Abstract
The ability to reproduce visually presented actions has been studied through neuropsychological observations of patients with ideomotor apraxia. These studies include attempts to understand the neural basis of action reproduction based on lesion–symptom mapping in different patient groups. While there is a convergence of evidence that areas in the parietal and frontal lobes within the left hemisphere are involved in the imitation of a variety of actions, questions remain about whether the results generalize beyond the imitation of tool use and whether the presence of a strong grasp component of the action is critical. Here we used voxel-based lesion–symptom mapping to assess the neural substrates of imitating meaningful (familiar, MF) and meaningless (unfamiliar, ML) tool-related (transitive) and non-tool related (intransitive) actions. The analysis showed that the left parietal cortex was involved in the imitation of transitive gestures, regardless of whether they were meaningful or not. In addition there was poor reproduction of meaningless actions (both transitive and intransitive) following damage of the right frontal cortex. These findings suggest a role of right frontal regions in processing of unfamiliar actions.
Keywords: Voxel-based morphometry, Ideomotor apraxia, Action imitation, Transitive gestures, Intransitive gestures
Highlights
We used voxel-based lesion–symptom mapping to assess imitation's neural substrates.
Transitive meaningful and meaningless actions involved the left parietal cortex.
Meaningless transitive and intransitive actions involved the right frontal cortex.
1. Introduction
Imitation is an innate tendency in humans (Meltzoff and Moore, 1977; Meltzoff and Moore, 1983; Meltzoff and Moore, 1989) as well as in newborn chimpanzees (Bard and Russell, 1999; Myowa, 1996; Myowa-Yamakoshi et al., 2005) and macaques (Ferrari et al., 2006). The ability to reproduce gestures may provide one of the foundations of social communication and it may have an important role in learning effective tool use (e.g. how to use a hammer).
Following a brain lesion people with apraxia can suffer from a deficit in reproducing and generating gestures (Mehler, 1987). In right handed people this syndrome has classically been described in relation to lesions of the left posterior parietal cortex (Liepmann, 1900; Liepmann, 1905; Rothi et al., 1991) though other studies point to the role of right brain areas as well as sub-cortical structures (Leiguarda et al., 1997; Leiguarda, 2001; De Renzi et al., 1980; Tessari et al., 2007; Bonivento et al., 2013), especially when finger configurations (Della Sala et al., 2006; Goldenberg and Strauss, 2002) or movement sequences (Canavan et al., 1989) have to be copied.
Prior neuropsychological evidence indicates that apraxia is not a unitary phenomenon, and frequently symptom dissociations have been reported in neuropsychological patients (Rumiati et al., 2009). For example, neuropsychological observations suggest a double dissociation between the production of meaningful and meaningless gestures, with some studies reporting patients who are more impaired when imitating meaningless (ML) compared to meaningful (MF) gestures (Tessari et al., 2007; Bartolo et al., 2001; Goldenberg and Hagmann, 1997; Peigneux et al., 2000) and others showing the opposite pattern (Tessari et al., 2007; Bartolo et al., 2001). Double dissociations have also been observed between finger and hand gestures, with patients able to imitate hand postures but not finger configurations and other patients with the opposite pattern of deficits (Goldenberg and Karnath, 2006).
A cognitive neuropsychological model of praxis was first proposed by Rothi et al. (1991) and then modified by other authors (Goldenberg and Hagmann, 1997; Buxbaum, 2001; Cubelli et al., 2000; Rumiati and Tessari, 2002). This model postulates (i) a semantic route to action, relying on long-term memory representations, which allow the reproduction of the MF (known) gestures, and (ii) a direct route, depending on a short-term memory representation of the action (i.e. the innervatory pattern in the original model from Rothi et al., 1991), which supports the reproduction of ML (new) gestures. For gesture reproduction the starting point of both routes is a visual analysis component, through which the visual properties of actions are processed. Also both the semantic and the direct processes end at the level of the motor system, which determines the actual implementation of the action.
Neuropsychological observations of unilateral brain damaged patients (Goldenberg and Hagmann, 1997; Peigneux et al., 2000; Buxbaum, 2001; Cubelli et al., 2000), as well as behavioural and imaging studies with healthy participants (Rumiati et al., 2009; Tessari and Rumiati, 2004; Peigneux et al., 2004), support the idea of these two separate neural systems for imitating MF and ML gestures but the results are inconsistent with respect to the neuro-anatomical structures involved (particularly concerning their hemispheric location). Lesions involving the parietal cortex, especially the angular gyrus, have been reported to cause a deficit in the imitation of meaningful (MF) as compared to meaningless (ML) actions in left-brain damaged patients (LBD; Tessari et al., 2007). On the other hand, Peigneux et al. (2000) reported a patient, with a lesion in the left occipito-parietal cortex, who imitated MF gestures better than ML gestures. Furthermore, Tessari et al. (2007) reported two right brain damaged patients (RBD), with lesions including the caudal portion of the pallidum, the putamen and the posterior limb of the internal capsule, who were more impaired in imitating ML than MF gestures. In the same study Tessari et al. (2007) presented data from LBD patients whose performance was worse with ML gestures, and this was associated with lesions to the superior temporal gyrus.
In studies using positron emission tomography (PET), the imitation of MF actions has been linked to activation in the left angular gyrus, the left middle frontal gyrus, the right supramarginal gyrus, and the right inferior parietal lobule (Peigneux et al., 2004), as well as the inferior temporal, angular and parahippocampal gyri in the left hemisphere (Rumiati et al., 2005). The parieto-occipital and occipito-temporal junctions on the right, the superior temporal gyrus on the left, and the superior parietal cortex bilaterally have also been shown to have increased activation linked to the imitation of ML actions (Rumiati et al., 2005).
Goldenberg et al. (2007) used lesion subtraction analysis to determine the locations specifically associated with defective pantomime of tool use in patients with left-brain damage and aphasia. Their results showed that the left inferior frontal cortex was associated with a deficit in pantomime — though the area of lesion overlap further extended into the underlying white matter so it is possible that damage to the white matter projections contributed to the observed deficit (Liepmann, 1905; Catani and Ffytche, 2005; Geschwind, 1975). The left inferior frontal and inferior parietal cortices were also found to be damaged in a group of stroke patients having a deficit in at least one of two tests assessing ideomotor apraxia (De Renzi et al., 1980) and ideational apraxia (De Renzi and Lucchelli, 1988) respectively, with the inferior frontal lesions being more frequent in those patients who were additionally impaired in recognizing transitive and intransitive actions (Pazzaglia et al., 2008).
Finally, a recent DTI study (Ramayya et al., 2010) with healthy right handed male volunteers investigated the connections between four regions that previous fMRI studies indicated to be involved in processing and performing complex tool use gestures: i) the posterior middle temporal cortex (linked to semantic representations of tools); ii) the posterior inferior parietal cortex (coding spatial invariant components of learned gestures to tools, such as the swing of the elbow to use a hammer); iii) the anterior inferior parietal cortex (proposed to integrate non-spatial semantic representations of tools with the spatial information relevant for effective tool use); and iv) the posterior inferior frontal and ventral premotor cortices that convert the planned gesture into the proper activation pattern for the movement implementation. The results highlighted the role of white matter pathways connecting the posterior middle temporal cortex and the anterior inferior parietal cortex, as well as the anterior inferior parietal cortex and the posterior inferior frontal and ventral premotor cortices. In both cases there was an asymmetry with significantly more subjects showing those connections in the left than the right hemisphere. Also the inferior posterior parietal cortex showed connections with the posterior middle temporal cortex, lateralized on the left, and with the inferior frontal and premotor cortices, lateralized in the right hemisphere. However in both cases the fibre terminations did not fall in the same location as the posterior parietal activations identified by previous functional neuroimaging studies and hence the role in tool use may be questioned (Ramayya et al., 2010).
The neuropsychological studies described above have typically relied on categorical subdivisions of patients based on a given cut-off as well as on observer-dependent lesion demarcations. The use of a cut-off, especially in tasks where each answer is scored either 1 or 0, introduces the risk that patients having scores differing only for one point are treated differently (scores that equal the cut-off will be considered as defective, while scores one point higher than the cut-off may be treated as not defective). Also manual demarcations of lesion sites can lead to under- or over- estimation of tissue differences according to the strictness of the observer's criteria. The present study aimed to investigate the neural substrates of action imitation using a procedure based on continuous rather than categorical scoring that is not biased by either its sampling of patients or lesion demarcation. To achieve this we employed whole-brain voxel-based morphometry (VBM) (Ashburner and Friston, 2000) based on the segmentation of grey matter (GM) and white matter (WM) tissue. VBM uses the general linear model to statistically assess the relations between brain tissue integrity and behavioural performance. Here, continuous MF and ML imitation scores were used as predictors of change in signal intensity within each voxel across the whole brain in a group of consecutively sampled patients, not pre-selected on the basis of having apraxia. This approach also allowed us to enter into the model covariates of no interest such as age, gender, handedness and lesion volume (i.e. the extent of the patients' lesions), which might otherwise confound the results.
For the first time in a single study we examined the neural substrates of MF and ML intransitive actions, relative to MF and matched ML transitive actions. We used the scores for patients obtained when imitating MF transitive and intransitive pantomimes, along with matched ML transitive and intransitive actions, as predictors of voxel signal intensity. The results are discussed in terms of previous findings, the mechanisms of apraxia and the neuronal underpinnings of action imitation.
2. Methods
2.1. Participants
2.1.1. Patients
Twenty-one consecutively-sampled patients (20 males and 1 female, 18 right-handed, 3 left-handed; mean age: 68.18; SD: 11.13. Age range: 36–82) were recruited from the panel of neuropsychological volunteers at the Behavioural Brain Science Centre, School of Psychology of the University of Birmingham. All patients had acquired brain lesions due to a stroke, were in the chronic stage (>9 months after the occurrence of the lesion) and had no contraindications to magnetic resonance imaging (MRI) scans. No other exclusion criteria were used. Seven patients had a lesion involving the left side of the brain (left brain damaged, LBD), eleven had a lesion on the right (right brain damaged, RBD) and three had bilateral brain lesions (BBL). According to the Edinburgh Handedness Inventory (Oldfield, 1971), 18 patients were right handed before the stroke and three were left-handed. Patients' demographic data and their Edinburgh Handedness Inventory scores are summarized in Supplementary Table 1.
2.1.2. Healthy controls
Eighteen English-speaking healthy participants (mean age = 65.56, SD = 10.18, age range = 37–78) with no reported history of neurological and/or psychiatric disease served as controls. Fifteen controls self-reported as right-handed while three reported as left-handed. All had normal or corrected-to-normal vision. Data from controls were used only to perform a preliminary categorization of each patient's imitation performance in order to describe the patient sample at a behavioural level. In addition to this, for the purpose of lesion identification (see below), we acquired T1-weighted images from 100 healthy controls (55 males and 45 females, mean age 54.5 years, range 20–87) with no history of stroke, brain damage or neurological disorders.
All patients and healthy volunteers provided written informed consent in agreement with ethics protocols at the School of Psychology and Birmingham University Imaging Centre (BUIC).
2.2. Imitation task
2.2.1. Transitive actions
For the transitive actions we presented 20 meaningful (MF) pantomimes of objects being used (e.g. hammering or drinking from a glass) and 20 unfamiliar meaningless (ML) control actions derived from the MF actions (e.g. an action maintaining the grasp and arm configuration for hammering but performed in an unusual direction; for details about the stimuli see Tessari and Rumiati, 2004). The stimuli employed here have been used in previously published studies on the topic, with both patients and controls, with participants from different nationalities (e.g. in Italy, Tessari and Rumiati, 2004, and in Germany, Rumiati et al., 2005; see Tessari and Rumiati, 2004 for the complete list of 20 MF and 20 ML actions). Pictures showing a sample of MF and ML transitive actions are provided in Supplementary Fig. 1.
Each transitive action was presented once to each participant and scored as 1 if performed correctly or 0 if it was performed incorrectly (maximum MF score = 20, ML = 20; total = 40).
The same list of actions was administered to patients and controls.
2.2.2. Intransitive actions
The stimuli were taken from the original set of 18 MF and 18 ML intransitive gestures by Tessari et al. (2011). The intransitive MF actions were a sample of the gestures commonly used for communication (e.g. waving “hello”) and were selected on the basis of being easily recognized by 10 independent Italian raters. The intransitive ML actions were created in order to match the MF actions for the complexity of their execution and they were judged as unrecognizable by the same 10 independent Italian raters as before (Tessari et al., 2011). The same list of MF and ML actions had already been used in previous studies on healthy Italian-speaking participants (Rumiati et al., 2009; Carmo and Rumiati, 2009). One half of the MF and ML actions involved movements of the hand (i.e. distal) while the other half involved the proximal use of an arm. In the present study, to adapt the task to the English patients and controls, 3 MF gestures were removed from the original MF list (see Tessari et al., 2011 for the complete actions' list) as 18 healthy English controls consistently failed to indicate that the gestures had a meaning. The three actions removed were the gestures for: i) starving (i.e. repeatedly tapping the stomach with the hand's side); ii) later (i.e. rotating the index finger); and iii) go away (i.e. oscillating the hand with the back of the hand towards the other person). The remaining 15 MF stimuli were easily recognized and named correctly by the native English raters. Three actions were also discarded from the original ML list i.e., i) touching one side of the body with the ipsilateral hand with the palm upward; ii) touching one cheek with the back of the contralateral hand; and iii) touching the chin with the finger tips, in order for this list to equal the MF list length. The discarded ML actions matched for complexity the gestures eliminated from the MF set according to the data from ten native English independent raters.
Two examples of the MF and ML intransitive actions used here as stimuli are illustrated in Supplementary Fig. 2.
Each intransitive action was shown only once and performance was scored 1 if the action was correctly executed and 0 if it was done incorrectly (maximum MF score = 15, ML = 15; total = 30).
2.2.3. Procedure — transitive and intransitive gestures
Each type of action was presented in a separate block to maximize the use of differential imitation processes, with the order of presentation randomized within each list. There were four blocks of stimuli: i) MF transitive; ii) ML transitive; iii) MF intransitive; and iv) ML intransitive. The block of MF pantomimes was administered before the ML pantomimes. After a short break, the intransitive actions were presented, again with the MF followed by the ML block. The MF actions were administered before the ML stimuli in order to reduce the likelihood of selecting the common ‘direct’ route for imitation of MF as well as ML actions, given that MF actions could be reproduced using the same, direct imitative route as ML actions (Tessari and Rumiati, 2004).
The experimenter demonstrated each action using the right (dominant) hand. This was done to maximize the consistency of stimulus presentation across participants. We avoided using video stimulus presentations as it could have affected patients' performance because of reasons unrelated to a praxic deficit (e.g. if patients could not clearly see the stimuli because of the relatively small size of the computer screen). Patients and controls were instructed to reproduce the action as similarly as possible to the model. The patients performed the pantomimes using either their dominant hand or the ipsilesional hand if there was paresis of the contralesional limb. Sixteen patients used their dominant limb while five used their non-dominant limb (see Supplementary Table 1).
Table 1.
Grey matter substrates of the imitation of transitive and intransitive MF and ML gestures (VBM analysis).
| Cluster level |
Voxel level |
Coordinates |
Brain structure |
||||
|---|---|---|---|---|---|---|---|
| Model | p-FWE | Size | Z-score | X | Y | Z | (location) |
| Model 1: transitive MF; transitive ML; intransitive MF; intransitive ML | |||||||
| 0.007 | 1955 | 3.92 | −6 | −58 | 46 | Left PCUN (BA7) | |
| 3.53 | −4 | −4 | 50 | Left SMA (BA6) | |||
| 3.49 | −6 | −76 | 30 | Left CUN (BA17) | |||
| Model 2: transitive ML | |||||||
| 0.000 | 7514 | 4.20 | −14 | −50 | 22 | Left PCUN (BA7) | |
| 4.10 | −24 | −60 | 54 | Left SPC (BA7) | |||
| 3.95 | −32 | −64 | 18 | Left Mid OCC (BA17) | |||
| Left Mid CC (BA 24) | |||||||
| 0.049 | 1424 | 3.89 | 20 | 8 | 40 | Right SFC (BA6) | |
| 3.59 | 12 | 34 | 50 | Right Med SFC (BA6) | |||
| 3.41 | 14 | −6 | 42 | Right Mid CC (BA24) | |||
| Model 3: transitive MF | |||||||
| 0.013 | 1996 | 3.91 | −8 | −38 | 26 | Left PCC (BA23) | |
| 3.88 | −12 | −44 | 54 | Left PCUN (BA7) | |||
| 3.63 | −12 | −44 | 22 | Left PCC (BA23) | |||
| Model 4: intransitive ML | |||||||
| 0.000 | 3993 | 4.77 | 8 | 46 | 40 | Right Med SFC (BA6) | |
| 4.25 | 8 | 28 | 40 | Right Med SFC (BA6) | |||
| 4.25 | 12 | 6 | 64 | Right SMA (BA6) | |||
| 3.92 | −6 | −58 | 46 | Left PCUN (BA7) | |||
| 3.53 | −4 | −4 | 50 | Left SMA (BA6) | |||
Abbreviations: BA, Brodmann Area; CUN, cuneus; Med SFC, medial superior frontal cortex; Mid CC, middle cingulate cortex; Mid OCC, middle occipital cortex; PCC, posterior cingulate cortex; PCUN, precuneus; SFC, superior frontal cortex; SMA, supplementary motor area; SPC, superior parietal cortex; VBM, voxel-based morphometry.
To control for the hand used, some controls were asked to use their dominant hand while others used their non-dominant hand when responding.1 Twelve controls imitated with their dominant hand while six used their non-dominant hand.
The performance of each participant was video-recorded and later scored by two independent raters blind to the experimental conditions.2 For both transitive and intransitive actions, an action was scored as incorrect if the participant performed a spatial error with the hand or the arm; a visual error (i.e. the action was: i) a combination of two items included in the list; ii) an action that was visually similar to the target; iii) a meaningful action, visually similar to the meaningless target), or an omission (for a detailed description of the errors see Tessari and Rumiati, 2004).
2.3. Other neuropsychological assessment
Patients were also administered some sub-tests from the BCoS Screen (Humphreys et al., 2012) assessing i) orientation — i.e. Personal Information and Time and Space; ii) frontal functions — the Rule Finding and Switching task; iii) language — i.e. Picture Naming; and iv) action planning and control — i.e. Multiple Step Object Use, Gesture Production, Imitation, Gesture Recognition and Figure Copy. The scores for all patients from the neuropsychological evaluation are reported in Supplementary Table 2.
2.4. Neuroimaging assessment
2.4.1. MRI scans
The MRI scans of patients and healthy controls were acquired at the Birmingham University Imaging Centre (BUIC) on a 3 T Philips Achieva MRI system with an 8 channel phased array SENSE head coil. A sagittal T1-weighted sequence (sagittal orientation, echo time/time to repetition, TE/TR = 3.8/8.4 ms, voxel size 1 × 1 × 1 mm3) was used to acquire the anatomical scans.
2.4.2. Pre-processing of the T1 data
The T1 scans were converted and reoriented with MRIcro (Rorden, 2005) and then preprocessed using SPM5 (Mechelli et al., 2005). The scans were transformed into the standard MNI (Montreal Neurological Institute) space using a modified unified-segmentation procedure (Friston et al., 2007; Ashburner and Friston, 2005) protocol, optimized for patients with brain lesions by including an extra tissue class that accounts for the “abnormal” voxels within lesions (Ashburner and Friston, 2005). The procedure generated four classified tissue maps for grey matter (GM), white matter (WM), cerebrospinal fluid (CSF) and abnormal tissue, on the basis of the intensity of the signal in each voxel using a priori knowledge of the expected location of that tissue, with each map representing the probability that a given voxel belonged to GM, WM, CSF or an abnormal tissue class (Ashburner and Friston, 2005). After segmentation, the resulting images were smoothed using an 8-mm FWHM Gaussian filter previously shown to be optimal for lesion detection and further analysis of segmented images (Ashburner and Friston, 2005; Seghier et al., 2008).
2.4.3. VBM analyses
Voxel-based morphometry was carried with SPM5 (Friston et al., 2007) using parametric statistics within the framework of general linear model (Ashburner and Friston, 2000).
The segmented scans (see above) for 21 patients were used to investigate the relationship between GM and WM integrity and continuous scores for the transitive MF and ML and intransitive MF and ML imitation tasks on a voxel-by-voxel basis. The patients' raw scores for imitating transitive MF and ML and intransitive MF and ML gestures were used as the covariates of interest. As the scores at the different imitation tasks were (unavoidably) highly correlated, after consulting a leading expert in the field, we decided to adopt the following approach: Two models were estimated: one including only GM and the other WM segmented images along with the four covariates of interest i.e. i) transitive MF, ii) transitive ML, iii) intransitive MF, and iv) intransitive ML. Also, eight further models were estimated, each including only one covariate of interest and either GM or WM: 1) transitive MF and GM; 2) transitive ML and GM; 3) intransitive MF and GM; 4) intransitive ML and GM; 5) transitive MF and WM; 6) transitive ML and WM; 7) intransitive MF and WM; and 8) intransitive ML and WM.
Age, handedness,3 education, gender and lesion volume were as used covariates of no interest in all the models.
T- contrasts were run on each model using a mixed peak and cluster threshold with at least 1000 voxels showing a Z > 2.6 (i.e. p ≤ 0.0054, uncorrected). Results are reported at the cluster level corrected for multiple comparisons (p = 0.05, FWE, corrected).
The anatomical localization of the lesions was performed using the AAL3 toolbox (Tzourio-Mazoyer et al., 2002) and confirmed using the Duvernoy human brain atlas (Duvernoy, 1991). The location of WM lesions with regard to specific pathways was assessed based on the JHU WM tractography atlas (Hua et al., 2008) and the MRI Atlas of Human White Matter by Mori (Mori et al., 2005). The brain coordinates are presented in the standardized MNI space.
2.4.4. Lesion overlap map
In the current study we also present a lesion overlap map illustrating the lesion distribution across the entire group of patients. Patients' lesions were identified using the automated lesion identification procedure described by Seghier et al. (2008). The 21 patients' MRI structural images were segmented, normalized and smoothed (8 mm). Then outlier voxels were detected by comparing the segmented GM and WM of the patient to the ones of 100 controls using fuzzy clustering (Seghier et al., 2008) and then the outlier voxels in each tissue class were assigned to the lesion. We next created the overlap of the lesions for all patients using the “image calculator” function of SPM5 with the expression: “i1 + i2 + i3 + i4 + … + i21” and displayed the overlap map with MRICRON (Rorden, 2005). This was done to represent the spatial distribution of lesions in our group of patients (Supplementary Fig. 3). The lesion volume for each patient was calculated using Matlab 7.5 (The MathWorks, Natick, MA, USA) based on individual lesions from the automated lesion identification procedure (see above). The estimated lesion volumes of all individual patients were used as covariates in the VBM analyses (see above).
3. Results
3.1. Behavioural results — transitive (MF & ML) and intransitive (MF & ML) actions
Fig. 1 illustrates the proportional scores for transitive and intransitive gestures for patients and control participants.
Fig. 1.
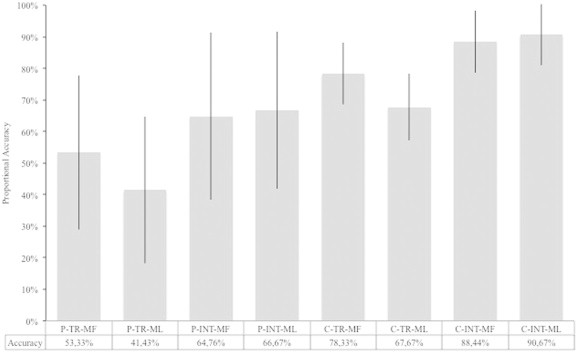
This figure shows the accuracy in imitation for patients and controls. The data for transitive and intransitive MF and ML actions are based on proportions as the total maximum scores varied across stimuli. Abbreviations: P-TR-MF, patients' mean proportional accuracy with MF transitive actions; P-TR-ML, patients' mean proportional accuracy with ML transitive actions; P-INT-MF, patients' mean proportional accuracy with MF intransitive actions; P-INT-ML, patients' mean proportional accuracy with ML intransitive actions; C-TR-MF, controls' mean proportional accuracy with MF transitive actions; C-TR-ML, controls' mean proportional accuracy with ML transitive actions; C-INT-MF, controls' mean proportional accuracy with MF intransitive actions; C-INT-ML, patients' mean proportional accuracy with ML intransitive actions.
Data for the controls were used to categorize each patient's performance. Cut-offs were established on the basis of the control means and standard deviations for the transitive (MF (mean = 15.78; SD = 1.93); ML (mean = 13.89; SD = 2.30); totals (mean = 29.67; SD = 3.50)) and intransitive actions (MF (mean = 13.44; SD = 1.42); ML (mean = 13.72; SD = 1.36); totals (mean = 27.17; SD = 2.66)), using the modified t-test of Crawford and Garthwaite (2002).
Fifteen out of twenty one patients had a deficit in the imitation of at least on type of gesture.
For the transitive action imitation task eleven patients (6 LBD, 4 RBD and 1 BBD) performed below the cut-off for MF (≤12), ML (≤9) and total action performance (≤23); one LBD had normal performance with MF but fell below the cut-off for the ML gestures and the total action scores; one RBD was defective only when imitating MF actions and one RBD was below the cut-off only for the ML actions. At the intransitive action imitation task eight patients (4 LBD, 3 RBD and 1 BBD) performed below the cut-off for MF (≤10) and ML (≤11) and total actions (≤22); two patients (1 LBD and 1 RBD) fell below the cut-off for MF and totals; one RBD was defective for the ML and total action scores and two (1 RBD and 1 BBD) were defective only for the ML actions.
Additionally, we investigated dissociations in performance (i.e. differences between performance at two tasks that were unlikely to be reported among healthy individuals) using the ‘revised standardized difference test’ (RSDT) by Crawford and Garthwaite (2005).
Supplementary Table 1 indicates for each patient if performance was below or above the cut-off and the presence or absence of a dissociation between the scores obtained with MF and ML gestures at each task.5 For the transitive gestures, out of the eleven patients failing imitation of both MF and ML gestures, eight patients (5 LBD, 2 RBD and 1 BBD) had better scores when they imitated the MF gestures, one RBD did better with the ML and two (1 BBD and 1 RBD) were equally impaired with both MF and ML. One RBD patient showed deficits imitating only the MF and imitated the ML gestures significantly better. Finally, one RBD patient received a score below the cut-off for the ML but not for the MF gestures, but there was not a reliable dissociation. For the intransitive gestures, eight patients had a deficit on both MF and ML actions; two of these patients had significantly better scores on the MF gestures, scoring at a control level with the MF gestures but below the cut-off with the ML gestures. Another two patients (1 LBD and 1 RBD) had the opposite dissociation, scoring below the cut-off for the MF gestures but within normal limits for ML gestures. Finally, one RBD patient was impaired for the ML gestures only relative to controls but did not show a dissociation between performance with the MF and ML gestures.
3.2. Neuroimaging results: grey matter
3.2.1. GM — transitive MF & ML and intransitive MF & ML
The model including the patients' continuous scores for imitating i) transitive MF ii) transitive ML iii) intransitive MF and iv) intransitive ML gestures revealed a cluster within the parietal lobe linked to performance, including the precuneus (PCUN) and extending posteriorly to the cuneus (CUN) and anteriorly to the supplementary motor area (SMA) in the left hemisphere (Fig. 2a; Table 1).
Fig. 2.
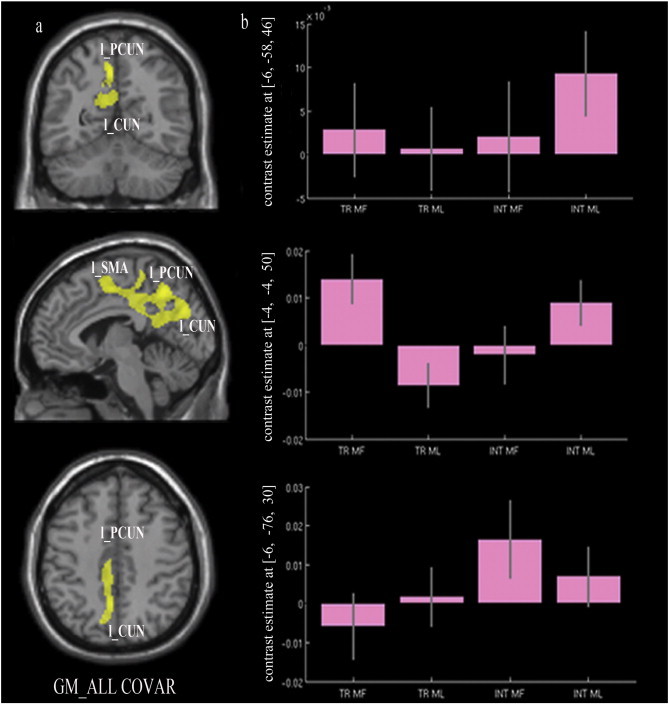
Grey matter substrates of the imitation of transitive and intransitive MF and ML gestures (results based on the model with all covariates).Abbreviations: l_PCUN, left precuneus; l_CUN, left cuneus; l_SMA, left supplementary motor area; MF TR, meaningful transitive actions; ML TR, meaningless transitive actions; MF INT, meaningful intransitive actions; ML INT, meaningless transitive actions.
A plot showing individual effects of each covariate indicated that, for the peak in the left PCUN, the imitation of intransitive ML gestures had the larger effect size (Fig. 2b). The peak on the left CUN related mainly to the transitive MF gestures, even though an effect of the intransitive gestures was also present. Finally intransitive MF gestures were most strongly related to the left SMA cluster.
3.2.1.1. GM — transitive ML
The analysis based on the model including the GM segmented images along with the ML transitive action scores as the only covariate of interest revealed one large cluster within the left superior parietal cortex including the left PCUN and extending anteriorly to the middle portion of the cingulate cortex (Mid CC) and posteriorly to the left middle occipital cortex (OCC) (Fig. 3a_1; Table 1). Also a cluster was found in the superior and middle right frontal cortex (SFC and Mid SFC) and right Mid CC (Fig. 3a_2; Table 1).
Fig. 3.
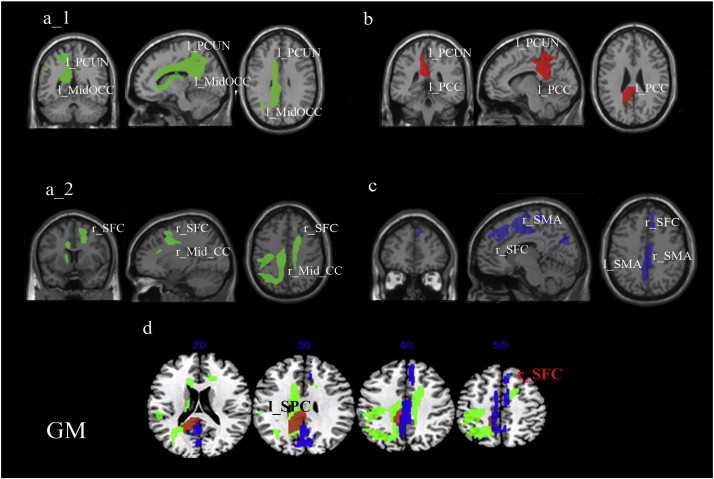
Grey matter substrates of the imitation of transitive and intransitive MF and ML gestures (results from models with single covariate of interest).Abbreviations: l_PCUN, left precuneus; l_MidOCC, left middle occipital cortex; r_SFC, right superior frontal cortex; r_Mid_CC, right middle cingulum; l_PCC, left posterior cingulum; l_SMA, left supplementary motor area; r_SMA, right supplementary motor area; l_SPC, left superior parietal cortex. Blobs colours: green = transitive ML; red = transitive MF; blue = intransitive ML.
3.2.1.2. GM — transitive MF
The analysis based on the model with the patients' scores at imitating MF transitive gestures as the sole covariate of interest highlighted a large cluster in the parietal cortex including the posterior cingulate cortex (PCC) and the PCUN in the left hemisphere (Fig. 3b; Table 1).
3.2.1.3. GM — intransitive ML
The analysis based on the model with scores for ML intransitive gestures as the sole covariate of interest and GM scans revealed a cluster in the midline having its peak in the SFC extending posteriorly to not only the SMA in the right hemisphere but also the SMA and PCUN in the left hemisphere (Fig. 3c, Table 1).
Next, to identify common cortical regions underlying imitation of transitive and intransitive gestures, we overlapped results obtained based on the three above models (see Fig. 3d).
3.2.1.4. GM — intransitive MF
The analysis with the model with only MF intransitive actions' scores revealed no significant clusters (no findings survived correction for multiple comparisons).
3.3. Neuroimaging results: white matter
The analyses with the models including: i) all the four covariates, ii) transitive MF gestures only, iii) intransitive MF gestures only, and iv) intransitive ML gestures only revealed no significant clusters (no findings survived correction for multiple comparisons).
3.3.1. WM — transitive ML
The contrast based on the model with transitive ML gestures as the only covariate of interest revealed a cluster in the left corticospinal tract, corpus callosum and white matter within the left superior temporal gyrus (Fig. 4; Table 2).
Fig. 4.
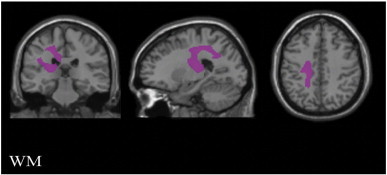
White matter substrates for transitive ML. Results indicate that deficits in transitive ML gestures were linked to damage within the left corticospinal tract, left corpus callosum and white matter within the left superior temporal gyrus.
Table 2.
White matter substrates of imitation of transitive ML gestures (VBM analysis).
| Cluster level |
Voxel level |
Coordinates |
Brain structure |
||||
|---|---|---|---|---|---|---|---|
| Model | p-FWE | Size | Z-score | X | Y | Z | (location) |
| Transitive ML | |||||||
| 0.011 | 2912 | 3.35 | −24 | −28 | 40 | Left CTRG, CC | |
| 3.29 | −24 | −22 | 16 | and WM within left STG | |||
| 3.08 | −48 | −34 | 22 | ||||
Abbreviations: CC, corpus callosum; CTRG, corticospinal tract; STG, superior temporal gyrus; VBM, voxel-based morphometry; WM, white matter.
3.4. Additional analyses
Additional analyses were carried out in order to rule out the possibility that our results were driven by outliers. For this purpose we carried out regression and correlation analyses on beta values extracted from the clusters that were revealed by the VBM contrasts and behavioural results. None of the regressions or correlations appeared to be driven by single patients (or outliers). The analyses and the results, along with the relevant plots, are presented in the supplementary material (see Supplementary analyses and Supplementary Figs. 4 and 5).
4. Discussion
The present study investigated the link between GM and WM lesions and performance on gesture imitation tasks, according to (i) the type of action to be executed (transitive or intransitive) and (ii) the familiarity of the action (MF or ML). The brain lesion data were analysed using voxel-based morphometry (Ashburner and Friston, 2000), an unbiased approach that treats patients' behavioural scores as predictors of change in the intensity of the signal from each voxel of segmented GM or WM tissue. As indicated above, two models including all the covariates of interest looked at the brain regions where the signal changes in GM and WM were predicted by the imitation scores, regardless of gesture type or meaning. After this, individual models were used to explore the effect of single gesture types. We decide to adopt this procedure after consulting experts in the field, who advised that this was appropriate when dealing with highly correlated covariates of interest.
4.1. GM changes
Reliable correlations were found between impairments in gesturing and neural changes on GM for the contrasts run on the model including all four imitation tasks, as well as on three of the models with individual tasks as unique covariate of interest (i.e. imitation of i) MF transitive, ii) ML transitive, and iii) ML intransitive gestures). A cluster in the left superior parietal cortex involving the PCUN, extending also posteriorly to the CUN in the occipital cortex and anteriorly to the SMA, appeared to relate to all four gesture imitation tasks. Plots were produced for each peak in order to explore the relative contribution of each task in predicting the signal. As can be seen from the histograms in Fig. 2b, intransitive ML gesture scores generated the highest beta values in the PCUN. The transitive MF gesture scores generated the highest beta values in the left SMA while the intransitive MF gesture scores had the highest betas in the left CUN. However, although a covariate may have a relatively higher contribution in specific peaks, each covariate contributed to the main finding. Clusters in the left PCUN were also highlighted in the analyses run with the models including the ML transitive, MF transitive and ML intransitive gestures as single covariates. The results suggest a general involvement of the PCUN in all the studied actions. To the best of our knowledge few lesion studies have investigated the cognitive and behavioural consequences of brain damage involving the PCUN but recent neuroimaging techniques have explored its functions (Cavanna and Trimble, 2006). The PCUN is known to belong to the associative cortices serving a variety of functions including visuo-spatial imagery, spatially guided behaviour, first-person perspective taking and experiencing agency (Cavanna and Trimble, 2006) i.e., functions that may all contribute to the execution of complex gestures. Alternatively, the PCUN may be directly involved in the production of the target action due to being involved in the apprehension of the spatial relations between body parts (Goldenberg, 2009). Further work is needed to test between these alternatives.
The common effect of all four of the covariates of interest extended further to the left superior parietal cortex. This is consistent with previous reports of lesions in this area causing the symptoms of ideomotor apraxia (Rothi et al., 1991). The result also fits with observations of Tessari et al. (2007) that unilateral LBD patients with lesions in superior parietal structures fail in the imitation of MF tool-use and ML gestures (Goldenberg and Hagmann, 1997; Peigneux et al., 2000). Moreover damage to the left superior parietal cortex has been linked not only to ideomotor apraxia (specifically a deficit in imitation) but also to ideational apraxia (i.e. having problems in the voluntary execution of multiple step actions, such as lighting a candle with a match (Pazzaglia et al., 2008)). The results suggest that superior parietal structures in the left hemisphere have a role not only in imitation but also more generally in voluntary movement execution.
In addition to the above, the model including all four gesture types suggested an association between imitation and the left SMA. This is in line with previous reports from imaging studies showing SMA activation during imitation of arm or hand movement (Rumiati et al., 2005; Chen et al., 2010). We note too that apraxia for learned transitive gestures has previously been reported in patients with left SMA lesions by Watson et al. (1986) and, consistent with this, the plot of our results showed that the MF transitive gestures had the strongest relation with signal strength in the SMA, although an effect on ML intransitive gestures was also present (Fig. 2b). When each gesture type was considered alone the cluster in the left SMA was highlighted for ML intransitive gestures only. However there was no relation between signal loss in this area and any of the other gesture types considered individually. This pattern of results within the left SMA does not allow straightforward interpretation and needs further investigation. However, we can hypothesize that the SMA is involved in processing gestures regardless of their familiarity or type (transitive vs. intransitive). Also, it is possible that the correlation between imitation and SMA damage stands for all the action types considered together but not for the MF and ML transitive gestures taken individually, since the transitive gestures relied less consistently on the SMA. One further possibility is that the MF and ML transitive gestures were processed through the SMA by some patients who may have contributed to the general main effect of this region.
Beside the main effect emerging from the unique model, we obtained significant results from three out of four models (i.e. for transitive MF and ML, along with intransitive ML gestures), including each gesture type when taken as a single covariate of interest at a time. These analyses gave different patterns of results according to the actions involved — with the results supporting an argument not only for them being neural substrates dedicated to preferentially processing ML gestures but also for the separate neural underpinnings of transitive and intransitive gestures. We found one cluster within the left inferior parietal cortex for ML and MF transitive actions, but not for ML intransitive actions. This is consistent with the previous suggestion that sub-regions of the left inferior parietal cortex have a role in tool use (Peigneux et al., 2004; Ramayya et al., 2010; Orban and Rizzolatti, 2012). However, the fact that an effect was found not only with MF but also with the ML transitive actions suggests that stored knowledge of the movement may be less critical than the presence of a grasping component (both MF and ML transitive gestures require taking a precise hand configuration, as for holding a tool). This would hold for ML and MF transitive actions alike but not for intransitive actions. We should also consider the large overlap between the region in the left parietal cortex linked to poor reproduction of MF transitive gestures and the cluster for the ML transitive gestures. This overlap suggests an affinity between the neural structures supporting MF and ML gestures and goes against a claim for the existence of separate routes (direct and semantic) for imitation. Rather than this, the data suggest that ML transitive gestures are more demanding and so they simply require additional neural processes. This need for more resources for ML transitive gestures would explain the larger region of parietal cortex linked to these gestures. However, if processing demands alone were critical, we should have found some evidence also with other gesture types, with the clusters decreasing in size as the complexity of gestures decreased. However, this was not the case. We propose instead that the current data highlight brain areas related to the first step of tool identification or correct grasping elaboration (that is common to MF pantomimes and matched ML gestures) but not the areas where semantic information of known transitive actions is stored.
In contrast to the results with transitive MF gestures, there were no brain areas linked to poor performance of intransitive MF gestures. It is possible that, in our sample, patients with impairments in the proposed semantic route to transitive actions were poorly represented, and so we were unable to isolate an effect of damage to that route. According to this proposal, most of the dissociations in performance were in the direction of better scores with the MF gestures (see Supplementary Table 1). This relative advantage cannot just be due to the MF gesture being more easily executed, as this would not account for the data where some patients have reverse dissociation.
The analyses also highlighted a correlation between imitation and damage to the right superior frontal cortex for both transitive and intransitive ML gestures, but not for the MF transitive actions. It has previously been reported that right hemisphere lesioned patients can have problems with ML transitive gestures (Tessari et al., 2007) although in their case the patients' lesions overlapped most on the basal ganglia. Nonetheless the basal ganglia and the frontal cortex are strictly and reciprocally connected (Draganski et al., 2008). Moreover, as mentioned in the introduction, a recent study by Ramayya et al. (2010) highlighted that a path connecting the frontal and the anterior inferior parietal cortex was vastly rightward lateralized. So it may be possible that the basal ganglia and the frontal cortex are both part of a right hemisphere circuit, which may also include the parietal cortex, which in turn supports the reproduction of ML gestures. However the major point here is that only the ML gestures, regardless of being transitive or intransitive, gave results overlapping on the right frontal cortex.
In addition to the clusters shared for different action types, some results were associated with specific categories of gesture. The cluster in the left hemisphere linked to poor reproduction of ML transitive gestures was extended beyond that associated with impairments in MF transitive and ML intransitive gestures and which ran anteriorly to the cingulate cortex (with the peak voxel located in the left Mid CC, and extending posteriorly to the left occipital cortex). As already proposed by Carmo and Rumiati (2009) and as indicated by our data, the ML transitive gestures may have been more difficult to imitate than the other gestures and thus required resources additional to those in the left hemisphere shared with the MF transitive gestures and with those in the right hemisphere shared with ML intransitive gestures — this included both additional visual processing and greater demands on task monitoring. Damage to the middle and anterior portions of the cingulate cortex in the right hemisphere also linked to problems with ML transitive gestures. This part of the cingulate cortex has been shown to be involved in tasks tapping attention and executive function, such as the colour naming Stroop task (Bush et al., 2000), and its relation to the imitation of ML transitive gestures may suggest a higher demand for task monitoring with those stimuli.
As discussed above, the involvement of the left SMA was only found for intransitive ML gestures. These clusters extended to the SMA in the right hemisphere. Previous studies have proposed that there is bilateral involvement of the SMA in planning gestures that require the sequencing of action subroutines (Roland et al., 1980a; Roland et al., 1980b). It may be plausible then that the intransitive ML gestures, more than the other types of action, require that movements are segmented into smaller components in order to be mirrored. Indeed, the ML intransitive gestures cannot rely on other strategies to be performed — such as the automatic activation of an existing representation of their meaning (as the MF gestures) or of an action schema triggered by the posture of the hand (as in the case of both MF and ML transitive). It follows that the SMA may be a key component of a direct (non-semantic) route to imitation (Riddoch et al., 1989). This would also fit with our findings of a main effect within the SMA when all the four types of gesture were considered together, despite a lack of an effect when MF transitive and ML transitive were analysed individually.
Unfortunately we did not obtain any significant findings from the individual analysis of the MF intransitive actions. Thus we do not know if the SMA was involved in processing those actions. More investigations are needed in order to clarify this point.
4.2. WM changes
In addition to the grey matter cortical lesions related to action imitation, white matter damage was also linked to poor performance of ML transitive actions (specifically lesions within the left temporal cortex extending to the corpus callosum and corticospinal tract). Ramayya et al. (2010) proposed that the temporal cortex contains semantic information about tools and is connected to other areas involved in tool-related actions through WM tracts. Our result for the ML transitive actions suggests the possibility that similar communication channels serve the recognition and categorization of general tool-related actions rather than the retrieval of information about specific tools. Damage to similar WM pathways may have a less detrimental effect on MF actions, which are coded more robustly and may be supported to store information about the meaning of the actions.
In summary, the results highlight the PCUN and SMA as contributing to all gesture types while a region in the left parietal cortex was shared by both MF and ML transitive gestures and ML gestures (both transitive and intransitive) were impaired after right frontal damage. In addition, the results dissociated SMA damage to ML intransitive gestures (particularly on the right) and damage to ML transitive gestures (linked to the left parietal and occipital cortex, also including the middle and anterior cingulate cortex as well as WM within the left temporal cortex). The results point to both common brain mechanisms involved in the processing of all gestures and to regions specialized for tool-related grasps (linked to both MF and ML tool-related actions) and to the direct imitation of action (for ML transitive and intransitive actions within the right frontal cortex).
The pattern of our findings suggests the opportunity to integrate the dual route model of motor performance (Rumiati and Tessari, 2002) with the processing of tool-related actions. It also suggests that clinicians should test all patients for ideomotor apraxia, not only those having lesions to the left hemisphere, as lesions to the right hemisphere may damage the direct route to imitation.
Acknowledgements
The work was supported by the Stroke Association (grant number: prog6) and the NIHR (grant number: CRF-2011-10017) (UK).
Footnotes
On average there was not an effect of the hand used (p > 0.1) on the performance of either the patients or the controls.
The Cohen's k agreement coefficient was calculated based on the scores provided by the two independent raters. The coefficient was computed for MF and ML actions taken separately and for the total action scores. As the coefficient was ≥0.80 in all the cases considered, the scores of only one rater (the same for patients and controls) were used.
Patients were divided into right-handed and left-handed according to the scores they received at the Edinburgh Handedness Inventory (Oldfield, 1971).
We used a p-value of ≤0.005 instead of ≤0.001 (uncorrected) in an attempt to lower as much as possible both the risk of obtaining results by chance and the risk of ignoring results because of too stringent criteria. We considered only results that were significant after correction, which take into consideration the large number of comparisons across vowels, thus lowering the actual p-value. Moreover, we set a quite stringent threshold for the minimum number of voxels (i.e. 1000). Altogether the criteria we adopted made it unlikely that we obtained results by chance even with the threshold adopted.
Dissociations between different gesture types (i.e. transitive vs. intransitive) were not calculated, as the intransitive gestures usually are easier to execute, despite attempts to match them for complexity to the transitive gestures.
Appendix A. Supplementary material
Supplementary material
Appendix A. Supplementary material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nicl.2014.09.010.
References
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. 15955494 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. 10860804 [DOI] [PubMed] [Google Scholar]
- Bard K.A., Russell C.L. Evolutionary foundations of imitation: social cognitive and developmental aspects of imitative processes in non-human primates. In: Nadel J., Butterworth G., editors. Imitation in Infancy. Cambridge University Press; Cambridge: 1999. pp. 89–123. [Google Scholar]
- Bartolo A., Cubelli R., Della Sala S., Drei S., Marchetti C. Double dissociation between meaningful and meaningless gesture reproduction in apraxia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2001;37:696–699. doi: 10.1016/s0010-9452(08)70617-8. 11804218 [DOI] [PubMed] [Google Scholar]
- Bonivento C., Rumiati R.I., Biasutti E., Humphreys G.W. The role of the basal ganglia in action imitation: neuropsychological evidence from Parkinson's disease patients. Experimental Brain Research. 2013;224(2):211–220. doi: 10.1007/s00221-012-3300-8. 23104400 [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. 10827444 [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J. Ideomotor apraxia: a call to action. Neurocase. 2001;7(6):445–458. doi: 10.1093/neucas/7.6.445. 11788737 [DOI] [PubMed] [Google Scholar]
- Canavan A.G.M., Passingham R.E., Marsden C.D., Quinn N., Wyke M., Polkey C.E. The performance on learning tasks of patients in the early stages of Parkinson's disease. Neuropsychologia. 1989;27:141–156. doi: 10.1016/0028-3932(89)90167-x. 2927625 [DOI] [PubMed] [Google Scholar]
- Carmo J.C., Rumiati R.I. Imitation of transitive and intransitive actions in healthy individuals. Brain and Cognition. 2009;69:460–464. doi: 10.1016/j.bandc.2008.09.007. 18976850 [DOI] [PubMed] [Google Scholar]
- Catani M., Ffytche D.H. The rises and falls of disconnection syndromes. Brain: A Journal of Neurology. 2005;128:2224–2239. doi: 10.1093/brain/awh622. 16141282 [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology. 2006;129:564–583. doi: 10.1093/brain/awl004. 16399806 [DOI] [PubMed] [Google Scholar]
- Chen X., Scangos K.W., Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2010;30(44):14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. 21048123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H. Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40:1196–1208. doi: 10.1016/s0028-3932(01)00224-x. 11931923 [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H. Testing for suspected impairments and dissociations in single-case studies in neuropsychology: evaluation of alternatives using Monte Carlo simulations and revised tests for dissociations. Neuropsychology. 2005;19(3):318–331. doi: 10.1037/0894-4105.19.3.318. 15910118 [DOI] [PubMed] [Google Scholar]
- Cubelli R., Marchetti C., Boscolo G., Della Sala S. Cognition in action: testing a model of limb apraxia. Brain and Cognition. 2000;44:144–165. doi: 10.1006/brcg.2000.1226. 11041987 [DOI] [PubMed] [Google Scholar]
- De Renzi E., Lucchelli F. Ideational apraxia. Brain: A Journal of Neurology. 1988;111(Pt 5):1173–1185. doi: 10.1093/brain/111.5.1173. 3179688 [DOI] [PubMed] [Google Scholar]
- De Renzi E., Motti F., Nichelli P. Imitating gestures. A quantitative approach to ideomotor apraxia. Archives of Neurology. 1980;37:6–10. doi: 10.1001/archneur.1980.00500500036003. 7350907 [DOI] [PubMed] [Google Scholar]
- Della Sala S., Faglioni P., Motto C., Spinnler H. Hemisphere asymmetry for imitation of hand and finger movements, Goldenberg's hypothesis reworked. Neuropsychologia. 2006;44(8):1496–1500. doi: 10.1016/j.neuropsychologia.2005.11.011. 16434067 [DOI] [PubMed] [Google Scholar]
- Draganski B., Kherif F., Klöppel S., Cook P.A., Alexander D.C., Parker G.J.M., Deichmann R., Ashburner J., Frackowiak R.S.J. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2008;28(28):7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. 18614684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H.M. The Human Brain: Surface, Blood Supply and Three-Dimensional Sectional Anatomy. Springer-Verlag; Wien: 1991. [Google Scholar]
- Ferrari P.F., Visalberghi E., Paukner A., Fogassi L., Ruggiero A., Suomi S.J. Neonatal imitation in rhesus macaques. PLoS Biology. 2006;4:e302. doi: 10.1371/journal.pbio.0040302. 16953662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J., Kiebel S.J., Nichols T.E., Penny W. Statistical Parametric Mapping: The Analysis of Functional Brain Images. first edition. Elsevier Academic; Amsterdam, The Netherlands: 2007. [Google Scholar]
- Geschwind N. The apraxias: neural mechanisms of disorders of learned movement. American Scientist. 1975;63:188–195. 1115438 [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47:1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. 18692079 [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Hagmann S. The meaning of meaningless gestures: a study of visuo-imitative apraxia. Neuropsychologia. 1997;35:333–341. doi: 10.1016/s0028-3932(96)00085-1. 9051681 [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Hermsdörfer J., Glindemann R. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cerebral Cortex (New York, N.Y.: 1991) 2007;17:2769–2776. doi: 10.1093/cercor/bhm004. 17339607 [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Karnath H.O. The neural basis of imitation is body part specific. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2006;26(23):6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. 16763035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G., Strauss S. Hemisphere asymmetries for imitation of novel gestures. Neurology. 2002;59:893–897. doi: 10.1212/wnl.59.6.893. 12297573 [DOI] [PubMed] [Google Scholar]
- Gonzalez Rothi L.J.G., Ochipa C., Heilman K.M. A cognitive neuropsychological model of limb praxis. Cognitive Neuropsychology. 1991;8:443–458. [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., Calabresi P.A., Pekar J.J., van Zijl P.C.M., Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. 17931890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.W., Bickerton W.L., Samson D., Riddock J. The Birmingham Cognitive Screen (BCoS) Psychology Press; London: 2012. [DOI] [PubMed] [Google Scholar]
- Leiguarda R. Limb apraxia: cortical or subcortical. Neuroimage. 2001;14:S137–S141. doi: 10.1006/nimg.2001.0833. [DOI] [PubMed] [Google Scholar]
- Leiguarda R.C., Pramstaller P.P., Merello M., Starkstein S., Lees A.J., Marsden C.D. Apraxia in Parkinson's disease, progressive supranuclear palsy, multiple system atrophy and neuroleptic-induced Parkinsonism. Brain: A Journal of Neurology. 1997;120(Pt 1):75–90. doi: 10.1093/brain/120.1.75. 9055799 [DOI] [PubMed] [Google Scholar]
- Liepmann H.K. Das Krankheitshildder der Apraxie (Motorischen/Asymbolie) Monatschrift für Psychiatrie und Neurologie. 1900;8:15–44. [Google Scholar]
- Liepmann H.K. Die linke Hand und das Handeln. Muenchener Medizinische Wochenschrift. 1905;52:2375–2378. [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- Mehler M.F. Visuo-imitative apraxia. Neurology. 1987;37:129. [Google Scholar]
- Meltzoff A.N., Moore M.K. Imitation in newborn infants: exploring the range of gestures imitated and the underlying mechanisms. Developmental Psychology. 1989;25:954–962. doi: 10.1037/0012-1649.25.6.954. 25147405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A.N., Moore M.K. Imitation of facial and manual gestures by human neonates. Science (New York, N.Y.) 1977;198:75–78. doi: 10.1126/science.198.4312.75. 17741897 [DOI] [PubMed] [Google Scholar]
- Meltzoff A.N., Moore M.K. Newborn infants imitate adult facial gestures. Child Development. 1983;54:702–809. 6851717 [PubMed] [Google Scholar]
- Mori S., Wakana S., Nagae-Poetscher L.M., van Zijl P.C.M. MRI Atlas of Human White Matter. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- Myowa M. Imitation of facial gestures by an infant chimpanzee. Primates. 1996;37:207–213. [Google Scholar]
- Myowa-Yamakoshi M., Yamaguchi M.K., Tomonaga M., Tanaka M., Matsuzawa T. Development of face recognition in infant chimpanzees (Pan troglodytes) Cognitive Development. 2005;20:49–63. [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Orban G.A., Rizzolatti G. An area specifically devoted to tool use in human left inferior parietal lobule. Behavioral and Brain Sciences. 2012;35(4):234. doi: 10.1017/S0140525X11001944. 22697603 [DOI] [PubMed] [Google Scholar]
- Pazzaglia M., Smania N., Corato E., Aglioti S.M. Neural underpinnings of gesture discrimination in patients with limb apraxia. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2008;28(12):3030–3041. doi: 10.1523/JNEUROSCI.5748-07.2008. 18354006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P., Van der Linden M., Andres-Benito P., Sadzot B., Franck G., Salmon E. Exploration neuropsychologique et par imagerie fonctionnelle cérébrale d'une apraxie visuo-imitative. Revue Neurologique. 2000;156:459–472. [PubMed] [Google Scholar]
- Peigneux P., Van der Linden M., Garraux G., Laureys S., Degueldre C., Aerts J., Del Fiore G., Moonen G., Luxen A., Salmon E. Imaging a cognitive model of apraxia: the neural substrate of gesture-specific cognitive processes. Human Brain Mapping. 2004;21:119–142. doi: 10.1002/hbm.10161. 14755833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramayya A.G., Glasser M.F., Rilling J.K. A DTI investigation of neural substrates supporting tool use. Cerebral Cortex (New York, N.Y.: 1991) 2010;20:507–516. doi: 10.1093/cercor/bhp141. 19608779 [DOI] [PubMed] [Google Scholar]
- Riddoch M.J., Humphreys G.W., Price C.J. Routes to action: evidence from apraxia. Cognitive Neuropsychology. 1989;6:437–454. [Google Scholar]
- Roland P.E., Larsen B., Lassen N.A. Supplementary motor area and other cortical areas in organization of voluntary movements in man. Journal of Neurophysiology. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. 7351547 [DOI] [PubMed] [Google Scholar]
- Roland P.E., Skinhøj E., Lassen N.A., Larsen B. Different cortical areas in man in organization of voluntary movements in extrapersonal space. Journal of Neurophysiology. 1980;43:137–150. doi: 10.1152/jn.1980.43.1.137. 7351548 [DOI] [PubMed] [Google Scholar]
- Rorden, C., (2005), MRIcro. http://www.sph.sc.edu/comd/rorden/mricro.html.
- Rumiati R.I., Carmo J.C., Corradi-Dell'Acqua C. Neuropsychological perspectives on the mechanisms of imitation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2009;364:2337–2347. doi: 10.1098/rstb.2009.0063. 19620105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiati R.I., Tessari A. Imitation of novel and well-known actions: the role of short-term memory. Experimental Brain Research. 2002;142:425–433. doi: 10.1007/s00221-001-0956-x. 11819052 [DOI] [PubMed] [Google Scholar]
- Rumiati R.I., Weiss P.H., Tessari A., Assmus A., Zilles K., Herzog H. Common and differential neural mechanisms supporting imitation of meaningful and meaningless actions. Journal of Cognitive Neuroscience. 2005;17:1420–1431. doi: 10.1162/0898929054985374. 16197695 [DOI] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J., Leff A.P., Price C.J. Lesion identification using unified segmentation–normalisation models and fuzzy clustering. Neuroimage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. 18482850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari A., Canessa N., Ukmar M., Rumiati R.I. Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain: A Journal of Neurology. 2007;130:1111–1126. doi: 10.1093/brain/awm003. 17293356 [DOI] [PubMed] [Google Scholar]
- Tessari A., Rumiati R.I. The strategic control of multiple routes in imitation of actions. Journal of Experimental Psychology. Human Perception and Performance. 2004;30:1107–1116. doi: 10.1037/0096-1523.30.6.1107. 15584818 [DOI] [PubMed] [Google Scholar]
- Tessari A., Toraldo A., Lunardelli A., Zadini A., Rumiati R.I. Prova standardizzata per la diagnosi del disturbo aprassico ideomotorio selettivo per tipo di gesto e tipo di effettore. Ricerche di Psicologia. 2011;3:311–339. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- Watson R.T., Fleet W.S., Gonzalez-Rothi L., Heilman K.M. Apraxia and the supplementary motor area. Archives of Neurology. 1986;43:787–792. doi: 10.1001/archneur.1986.00520080035016. 3729758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


