complications (stroke or encephalopathy) as determined by a Johns Hopkins risk score >0.1.6 Exclusion criteria were renal failure requiring dialysis, non-English speaking, contraindications to MRI (e.g. pacemaker) and emergency surgery. Patients who did not have the capacity to consent based on an interview with research staff (and thus potentially delirious) were not enrolled. As part of the main randomized controlled trial, patients were randomized 1:1 to blood pressure targets during cardiopulmonary bypass based on measures of cerebral autoregulation vs. standard of care targets. Data on postoperative outcomes (major morbidity/mortality and acute kidney injury) in a subset of these patients has been reported separately.5,7 During the time period of the nested cohort study, 1337 patients were screened, of which 777 (58.1%) did not meet enrollment criteria, 246 (18.4)% were not approached for logistical reasons (such as staff availability on weekends or emergent procedure), 134 (10.0%) declined participation, and 180 (13.5%) were enrolled. Baseline olfaction data were missing for 15 patients, leaving a total of 165 patients included in this nested cohort study.
Perioperative Care
Patients received standard institutional monitoring including radial arterial blood pressure. General anesthesia was induced and maintained with midazolam (0.15 mg kg−1), fentanyl (5–20 µg kg−1) and isoflurane, with pancuronium or vecuronium for muscle relaxation. Cardiopulmonary bypass was with a non-occlusive roller pump, a membrane oxygenator, and the circuit included a ≤40-µm arterial line filter. Non-pulsatile flow was maintained between 2.0 and 2.4 L/min m−2. Patients were managed using alpha-stat pH management, and rewarming was based on institutional standards with a goal of maintaining nasopharyngeal temperature < 37°C. Postoperative sedation was with propofol 20 – 75 µg kg−1 min−1 until appropriate for tracheal extubation or for 24 hours after surgery. Patients requiring > 24 hours of mechanical ventilation received fentanyl and midazolam.
Olfaction Testing
Olfaction was objectively assessed before surgery (median 1 day prior to surgery, inter-quartile range 0–3 days) with the Brief Smell Identification Test™ (BSIT, Sensonic, Inc., Haddon Heights, NJ)8, a 12-item test of olfaction that uses microencapsulated odorants embedded in 10–50 µm ureaformaldehyde polymer microcapsules fixed to a strip on a single page binder. Scents are released when the strips are scratched. The patient is shown multiple-choice questions with 4 possible responses to identify the odorant (forced-choice test). The test-retest reliability of the BSIT in subjects tested twice within a 1-week interval was 0.71.9
Neuropsychological Testing, Delirium Assessment, and Neurologic Evaluation
Neuropsychological testing was generally performed 1–3 days before surgery and 4–6 weeks after surgery. The tests assessed a number of cognitive domains known to be associated with vascular disease, and thus potentially affected in patients after cardiac surgery.10–12 The testing battery consisted of the Rey Auditory Verbal Learning Test,13 a test of verbal learning and memory; Rey Complex Figure Test,14 a test of visuospatial ability and executive function; Controlled Oral Word Association Test,15 a test of executive functions; Symbol Digit Modalities Test,16 a test of psychomotor speed and attention control; Trail Making B,17 a test of visuomotor speed, attention, and executive functions; and the Grooved Pegboard Test,18 a measurement of fine motor dexterity and speed.
Delirium was assessed using a validated chart-review method.19 Because clinical judgment was required, a research assistant trained in formal delirium assessment by one of the authors (KN, a psychiatrist) performed all the chart abstractions using a chart-based instrument. According to the methodology of Inouye et al.,19 the abstractor searched for any mention of key terms, with evidence of acute onset or change, to support a diagnosis of delirium. Key terms and pertinent evidence were based on the following question: “Is there any evidence from the chart of acute confusional state (e.g. delirium, mental status change, inattention, disorientation, hallucinations, agitation, inappropriate behavior, etc.)?”19 All sections of the chart were searched, including but not limited to progress notes, nursing notes, social work notes, physical/occupational therapy notes, and consultant notes. For each possible episode of delirium, the abstractor recorded the source of information, time of onset, and a verbatim description of the episode. A three-person panel (KN, CB, LM) with training in formal delirium assessment and active involvement in delirium research reviewed the chart abstractions and determined the final diagnosis of delirium.
The neurologic status of patients was also evaluated on postoperative day 5 or at hospital discharge (whichever occurred first) with the National Institutes of Health Stroke Scale (NIHSS). Covariates and other variables were recorded from the anesthesia preoperative or intraoperative record and from other clinical documentation by research staff. In particular, staff specifically searched for evidence of baseline neurologic disease, including but not limited to Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment, seizures, and head trauma.
Data Analysis
Statistical analyses were conducted using Stata version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP)
Patients were classified as having impaired olfaction using prior methodology1 in which a cutoff was identified as a score on the BSIT below the 5th percentile from subjects < 55 y/o, using previously published normative data.20 Olfaction score on the BSIT was also considered as a continuous variable. The two primary outcomes were incident delirium and change in composite cognitive Z-score (defined as follow-up minus baseline score). To calculate the composite cognitive Z-score, individual cognitive test scores were converted to Z-scores, using the mean and standard deviation of the baseline scores of the control group from the randomized parent study as the reference. After each individual cognitive test score was converted to a Z-score, the scores were combined into an average Z-score, calculated from the average of the non-missing individual test Z-scores. Finally, the average Z-scores were re-normalized using the control group’s mean and standard deviation to develop a composite cognitive Z-score outcome.21
Our sample size has a power of 85% with an alpha of 0.05 to detect a 0.5 standard deviation difference in the composite cognitive outcome Z-score between individuals with versus those without impaired olfaction at baseline. Epidemiologic studies have suggested that a decline in cognitive Z-score of 0.5 is clinically important.22 Linear regression models were used to assess the association between impaired olfaction and the change in the composite cognitive Z-score from baseline. Generalized linear regressions for the binomial family and robust variance estimator23,24 were used to estimate the relative risk of post-operative delirium comparing patients with and without abnormal olfaction after adjusting for the relevant covariates. Variables to include in adjusted models were considered a priori and included age, history of stroke, baseline cognitive status (for the model examining delirium only), and randomization status. We did not include baseline cognitive status in the primary model examining change in cognitive outcomes because of the potential for biased results.25 However, we subsequently examined effect modification by baseline cognitive score on the association between baseline olfaction status and change in composite cognitive score, using interaction terms in the regression model, which by necessity include baseline cognitive status. Thus, this analysis may be limited by potential measurement error of the cognitive outcome, which could introduce bias when baseline cognition is included in the model, as well as the potential for factors leading to cognitive change prior to baseline to continue to operate during follow-up, which could also introduce bias.25
Reasons for missing data and their patterns were explored and tabulated, and several sensitivity analyses were conducted. First, to account for missing olfaction data and other covariates, assuming missingness at random, multiple imputation using Markov chain Monte Carlo (MCMC) data augmentation method for multivariate normal model26 was performed using all data from the parent study and using the missing data module in Stata. Sensitivity of the inferences to the model specification and the multiple imputation method was assessed by 1) increasing the number of imputed datasets up to 40, and 2) including various sets of variables and changing imputation equations. Second, post-operative cognitive outcome data was completely missing in 43/165 (26%) of patients. Sensitivity analysis of incomplete outcome data was conducted by assigning missing data to random draw from either upper 10% or lower 10% of the distribution of the postoperative composite cognitive scores in the entire sample assuming that those with missing data either had the “best” or the “worst” outcomes. Results were assessed for complete case and the 2 additional assumptions regarding the missing outcome data. Although patients with delirium at the time of consent were ineligible for this study, we also conducted a sensitivity analysis adjusted for age and cognition in which we excluded patients with evidence of delirium at any time during the surgical hospitalization prior to surgery.
RESULTS
Olfaction testing was available from 165 patients of whom 54 (33%) had impaired olfaction prior to surgery. The prevalence of impaired olfaction increased with increasing age: for <60 y/o, 24%; for 61–70 y/o, 26%; for 71–80 y/o, 41%; and for > 80 y/o, 44%, although this trend did not reach statistical significance (P = 0.191 for Fisher’s exact test). Characteristics of patients with and without impaired olfaction are listed in Table 1. In the overall cohort, no patients had diagnoses of Alzheimer’s disease, Parkinson’s disease, or mild cognitive impairment. Patients with and without impaired preoperative olfaction were similar with the exception of a higher frequency of prior transient ischemic attacks in the normal olfaction group and a trend towards increased age (P =0.05) in the impaired olfaction group. As shown in Table 2, patients with preoperative impaired olfaction scores had lower scores on all baseline individual cognitive tests and the composite cognitive Z-score than patients with preoperative normal olfaction scores.
Table 1.
Patient Characteristics.
| Variable | Normal Baseline Olfaction N=111 |
Impaired Baseline Olfaction N=54 |
P-Value |
|---|---|---|---|
| Age (mean±SD) | 69±8 | 72±8 | 0.053a |
| Male/Female, n (%) | 71/40 (64/36) | 36/18 (66.7/33.3) | 0.733b |
| Race | |||
| caucasian, n (%) | 89 (80.2) | 41 (75.9) | 0.536c |
| African American, n (%) | 15 (13.6) | 10 (18.9) | |
| Hispanic, n (%) | 2 (1.8) | 0 | |
| Asian (Indian), n (%) | 1 (0.9) | 0 | |
| Asian (Oriental), n (%) | 2 (1.8) | 0 | |
| Other, n (%) | 2 (1.8) | 3 (5.7) | |
| Prior Stroke, n (%) | 11 (9.9) | 5 (9.3) | 1.000c |
| Prior Transient Ischemic Attack, n (%) | 14 (12.6) | 0 (0.0) | 0.005c |
| Prior Carotid Artery Endarterectomy, n (%) | 5 (4.5) | 3 (5.6) | 0.717c |
| Subjective Memory Loss, n (%) | 1 (0.9) | 1 (1.9) | 0.549c |
| Concussion History, n (%) | 0 (0) | 1 (1.9) | 0.327c |
| Tremor History, n (%) | 2 (1.8) | 1 (1.9) | 1.000c |
| Seizures, n (%) | 0 (0) | 1 (1.9) | 0.327c |
| Evidence of preoperative delirium, n (%) | 3 (2.7) | 3 (5.6) | 0.394c |
| Chronic Obstructive Lung Disease, n (%) | 15 (13.5) | 7 (13.0) | 1.000c |
| Current Smoker, n (%) | 10 (9.0) | 7 (13.0) | 0.427c |
| Previous Smoker, n (%) | 47 (42.3) | 25 (46.3) | 0.631b |
| Coronary Artery Disease, n (%) | 88 (79.3) | 41 (75.9) | 0.625b |
| Prior Myocardial Infarction, n (%) | 27 (24.3) | 17 (31.5) | 0.329b |
| Peripheral Arterial Disease, n (%) | 16 (14.4) | 14 (25.9) | 0.072b |
| Hypertension, n (%) | 96 (86.5) | 45 (83.3) | 0.640c |
| Diabetes, n (%) | 60 (54.1) | 24 (44.4) | 0.247b |
| Congestive Heart Failure, n (%) | 16 (14.4) | 9 (16.7) | 0.817c |
| Atrial Fibrillation, n (%) | 16 (14.4) | 7 (13.0) | 1.000c |
| Prior Cardiac Surgery, n (%) | 9 (8.1) | 5 (9.3) | 0.774c |
| Chemotherapy, n (%) | 3 (2.7%) | 3 (5.6%) | 0.394c |
| Sinus Disease, n (%) | 3 (2.7%) | 1 (1.9%) | 1.000c |
| Medication | |||
| Aspirin, n (%) | 87 (78.4%) | 42 (77.8%) | 0.930b |
| Heparin, n (%) | 32 (28.8) | 12 (22.2) | 0.368b |
| Diuretics, n (%) | 45 (40.5) | 22 (40.7) | 0.980b |
| Nitrates, n (%) | 23 (20.7) | 11 (20.4) | 0.958b |
| HMG-Coenzyme A Reductase Inhibitors, n (%) | 70 (63.1) | 31 (57.4) | 0.484b |
| Beta-adrenergic receptor blockers, n (%) | 69 (62.2) | 31 (57.4) | 0.558b |
| Calcium Channel Blockers | 29 (26.1) | 14 (25.9) | 0.978b |
| Angiotensin Converting Enzyme Inhibitors, n (%) | 39 (35.1) | 21 (38.9) | 0.638b |
| Surgical Procedure | |||
| CABG, n (%) | 65 (58.6) | 32 (59.3) | 0.411c |
| CABG/AVR, n (%) | 11 (9.9) | 6 (11.1) | |
| CABG/MVR, n (%) | 8 (7.2) | 4 (7.4) | |
| AVR, n (%) | 17 (15.3) | 5 (9.3) | |
| MVR, n (%) | 2 (1.8) | 4 (7.4) | |
| Aortic Root Repair, n (%) | 6 (5.4) | 1 (1.9) | |
| Ascending Aneurysm Repair, n (%) | 1 (0.9) | 0 | |
| Other, n (%) | 1 (0.9) | 2 (3.7) | |
| Cardiopulmonary bypass duration (min), median (IQR) | 98 (79–130) | 106 (77–139) | 0.823d |
| Aortic cross-clamping duration (min), median (IQR) | 63 (49–83) | 61 (50–88) | 0.722d |
Abbreviations: SD=standard deviation; CABG=coronary artery bypass graft; AVR=aortic valve replacement; MVR=mitral valve repair/replacement; IQR=interquartile-range
T-test with equal variance
Chi-square test;
Fisher’s exact test;
Wilcoxon test
Table 2.
Preoperative and Postoperative (4–6 weeks after surgery) Cognitive Test Scores for Patients with Normal Versus Impaired Olfaction.
| Preoperative Cognitive Testsa | Postoperative Cognitive Testsa | |||||
|---|---|---|---|---|---|---|
| Test | Normal Olfaction N=111 |
Impaired Olfaction N=54 |
P-Value | Normal Olfaction N=89 |
Impaired Olfaction N=33 |
P-Value |
| Composite Z-Score Cognitive Outcome | 0.3 ± 1.0 | −0.5 ± 0.8 | <0.001b | 0.2±0.9 | −0.3±0.9 | 0.006b |
| RAVLT Trial 5 (range 0–15) | 9.0 ± 2.7 | 7.8 ± 2.5 | 0.004b | 9.6±2.8 | 7.8±3.1 | 0.003b |
| RAVLT Trial 9 (range 0–15) | 11.8 ± 2.8 | 9.9 ± 3.9 | 0.001b | 12.7±2.0 | 11.1±3.6 | 0.005b |
| Rey Complex Figure (range 0–36) | 31.0 ± 5.5 | 27.1 ± 6.9 | <0.001b | 30.8±5.8 | 28.8±5.8 | 0.117b |
| Controlled Oral Word Association Test | ||||||
| Letter F | 11.6 ± 4.4 | 9.2 ± 3.7 | 0.001b | 11.6±4.9 | 10.0±4.1 | 0.109b |
| Letter A | 9.9 ± 4.7 | 7.3 ± 3.6 | 0.001b | 9.0±4.7 | 7.4±4.1 | 0.090b |
| Letter S | 12.7 ± 5.4 | 9.9 ± 4.7 | 0.002b | 11.4±4.9 | 9.7±4.0 | 0.063b |
| Symbol Digit Modalities Test (range 0–110) | 34 ± 11 | 26 ± 11 | <0.001b | 35.0±11.9 | 31.2±11.4 | 0.127b |
| Trail Making B | 119 ± 57 | 147 ± 67 | 0.014c | 112±51 | 121±58 | 0.428c |
| Grooved Peg Board, Dominant Hand | 117 ± 46 | 141 ± 68 | 0.010c | 111±46 | 125±57 | 0.207c |
| Grooved Peg Board, Non-Dominant Hand | 137 ± 49 | 168 ± 84 | 0.008c | 130±61 | 144±74 | 0.616c |
Abbreviations: RAVL=Rey Auditory Verbal Learning Test
Data are reported as number correct (mean±SD) with the exception of Trail Making B and Grooved Peg Board, that are timed tests reported in seconds, and the composite outcome, which is reported as a Z score;
T-test with equal variance;
Wilcoxon test
Patients with normal vs. impaired olfaction had similar postoperative complications—for postoperative stroke, 3.6% vs. 5.6%, P=0.68; for acute kidney injury categories: risk, 35% vs. 29.6%, injury, 9.0% vs. 3.7%, and failure, 0.9% vs. 0%, overall P=0.32; for atrial fibrillation, 38.7% vs. 42.6%, P=0.64; for inotropic drugs > 24 hours, 13.5% vs. 11.1%, P=0.81; for mechanical lung ventilation > 48 hours, 8.1% vs. 9.3%, P=0.77; and for in-hospital mortality 3.6% vs. 5.6%, P=0.68 for Fisher’s exact test. However, the incidence of postoperative delirium was greater in patients with impaired vs. normal baseline olfaction (46.3% vs. 23.4%, P=0.003, unadjusted).
Cognitive Testing
Postoperative cognitive tests were available from 122 patients (Table 2). In unadjusted analyses, patients with impaired baseline olfaction had a lower composite cognitive Z-score at 4–6 weeks after surgery (P =0.006), and lower scores on the RAVLT test trial 5 correct (P =0.003) and RAVLT test 9 correct (P =0.005) compared to those with normal olfaction.
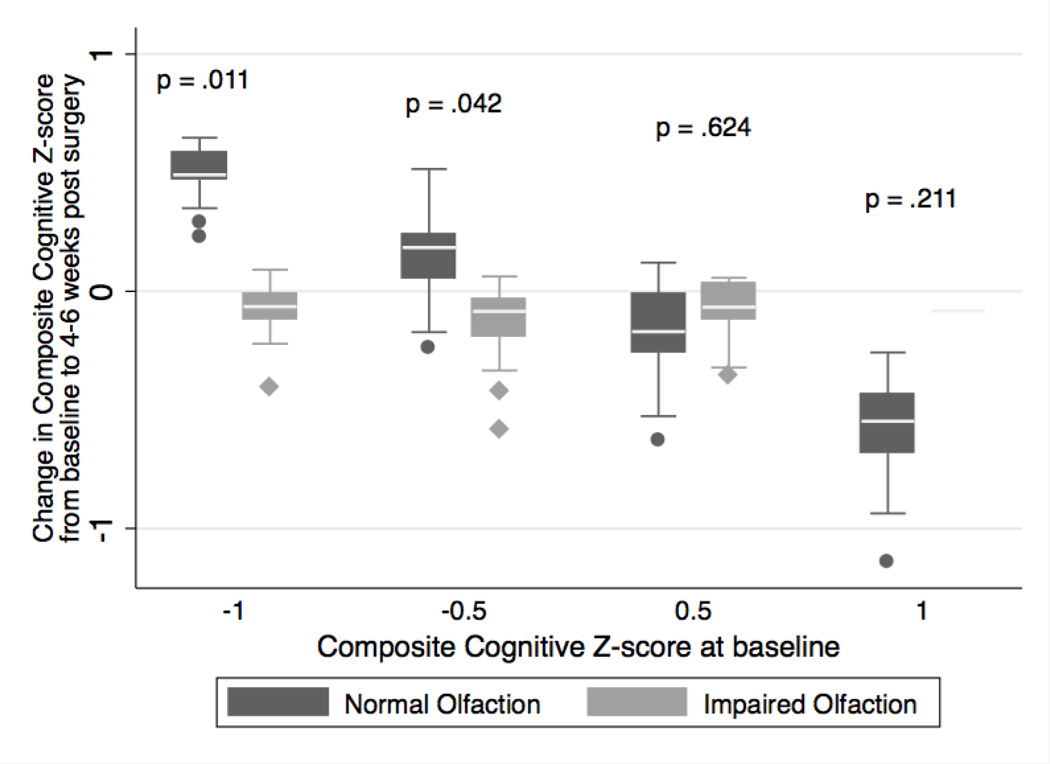
The change in the composite cognitive Z-score from baseline for the impaired olfaction compared to the normal olfaction group is listed in Table 3. After adjusting for age, history of stroke, and randomization status, there was no association between impaired olfaction and the change in composite cognitive Z-score outcome (coefficient, 0.03; 95%CI, −0.23 to 0.29; P=0.80). Because we hypothesized that patients with low baseline cognitive scores and impaired olfaction might be especially vulnerable to further cognitive decline after surgery, we added baseline cognition as an interaction term to the adjusted model and found that baseline cognitive score modified the association between impaired olfaction and change in the composite cognitive score after surgery (P interaction = 0.014). The results are shown in Figure 1 as the distribution of predicted change in the composite cognitive score from the multivariable linear model for impaired and normal olfaction at various levels of baseline composite cognitive score. When baseline cognitive score was low, patients with impaired olfaction had more decline in composite cognitive Z-score post-operatively, compared with patients with normal olfaction. In contrast, at higher baseline cognitive scores, the decline in composite cognitive Z-score was not different between the normal and impaired olfaction groups.
Table 3.
The Association between Impaired Olfaction and Postoperative Neurological Outcomes
| Change in Cognitive Z-Scorea | Delirium | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedc | |||||
| β-coefficient (95%CI) | P- value |
β-coefficient (95%CI) |
P- value |
Relative Risk (95%CI) |
P- value |
Relative Risk (95%CI) |
P- value |
|
| Impaired vs. normal olfaction | 0.03 (−0.21, 0.28) | 0.80 | 0.03 (−0.23, 0.29) | 0.80 | 1.98 (1.27, 3.08) | 0.003 | 1.90 (1.17, 3.09) | 0.009 |
| Olfaction test score (range 0–12) (per one fewer correct item) | 0.02 (−0.03, 0.07) | 0.45 | 0.02 (−0.03, 0.08) | 0.44 | 1.12 (1.03, 1.23) | 0.009 | 1.11 (1.01, 1.21) | 0.026 |
Abbreviations: CI=Confidence Interval
Change is defined as follow-up score minus baseline score, so “positive” values indicate improvement.
Adjusted for age, randomization group, and prior stroke, (Note: age was modeled as a non-linear predictor)
Adjusted for age, randomization group, prior stroke, and baseline cognition (Note: age was modeled as a linear predictor)
Figure 1. Change in cognitive Z-scores between patients with impaired and normal baseline olfaction scores, according to baseline cognitive status.
Distribution of predicted change in composite cognitive Z-score from baseline to 4–6 weeks after surgery between patients with impaired and normal olfaction scores, at various levels of baseline cognitive Z-score. Presented p-values are associated with the adjusted comparison of change in composite cognitive score for impaired vs. normal olfaction groups, at several selected levels of baseline cognition.
Delirium
The incidence of delirium in the study sample was 31%. Impaired olfaction was associated with an estimated 1.90-fold increased risk of postoperative delirium, after adjusting for age, history of stroke, randomization status, and baseline composite cognitive Z-score (relative risk [RR], 1.90; 95%CI, 1.17−3.09; P =0.009). As context, the increased risk of delirium with impaired olfaction in our model is similar to an 18-year age increase. Additionally, for every 1-unit decrease in baseline olfaction score (range 3–12) the adjusted risk of delirium increased by an estimated 11% (RR, 1.11; 95%CI, 1.01–1.21; P=0.03).
Sensitivity Analyses
In a multiple imputation analysis to account for missing olfaction or covariate data, the increased risk of delirium was still significant for the impaired vs. normal olfaction groups, but was not significant when the effect of a 1-unit decrease in olfaction score was considered. The results of sensitivity analysis of missing change in composite Z-score data under 2 scenarios—best post-operative Z-score or worst post-operative Z-score—were not different from the complete case analysis. Additionally, there remained an increased risk of delirium in patients with impaired olfaction when patients (n=6) with evidence of delirium during the surgical hospitalization prior to surgery were excluded.
DISCUSSION
In this study we identified impaired olfaction in 33% of patients before cardiac surgery. The presence of pre-existing impaired olfaction compared to normal olfaction was associated with an increased risk for postoperative delirium and worse baseline cognitive score, but no difference in the adjusted change in postoperative cognitive performance from baseline.
The results of this study highlight the prevalence of impaired olfaction in patients undergoing cardiac surgery. The high prevalence of impaired olfaction has not been well described during the perioperative period, although anecdotal reports of impaired olfaction following surgery have been noted.27 The prevalence of impaired olfaction in our study is slightly higher than that reported in a community-based study of 2491 individuals age 53 to 97 years, in which impaired olfaction was found in 24.5% of subjects.1 In that study, factors found to be independently associated with impaired olfaction were age, male sex, current smoking, nasal congestion, stroke, and epilepsy. Our results suggest an association between impaired olfaction and increasing age (P=0.05).
Although impaired olfaction might be considered trivial in the context of cardiac surgery, our results demonstrate that impaired olfaction is associated with a severe postoperative complication—delirium. Postoperative delirium is a common disorder in the elderly which is associated with prolonged hospitalization, medical complications, decreased independence, and mortality.28,29 The mechanism of postoperative delirium is not known but likely results from the interaction between pre-existing patient vulnerability (such as age, neurodegeneration, and education) and acute precipitating factors.30 Since prior studies have demonstrated associations between impaired olfaction and neurodegenerative disease, such as Alzheimer’s and Parkinson’s diseases,3,4 assessment of olfaction might add to current methods of evaluating patient’s neurologic status. Olfaction impairment may identify patients with an increased risk of unrecognized neurologic impairment, which renders them vulnerable to perioperative stress and subsequent delirium. Importantly, the increased risk of delirium among patients with impaired olfaction was independent of baseline cognitive scores. Because treatment of established delirium is difficult, prevention is important, and early identification of high-risk patients would help target prevention efforts and potentially reduce negative consequences of delirium-prevention efforts, such as medication side effects or increased resource utilization.
Our results also demonstrate an association between impaired baseline olfaction and poor baseline cognition, further supporting the hypothesis that impaired olfaction might identify patients with unrecognized or subclinical neurologic impairment. However, in adjusted models, impaired olfaction at baseline was not associated with an overall change in the composite cognitive Z-score from baseline to 4–6 weeks postoperatively, similar to results from a previous study in non-cardiac surgery patients.31 Interestingly however, we did find that in the subset of patients with low cognitive scores at baseline, the presence of impaired olfaction before surgery was associated with worse cognitive performance after surgery, an association not seen in patients with high baseline cognitive scores. These results suggest that impaired olfaction may indicate greater cognitive vulnerability among patients already at low baseline cognitive status. These results must be interpreted cautiously since the significant difference in cognitive outcomes among patients with low baseline cognitive scores was largely due to improvement in postoperative cognitive scores among patients with normal olfaction (perhaps due to learning effect), as opposed to a large decline in postoperative cognitive scores among patients with impaired olfaction.
While our data does not allow mechanistic explanation of the observation that impaired olfaction is associated with delirium, one potential explanation is that impaired olfaction identifies patients at higher risk of undiagnosed or preclinical neurodegenerative disease, who are vulnerable to delirium. The earliest pathology of Alzheimer’s disease, including neurofibrillary tangles and amyloid plaques, occurs in areas that are critical for olfactory processing, including the anterior olfactory nucleus, the prepiriform cortex, the entorhinal cortex, the amygdaloid areas, and the hippocampal areas,32 and neuronal loss has been reported in both the olfactory bulb and olfactory epithelium in patients with Alzheimer’s disease compared to controls.33 Further, olfactory dysfunction has been shown to increase in parallel with progression of Alzheimer’s disease.34 Recent evidence has emerged that the microglia in elderly patients with chronic neurodegenerative disease may be “primed” for an inflammatory insult.35 After exposure of primed microglia to an inflammatory insult, such as surgery and cardiopulmonary bypass, an exaggerated microglial response with increased cytokine expression and expression of damaging molecules, such as inducible nitric oxide synthase, may result in delirium and accelerate further neurodegeneration.35
There are several limitations to our study, and thus our results should be considered preliminary and need to be confirmed. Our methods provide only a broad assessment of olfaction, since time constraints of olfactory testing of surgical patients who are also undergoing neuropsychological testing and examination are not conducive to more detailed testing. Thus, our methods do not allow for distinguishing the location (peripheral vs. central) of olfaction impairment, nor do they allow for distinguishing which component of olfaction was impaired, such as threshold, detection, or odor discrimination. Odor identification and discrimination involve high-order cognitive processes while threshold detection requires only low-level perception.36 We also defined impaired olfaction based on comparison of individual test scores with normative data derived from volunteers, and the olfaction testing in this study used a forced answer format, which might introduce a source of misclassification error.
The incidence of delirium in our study was 31%, which is consistent with prior studies of patients undergoing cardiac surgery.37 We used a rigorous validated method of delirium assessment, but compared to the well-known Confusion Assessment Method,38 sensitivity of our method has been shown to be 74% and specificity 83%.19 Thus, misclassification error may be present, although we do not expect that misclassification would be differential by olfaction status. We did find that in the subset of patients with low cognitive scores at baseline, the presence of impaired compared to normal olfaction before surgery was associated with worse cognitive performance after surgery. As discussed in the methods section, a limitation of this analysis is potential bias that is introduced by the necessary inclusion of baseline cognitive status in the model as an interaction term. Additionally, there appeared to be a significant learning effect among patients with normal olfaction at baseline. Therefore, an alternative explanation is that patients with impaired olfaction and low baseline cognitive scores did not experience the same learning effect after surgery as patients with normal olfaction.
Because of the limitations of both the olfaction testing and the chart-based delirium assessment as described, these results should be considered as preliminary and suggestive, and need to be confirmed using more rigorous measures in future studies. Further studies are also needed to identify whether adding impaired olfaction to known predictive models of delirium after cardiac surgery can improve model accuracy and better identify patients at increased risk of postoperative delirium.
ACKNOWLEDGMENTS
Conflict of Interest
Funding: This work was supported by National Institutes of Health (NIH) KL-2 Clinical Research Scholars Program, NIH (RO3 AG042331), and the Jahnigen Career Development Award (CB); Mid-Atlantic Affiliate of the American Heart Association and the NIH (RO1 HL092259) (CH), and the American Heart Association Scientist Development Grant (RG).
Karin Neufeld has received research support from Ornim Medical.
Charles Hogue has received research support from Covidien, Inc. and served on the advisory board for Ornim Medical.
Footnotes
Author Contributions: The corresponding author affirms that everyone who has significantly contributed to this work is listed as an author.
Charles Brown was involved in study design, methods, analysis assistance, preparation of paper, and approved the final version.
Candice Morrissey was involved in study design, methods, analysis assistance, preparation of paper, and approved the final version.
Masahiro Ono was involved in data collection, preparation of paper, and approved the final version.
Gayane Yenokyan was involved in data analysis, preparation of paper, and approved the final version.
Ola A. Selnes was involved in study design, methods, preparation of paper, and approved the final version.
Jeremy Walston was involved in data interpretation, preparation of paper, and approved the final version.
Laura Max was involved in data collection, preparation of paper, and approved the final version.
Andrew LaFlam was involved in data collection, preparation of paper, and approved the final version.
Karin Neufeld was involved in data collection, preparation of paper, and approved the final version.
Rebecca Gottesman was involved in study design, methods, preparation of paper, and approved the final version.
Charles Hogue was involved in study design, subject recruitment, analysis assistance, preparation of paper, and approved the final version.
Sponsor’s Role: None
REFERENCES
- 1.Murphy C, Schubert CR, Cruickshanks KJ, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 2.Murphy C, Doty RL, Duncan HJ. Clinical disorders of olfaction. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2nd ed. New York, NY: Wiley; 2002. pp. 461–478. [Google Scholar]
- 3.Haehner A, Hummel T, Reichmann H. Olfactory Loss in Parkinson's Disease. Parkinsons Dis. 2011;2011:1–6. doi: 10.4061/2011/450939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers M, Tabert M, Devanand D. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep. 2006;6:379–386. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- 5.Ono M, Brady K, Easley R, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKhann GM, Grega MA, Borowicz LM, et al. Encephalopathy and stroke after coronary artery bypass grafting: Incidence, consequences, and prediction. Arch Neurol. 2002;59:1422–1428. doi: 10.1001/archneur.59.9.1422. [DOI] [PubMed] [Google Scholar]
- 7.Ono M, Arnaoutakis G, Fine D, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doty RL. The Brief Smell Identification Test Administration Manual. White Horse Pike NJ: Sensonic Inc; 2001. [Google Scholar]
- 9.Doty R, McKeown D, Lee W, et al. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995;20:645–656. doi: 10.1093/chemse/20.6.645. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk D, Keizer AM, Diephuis JC, et al. Neurocognitive dysfunction after coronary artery bypass surgery: A systematic review. J Thorac Cardiovasc Surg. 2000;120:632–639. doi: 10.1067/mtc.2000.108901. [DOI] [PubMed] [Google Scholar]
- 11.Stump DA. Selection and clinical significance of neuropsychologic tests. Ann Thorac Surg. 1995;59:1340–1344. doi: 10.1016/0003-4975(95)00108-w. [DOI] [PubMed] [Google Scholar]
- 12.Kneebone AC, Andrew MJ, Baker RA, et al. Neuropsychologic changes after coronary artery bypass grafting: use of reliable change indices. Ann Thorac Surg. 1998;65:1320–1325. doi: 10.1016/s0003-4975(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 13.Powell J, Cripej L, Dodril lC. Assessment of brain impairment with the Rey Auditory-Verbal Learning Test. Arch Clin Neuropsychol. 1991;6:241–249. [PubMed] [Google Scholar]
- 14.Meyers JE, Meyers KR. Rey complex figure test under four different administration procedures. Clin Neuropsychol. 1995;9:63–67. [Google Scholar]
- 15.Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 16.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 1998. [Google Scholar]
- 17.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 18.Costa LD, Vaughan HG, Jr, Levita E, et al. Purdue Pegboard as a predictor of the presence and laterality of cerebral lesions. J Consult Psychol. 1963;27:133–1337. doi: 10.1037/h0040737. [DOI] [PubMed] [Google Scholar]
- 19.Inouye S, Leo-Summers L, Zhang Y. A chart-based method for identification of delirium: Validation compared with interviewer ratings using the Confusion Assessment Method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Marcus A, Lee W. Development of the 12-Item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Selnes O, Grega M, Borowicz L, Jr, et al. Cognitive outcomes three years after coronary artery bypass surgery: a comparison of on-pump coronary artery bypass graft surgery and nonsurgical controls. Ann Thorac Surg. 2005;79:1201–1219. doi: 10.1016/j.athoracsur.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Goldsborough M, Borowicz L, et al. Cognitive outcome after coronary artery bypass: A One-Year Prospective Study. Ann Thorac Surg. 1997;63:510–515. doi: 10.1016/s0003-4975(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 23.Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9(2):175. [Google Scholar]
- 24.Wacholder S. Binomial regression in GLIM: Estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 25.Glymour M, Weuve J, Berkman L, et al. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162(3):267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 26.Allison P. Missing Data. Thousand Oaks, London, UK: Sage Publications; 2002. [Google Scholar]
- 27.Henkin RI. Altered taste and smell after anesthesia: Cause and effect? Anesthesiology. 1995;83:648–649. doi: 10.1097/00000542-199509000-00041. [DOI] [PubMed] [Google Scholar]
- 28.Zakriya K, Sieber FE, Christmas C, et al. Brief postoperative delirium in hip fracture patients affects functional outcome at three months. Anesth Analg. 2004;98:1798–1802. doi: 10.1213/01.ANE.0000117145.50236.90. [DOI] [PubMed] [Google Scholar]
- 29.Sieber F. Postoperative delirium in the elderly surgical patient. Anesthesiol Clin. 2009;27:451–464. doi: 10.1016/j.anclin.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Inouye S, Charpentier P. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–857. [PubMed] [Google Scholar]
- 31.Rentowl P, Hanning CD. Odour identification as a marker for postoperative cognitive dysfunction: A pilot study. Anaesthesia. 2004;59:337–343. doi: 10.1111/j.1365-2044.2004.03678.x. [DOI] [PubMed] [Google Scholar]
- 32.Nordin S, Murphy C. Impaired sensory and cognitive olfactory function in questionable Alzheimer's disease. Neuropsychology. 1996;10:113–119. [Google Scholar]
- 33.Kovacs T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3:215–232. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- 35.Perry V, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 36.Hedner M, Larsson M, Arnold N, et al. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32:1062–1067. doi: 10.1080/13803391003683070. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado JR, Wysong A, Van der Starre P, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 38.Inouye S, van Dyck C, Alessi C, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]