Abstract
Background
Recent neuroimaging work suggests that increased amygdala responses to emotional stimuli and dysfunction within regions mediating top down attentional control (dorsomedial frontal, lateral frontal and parietal cortices) may be associated with the emergence of anxiety disorders, including posttraumatic stress disorder (PTSD). This report examines amygdala responsiveness to emotional stimuli and the recruitment of top down attention systems as a function of task demands in a population of U.S. military service members who had recently returned from combat deployment in Afghanistan/Iraq. Given current interest in dimensional aspects of pathophysiology, it is worthwhile examining patients who, while not meeting full PTSD criteria, show clinically significant functional impairment.
Methods
Fifty-seven participants with sub-threshold levels of PTSD symptoms completed the affective Stroop task while undergoing fMRI. Participants with PTSD or depression at baseline were excluded.
Results
Greater PTSD symptom severity scores were associated with increased amygdala activation to emotional, particularly positive, stimuli relative to neutral stimuli. Furthermore, greater PTSD symptom severity was associated with increased superior/middle frontal cortex response during task conditions relative to passive viewing conditions. In addition, greater PTSD symptom severity scores were associated with: (i) increased activation in the dorsolateral prefrontal, lateral frontal, inferior parietal cortices and dorsomedial frontal cortex/dorsal anterior cingulate cortex (dmFC/dACC) in response to emotional relative to neutral stimuli; and (ii) increased functional connectivity during emotional trials, particularly positive trials, relative to neutral trials between the right amygdala and dmFC/dACC, left caudate/anterior insula cortex, right lentiform nucleus/caudate, bilateral inferior parietal cortex and left middle temporal cortex.
Conclusions
We suggest that these data may reflect two phenomena associated with increased PTSD symptomatology in combat-exposed, but PTSD negative, armed services members. First, these data indicate increased emotional responsiveness by: (i) the positive relationship between PTSD symptom severity and amygdala responsiveness to emotional relative to neutral stimuli; (ii) greater BOLD response as a function of PTSD symptom severity in regions implicated in emotion (striatum) and representation (occipital and temporal cortices) during emotional relative to neutral conditions; and (iii) increased connectivity between the amygdala and regions implicated in emotion (insula/caudate) and representation (middle temporal cortex) as a function of PTSD symptom severity during emotional relative to neutral trials. Second, these data indicate a greater need for the recruitment of regions implicated in top down attention as indicated by (i) greater BOLD response in superior/middle frontal gyrus as a function of PTSD symptom severity in task relative to view conditions; (ii) greater BOLD response in dmFC/dACC, lateral frontal and inferior parietal cortices as a function of PTSD symptom severity in emotional relative to neutral conditions and (iii) greater functional connectivity between the amygdala and inferior parietal cortex as a function of PTSD symptom severity during emotional relative to neutral conditions.
Keywords: Post-traumatic stress disorder, Emotion attention, Amygdala, Top down attention
Highlights
-
•
Greater PTSD symptoms associated with increased amygdala activation to emotional stimuli
-
•
PTSD symptoms associated with greater top down attention response in task and emotion conditions
-
•
PTSD symptoms were associated with slower reaction times.
-
•
Increased top down attention recruitment may compensate for heightened emotional responses.
1. Introduction
It has been argued that dysfunctional emotional regulation is a risk factor for the development of mood and anxiety disorders (Etkin et al., 2010; Rive et al., 2013). However, emotional regulation does not appear to consist of a single cognitive process (Gyurak et al., 2011). Emotion regulation has been described as two sets of control processes (Ochsner and Gross, 2005; Phillips et al., 2003). The first of these processes involves ventral prefrontal systems (ventral orbitofrontal and ventral prefrontal cortices) and has been implicated in the representation of emotional value and/or emotional conflict adaptation (Ochsner and Gross, 2005). It is argued that these regions can directly modulate emotional representations in the amygdala and nucleus accumbens via direct reciprocal projections (Ochsner and Gross, 2005). The second of these processes involves dorsal regions (dorsolateral prefrontal cortex, parietal cortex) involved in the priming of task relevant representations at the expense of task irrelevant representations. Through this priming, the representational competition between task relevant and task irrelevant representations is resolved and attentional control is asserted (Desimone and Duncan, 1995). Directed attention towards task relevant stimuli will result in reduced representation of emotional distracters (task irrelevant representations) and consequently emotional responses to the distracters will be inhibited (Blair et al., 2007; Mitchell et al., 2007). It has been argued that these processes can be recruited explicitly during cognitive reappraisal paradigms, where subjects consciously attempt to alter stimulus representations by priming non-emotional stimulus features (see, for reviews, Kalisch, 2009; Ochsner and Gross, 2005). During attention distraction paradigms it has been suggested that these processes are recruited implicitly (Blair et al., 2007; Pessoa et al., 2002; Pessoa et al., 2005).
A number of different tasks have been used to examine the role of top down attention during the implicit emotion regulation that occurs when responding to emotional distracters (Blair et al., 2007; Miller and Lynam, 2003; Mitchell et al. 2007, Mitchell et al. 2008; Mitchell et al., 2006; Vythilingam et al., 2007). The current task utilized the affective Stroop task (aST; Blair et al., 2007). In this task, participants are required to determine the quantity of digits presented. The number displays are temporally bracketed by emotional or neutral distracter images. In a number of studies, the aST has shown that increased activation within regions associated with top down attentional control (e.g. lateral frontal and parietal cortices) is associated with successful task performance (Blair et al., 2007; Mitchell et al. 2007, Mitchell et al. 2008). Emotional distracter stimuli are associated with increased amygdala activation relative to neutral distracters during trails where no task performance is required (see task description below), but this differential amygdala activation is attenuated when participants are required to perform that task (e.g. Blair et al., 2012; Blair et al., 2007; Mitchell et al. 2007, Mitchell et al. 2008). Critically, the recruitment of top down attention control is not thought to directly inhibit the amygdala, but, following Desimone and Duncan (1995), to prime temporal cortex representations of task relevant stimuli within temporal cortex. This priming augments their representation and consequently inhibits the temporal cortex representation of emotional distracters following representational competition (Desimone and Duncan, 1995).
Considerable fMRI work has shown that patients with post-traumatic stress disorder (PTSD) exhibit increased amygdala responses to emotional stimuli (Blair et al., 2013; Bremner et al., 2004; El Khoury-Malhame et al., 2011; Felmingham et al., 2010; Hayes et al., 2012; Rauch et al., 2006; Shin and Liberzon, 2010). As such, difficulties in concentration and hyper-vigilance associated with PTSD (APA, 2013) might reflect heightened priming of representations of emotional distracters within the temporal cortex. In addition, work has indicated that patients with PTSD show disrupted recruitment of regions implicated in top-down attention (lateral frontal cortices and parietal cortices; Aupperle et al., 2012; Esterman et al., 2013), particularly in the presence of emotional distracters (Blair et al., 2013; New et al., 2009; Pannu Hayes et al., 2009). This would be expected to lead to increased difficulties in asserting top down attentional control. Interestingly, there are also indications that individuals who have experienced trauma but have not developed PTSD show increased recruitment of regions implicated in top down attention as a function of task demands (Blair et al., 2013; New et al., 2009).
The goal of the current study was to examine the recruitment of top down attention systems as a function of task demands and amygdala responsiveness to emotional stimuli in a population of individuals recently exposed to potential trauma (U.S. military service members recently returned from combat deployment in Afghanistan or Iraq). The participants were selected such that while they might present with PTSD symptomatology, they did not meet full diagnostic criteria for PTSD. Given current interest in dimensional aspects of pathophysiology (Cuthbert and Insel, 2013; Regier, 2007), it is worthwhile examining patients who, while not meeting full PTSD criteria, show clinically significant functional impairment (Mylle and Maes, 2004; Pietrzak et al., 2009; Roy et al., 2012). In addition, current data indicate that 6–18% of Afghanistan/Iraq veterans will meet criteria for PTSD more than 3 months after returning from deployment (Hoge et al., 2004). As such, it is predicted that a significant proportion of these individuals will develop PTSD and the eventual aim of the project that these participants are enrolled in is to determine biomarkers indicative of the subsequent development of PTSD. However, our interim goal in this study was to determine the relationship between recruitment of systems implicated in top down attention and emotion and self-reported PTSD symptoms as indexed by the post-traumatic stress disorder Checklist-Military Version (PCL; Weathers et al., 1993) very soon after returning from active duty. Due to the presence of clinically significant functional impairment and the short interval between returning from deployment and scanning, we hypothesized that, consistent with previous work in PTSD patients, greater levels of PTSD symptoms would be associated with increased responses within the amygdala to emotional stimuli (Blair et al., 2013) and with reduced recruitment of regions implicated in top-down attentional control, specifically superior frontal, lateral frontal and parietal cortices (Blair et al., 2013; New et al., 2009; Pannu Hayes et al., 2009). Additionally, consistent with previous work in PTSD patients (Blair et al., 2013; New et al., 2009; Pannu Hayes et al., 2009), we hypothesized that stronger, inverse functional connectivity would be observed between the amygdala and regions associated with top down attention as PTSD symptom severity increased.
2. Methods
2.1. Participants
Study participants were 57 military service members (9 female) who were scanned within 8 weeks of returning from at least a 90-day deployment to either Iraq or Afghanistan. Participants were 42 European-Americans (73.68%), 4 African-Americans (7.02%), 3 Asian-American/Pacific Islander (5.26%) and 2 Hispanic Americans (3.51%) with an average age of 29.18 (19.9–51.6 years, standard deviation = 7.73). All participants were exposed to relatively severe traumatic experiences that met Criterion A of PTSD (according to DSM-IV or DSM-5). The most commonly reported type index trauma was being on a base that was being attacked (e.g. mortar or rocket fire, n = 26), followed by being in combat (e.g. firefights, hit by improvised explosive device [IED], n = 18), witnessing combat related violence (e.g. watching truck in convoy be hit by an IED, n = 8) and dealing with comrades being either killed-in-action or missing-in-action (n = 5). Participants endorsed a moderate number of PTSD symptoms (mean PTSD Checklist-Military Version = 26.46, standard deviation = 7.54 range = 16–48). Exclusion criteria included Glasgow Coma Scale scores of less than 14, any loss of consciousness greater than 60 min, or post-concussive syndrome. Participants were also excluded if they met criteria for PTSD, major depression or active/past psychosis as determined by a clinician. Participants were also excluded if they were taking calcium channel or alpha-blockers. The Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center Institutional Review Boards approved this study.
2.2. Study measures
2.2.1. The affective Stroop task
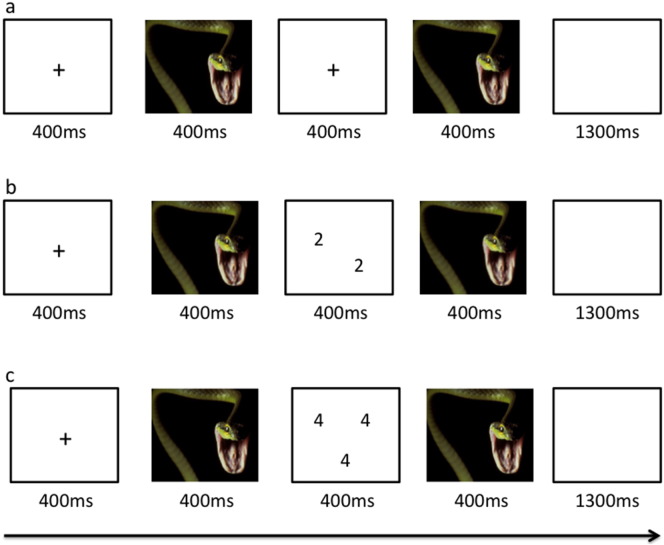
The affective task used here was an adapted version of the paradigm described in previous work (Blair et al., 2007). Each trial began with a fixation cross centrally presented for 400 ms (see Fig. 1). This was followed by a 400 ms image presentation. In the view trials, participants were then presented with a blank screen for 400 ms. During task trials, a numerical display was presented for 400 ms. For both view and task trials, there was then a second 400 ms period when the first image was presented again. This was followed by a blank screen for 1300 ms. The subjects had to determine the quantity of digits in the numerical display i.e., how many of the numbers were displayed, not the actual value of the numbers. For congruent trials, the quantity of numbers displayed was the same as the number value (e.g. three 3 s and four 4 s). For incongruent trials, the quantity of numbers displayed did not equal the number values (e.g. three 2 s and four 3 s). Participants could respond at any time from the presentation of the numerical display until the end of the blank screen. View trials required no response.
Fig. 1.
The affective Stroop task. Participants were exposed to neutral, positive or negative images followed either by a fixation cross (a), a screen showing digits congruent in their value and quantity (e.g. two 2 s; b) or a screening digits incongruent in their value and quantity (e.g. three 4 s; c). The fixation/numeric images were followed by a second exposure to the neutral positive or negative image. A negative image is depicted here.
The individual numerical stimuli consisted of three, four, five, or six 3 s, 4 s, 5 s, or 6 s randomly presented within a 9-point grid (see Fig. 1). The emotional stimuli consisted of 32 positive, 32 negative, and 32 neutral pictures selected from the IAPS. The normative mean valence and arousal values on a 9-point scale were respectively 3.35 ± 0.77 and 5.97 ± 1.07 for negative pictures, 7.43 ± 0.52 and 4.99 ± 1.10 for positive pictures, and 4.87 ± 0.28 and 2.66 ± 0.54 for neutral pictures. There were nine trial types: view, congruent, and incongruent trials involving negative, positive, and neutral emotional stimuli. Subjects were exposed to 2 runs each consisting of 16 trials of each of the nine trial classes and 48 fixation point trials to generate a baseline. Each image was presented once in a congruent trial, once in an incongruent trial and once in a view trial. Each image appeared only in one run. There were a total of 32 trials of each of the nine conditions presented and 96 fixation point trial. Trials were randomized within each run for each participant and counterbalanced between participants.
2.2.2. Post-traumatic stress disorder Checklist-Military Version (PCL; Weatherset al., 1993)
The PCL is a self-administered screen for PTSD. The PCL has demonstrated good internal consistency (Cronbach's alpha = .96) and convergent validity with both self- and clinician-report measures (Forbes et al., 2001; Weathers et al., 1993).
2.3. MRI parameters
Participants were scanned using a 3 T Siemens Magnetom scanner. A total of 166 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time = 2900 ms; echo time = 27 ms; 64 × 64 matrix; 90° flip angle; 22 cm field of view). Whole-brain coverage was obtained with 44 axial slices (thickness, 2.5 mm. 5 mm spacing; in-plane resolution, 3.44 × 3.44 mm). A high-resolution anatomical scan (3-dimensional spoiled gradient recalled acquisition in a steady state; repetition time = 2530 ms; echo time = 3.03 ms; 25.6 cm field of view; 7° flip angle; 176 axial slices; thickness, 1.0 mm; 256 × 256 matrix) in register with the EPI dataset was obtained covering the whole brain.
2.4. Imaging data preprocessing
Data were analyzed within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI; Cox, 1996). Both individual and group-level analyses were conducted. Each scan series began after equilibrium magnetization was reached through a Siemens automated process. Motion correction was performed by registering all volumes in the EPI dataset to a volume collected close to acquisition of the high-resolution anatomical dataset.
The EPI datasets for each subject were spatially smoothed (isotropic 6 mm kernel) to reduce variability among individuals and generate group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run, and multiplying the result by 100, producing regression coefficients representing percent-signal change.
2.5. General linear model (GLM) analysis
The model involved six motion regressors and the following task regressors: negative congruent, negative incongruent, negative view, neutral congruent, neutral incongruent, neutral view, positive congruent, positive incongruent and positive view. A regressor modeling incorrect responses was also included. This final regressor was included only to avoid contamination of the baseline and was not incorporated into the ANOVA described below. All regressors were convolved with a canonical hemodynamic response function (HRF) to account for the slow hemodynamic response. No significant collinearity between stimulus types was observed.
The participants' anatomical scans were individually registered to the Talairach and Tournoux atlas (Talairach and Tournoux, 1988). The individuals' functional EPI data were then registered to their Talairach anatomical scan within AFNI. Linear regression modeling was performed using the 10 regressors described earlier, plus regressors to model a first-order baseline drift function. This produced β coefficients and associated t statistics for each voxel and regressor.
2.6. fMRI data analysis
The group analysis of the BOLD data was then performed on regression coefficients from individual subject analyses using a 3 (emotion; negative, positive, neutral) × 3 (condition; congruent, incongruent, view) whole-brain repeated measures Analysis of Covariance (ANCOVA) with PCL scores as a covariate. PCL scores were mean centered before being entered into the ANCOVA. Due to its small size and theoretical importance, the amygdala was interrogated using an anatomically defined mask (Eickhoff–Zilles Architectonic Atlas 50% probability in AFNI) and an uncorrected threshold of p = .05. The AFNI ClustSim program was used to establish a p = .05 FWE corrected threshold (22 voxel clusters at initial threshold of p = .005, FWHM = 6 mm) for a whole-brain analysis. All reported regions in the whole brain analysis exceed this threshold. Post-hoc analyses were performed to facilitate interpretations. For these analyses, average percent signal change was measured across all voxels within each region of interest (ROI) generated from the functional masks, and data were analyzed using appropriate follow-up tests within SPSS. In order to minimize the likelihood of type I error in the comparison of correlation coefficients in dependent samples, Steiger's z was utilized (Steiger, 1980).
In addition, a connectivity analysis was conducted to examine differential functional connectivity between task conditions using an amygdala seed region. Critically, this analysis is an examination of the differences between the simple correlations between the amygdala ROI and the rest of the brain in each task condition, not a psychophysiological interaction analysis. The seed region was generated by creating a 5 mm-radius spheres centered on the peak voxel coordinates from the right amygdala finding in the main ANOVA (see Table 2). The average activation from the seed region was extracted across the time series. Interaction regressors were created by multiplying the average time series with nine task time course vectors (one for each task condition), which were coded: 1 = task condition present and 0 = task condition not present. The average activation for the seed region was entered into a linear regression model along with the nine interaction regressors (one per task condition) and 6 motion regressors. The differences in correlation between task conditions were then examined in a 3 (emotion; negative, positive, neutral) × 3 (condition; congruent, incongruent, view) whole-brain repeated measures ANCOVA with PCL scores as a covariate.
Table 2.
Differential connectivity analysis: Brain regions demonstrating a significant PCL score-by-emotion interaction.
| Coordinates of peak activationa |
||||||||
|---|---|---|---|---|---|---|---|---|
| Region | Left/right | BA | x | y | z | F value | p | Voxels |
| PCL score-by-emotion interaction | ||||||||
| Dorsomedial prefrontal/dorsal anterior cingulate cortex | Right | 32 | 7.5 | 13.5 | 38.5 | 9.135 | <.0001 | 25 |
| Inferior parietal cortex | Right | 40 | 61.5 | −28.5 | 26.5 | 10.09 | <.0001 | 47 |
| Inferior parietal cortex | Left | 40 | −49.5 | −40.5 | 29.5 | 11.67 | <.0001 | 44 |
| Caudate/anterior insula cortex | Left | 13 | −19.5 | 10.5 | 14.5 | 12.23 | <.0001 | 119 |
| Claustrum/anterior insula cortex | Left | −37.5 | −16.5 | −0.5 | 13.81 | <.0001 | 54 | |
| Caudate/lentiform nucleus | Right | 22.5 | 4.5 | 2.5 | 8.397 | <.0001 | 42 | |
| Middle temporal cortex | Left | 22 | −49.5 | −43.5 | 2.5 | 14.36 | <.0001 | 35 |
| Superior temporal gyrus | Right | 38 | 34.5 | −16.5 | −0.5 | 10.63 | <.0001 | 54 |
| Middle occipital/temporal cortex | Left | 19 | −34.5 | −67.5 | 29.5 | 9.667 | <.0001 | 76 |
| Middle frontal gyrus | Right | 6 | 34.5 | −4.5 | 47.5 | 8.723 | <.0001 | 23 |
Based on the Talairach–Tournoux Atlas; BA = Brodmann's Area.
3. Results
3.1. Behavioral results
Initially, we examined the impact of emotion and task condition on accuracy and response latencies across the sample, through two 3 (emotion; negative, positive, neutral) × 2 (condition; congruent, incongruent) repeated measures ANOVAs. With respect to accuracy, there were significant main effects of both task condition [F(1,56) = 15.93, p < .001] and emotion [F(1,56) = 3.82, p = .025]. Participants responded more accurately to congruent relative [M = 95.92%(SD = .06)] to incongruent stimuli [M = 92.31(SD = .09), t(56) = 3.99, p < .001] and to neutral stimuli [M = 94.95(SD = .06)] relative to both positive [M = 93.66(SD = .06)] and negative stimuli [M = 93.74(SD = .07), t(56) = 2.48 & 2.41 respectively, p < .05]. No significant condition-by-emotion interaction was observed [F(1,56) = 1.21, p = .302]. With respect to response latencies for correct responses, there were also main effects of task condition [F(1,56) = 208.95, p < .001] and emotion [F(1,56) = 31.57, p < .001]. Participants responded more quickly to congruent [M = 720.97 (SD = 117.28)] relative to incongruent trials [M = 781.58, (SD = 117.09), t(56) = 14.46, p < .001] and responded faster to neutral trials [M = 736.44(SD = 113.0)] relative to positive [M = 753.19(SD = 118.66)] and negative trials [M = 764.18(SD = 119.60), t(56) = 5.18 & 3.10 respectively, ps < .001]. No significant condition-by-emotion interaction was observed [F(1,56) = .711, p = .493].
Subsequent ANCOVAs using PCL scores as the covariate revealed no significant main effects or interactions for the accuracy data. However, a significant main effect of the PCL score covariate for the response latency ANCOVA was observed; greater PCL scores were associated with increased response times [r = .275, p = .039]. There were no interactions of the covariate, though, with either emotion or condition.
3.2. fMRI results
The goal of the current study was to assess whether sub-threshold PTSD symptom severity was related to the recruitment of regions associated with top-down attentional control implicated in the pathophysiology of PTSD. We examined this through a 3 (emotion; negative, positive, neutral) × 3 (condition; congruent, incongruent, view) ANCOVA conducted on the BOLD data using PCL scores as a covariate. This revealed regions showing significant interaction between PCL score and both emotion and task (Table 1). No regions showed significant PCL scores-by-emotion-by-task interactions or a main effect of PCL score (see Supplemental results for complete results).
Table 1.
Brain regions demonstrating significant PCL score-by-emotion and PCL score-by-task condition interactions.
| Coordinates of peak activationa |
||||||||
|---|---|---|---|---|---|---|---|---|
| Region | Left/right | BA | x | y | z | F value | p | Voxels |
| PCL score-by-task condition interaction | ||||||||
| Superior/middle frontal gyrus | Right | 8 | 22.5 | 22.5 | 44.5 | 10.85 | <.0001 | 39 |
| PCL score-by-emotion interaction | ||||||||
| Dorsomedial frontal cortex | Left | 6 | −1.5 | 1.5 | 50.5 | 10.91 | <.0001 | 71 |
| Dorsolateral prefrontal cortex | Right | 46 | 49.5 | 22.5 | 23.5 | 10.35 | <.0001 | 61 |
| Lateral frontal cortex | Right | 10/46 | 40.5 | 40.5 | 11.5 | 9.60 | .0001 | 53 |
| Inferior parietal cortex | Right | 40 | 46.5 | −43.5 | 32.5 | 8.53 | .0004 | 29 |
| Cadudate/claustrum/putamen | Right | 25.5 | 10.5 | 17.5 | 9.68 | .0001 | 66 | |
| Lentiform nucleus/putamen | Left | −19.5 | 7.5 | 11.5 | 8.10 | .0005 | 40 | |
| Middle temporal gyrus | Right | 21 | 61.5 | −52.5 | 8.5 | 9.59 | .0002 | 30 |
| Middle occipital gyrus | Left | 18 | −13.5 | −91.5 | 14.5 | 13.75 | <.0001 | 108 |
| Middle occipital gyrus | Left | 18 | −37.5 | −82.5 | 2.5 | 9.06 | .0002 | 46 |
| Middle occipital gyrus | Right | 19 | 31.5 | −88.5 | 8.5 | 9.91 | .0001 | 29 |
| Fusiform gyrus | Right | 37 | 55.5 | −55.5 | −15.5 | 9.62 | .0001 | 26 |
| Thalamus | Left | −16.5 | −16.5 | −0.5 | 10.98 | <.0001 | 25 | |
| Lingual gyrus/posterior cingulate cortex | Medial | 18/19 | 13.5 | −58.5 | 5.5 | 11.98 | <.0001 | 161 |
| Precentral gyrus | Right | 4 | 61.5 | −10.5 | 26.5 | 8.99 | .0002 | 27 |
| Declive | Right | 31.5 | −55.5 | −15.5 | 11.68 | <.0001 | 65 | |
Based on the Talairach–Tournoux Atlas; BA = Brodmann's Area.
3.2.1. Amygdala ROI
A significant PCL score-by-emotion interaction was observed in the right amygdala (k = 4, x,y,z: 25.5,−7.5,−21.5). A stronger positive relationship between PCL scores and BOLD response in the amygdala was observed for positive relative to neutral conditions [Stieger's Z = 3.03, p = .003] and for negative relative to neutral conditions at trend levels [Stieger's Z = 1.58, p = .11]. A stronger positive relationship between PCL scores and BOLD response in the amygdala was also observed for positive relative to negative stimuli at trend levels [Stieger's Z = 1.61, p = .10]. In short, increased PTSD symptoms were associated with larger increases in amygdala response to positive relative to neutral stimuli and, at least at trend levels, for negative relative to neutral stimuli and positive relative to negative stimuli.
3.2.2. PCL score-by-task condition interaction
A PCL score-by-task condition interaction was observed in the right superior/middle frontal gyrus (see Table 1 and Fig. 2). In this region, PCL scores showed a stronger positive association with BOLD response to congruent and incongruent trials relative to view trials [Stieger's Zs = 3.34 & 2.19 respectively, p < .01]. In other words, individuals with more PTSD symptoms showed greater increases in their BOLD responses within the right superior/middle frontal gyrus during congruent and incongruent trials relative to view trials.
Fig. 2.
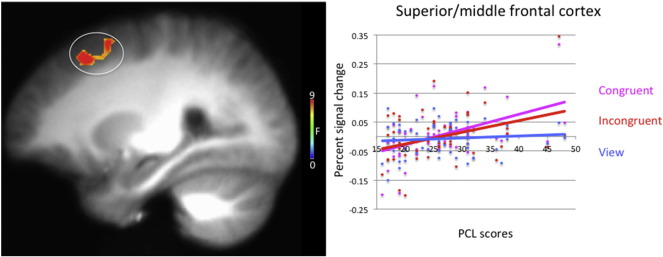
Task condition-by-PTSD checklist score interaction in the right superior/middle frontal cortex in 57 trauma-exposed combat veterans. Participants showed greater levels of increased activation in the right superior/middle frontal gyrus as a function of increased PTSD symptom severity during both congruent and incongruent trials relative to passive viewing trials.
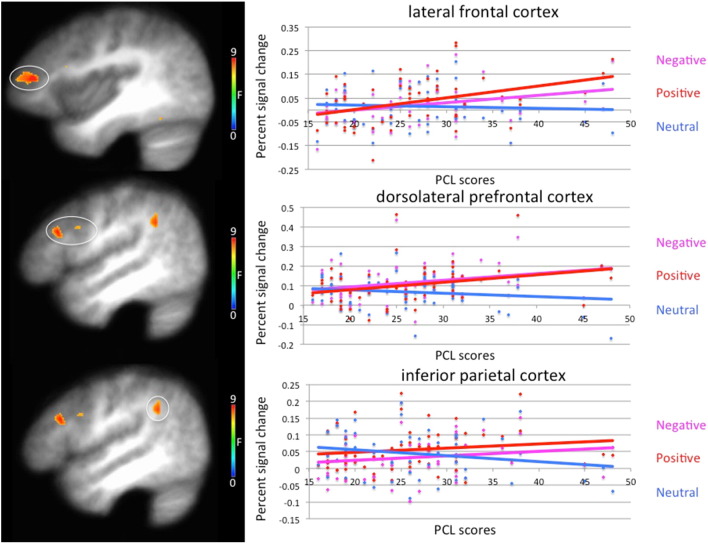
3.2.3. PCL score-by-emotion interaction
Regions showing a significant effect of the PCL score-by-emotion interaction included the right dorsolateral prefrontal cortex (dlPFC), right lateral frontal cortex (lFC), right inferior parietal cortex (iPC; see Fig. 3) and left dorsomedial frontal cortex/dorsal anterior cingulate cortex (dmFC/dACC), as well as bilateral striatal regions and regions of both temporal and occipital cortices (see Table 1). In all regions, PCL scores showed a stronger positive association with BOLD response for negative and positive trials relative to neutral trials [Stieger's Zs = 2.67–4.33, p < .008]. The association between PCL scores and BOLD response did not differ between negative and positive stimuli [Stieger's Zs = .08–1.37, p > .17], though trends towards a stronger, more positive relationship between PCL scores and BOLD response during positive relative to negative conditions were observed in the left middle occipital gyrus and right caudate/lentiform nucleus [Stieger's Zs = 1.90 and 1.91 respectively, p = .06]. In other words, greater PCL scores were associated with greater increases in the BOLD response in dmFC/dACC, dlPFC, lFC, iPC, striatum, occipital and temporal cortices during emotional trials relative to neutral trials.
Fig. 3.
Emotion-by-PTSD checklist score interaction in the right lateral frontal cortex and right dorsolateral prefrontal cortex in 57 trauma-exposed combat veterans. Participants showed greater levels of increased activation in the right lateral frontal cortex and dorsolateral prefrontal cortex as a function of increased PTSD symptom severity to both positive and negative stimuli relative to neutral stimuli.
3.3. Functional connectivity results
In order to investigate our hypothesis that stronger, inverse functional connectivity would be observed between the amygdala and regions associated with top down attention as PTSD symptom severity increased, a functional connectivity analysis was conducted. Differential connectivity as a function of task condition using the right amygdala as a seed region was examined using a 3 (emotion; negative, positive, neutral) × 3 (condition; congruent, incongruent, view) repeated measures whole brain ANCOVA with PCL scores as a covariate (see Table 2, for complete results see Supplementary results).
3.3.1. PCL score-by-task condition interaction
No regions survived correction for multiple comparisons in this contrast.
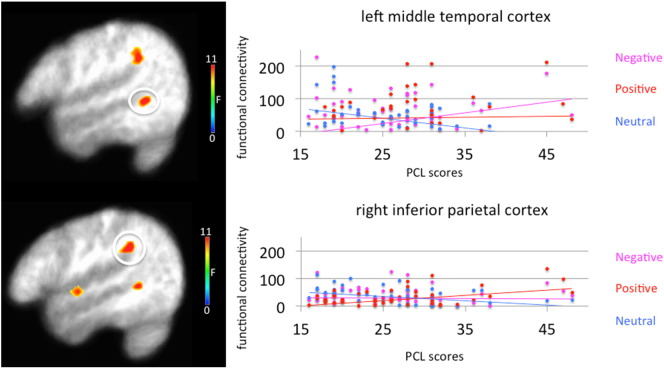
3.3.2. PCL score-by-emotion interaction
A significant PCL score-by-emotion interaction was observed in regions including dmFC/dACC, left caudate/anterior insula cortex, left claustrum/anterior insula cortex, right caudate/lentiform nucleus, bilateral inferior parietal cortex, right superior temporal gyrus and left middle temporal cortex (see Table 2 and Fig. 4). In all regions, a stronger, more positive correlation between PCL scores and functional connectivity was observed to positive stimuli relative to both neutral stimuli [Steiger's Z = 2.09–4.43, p < .037] and negative stimuli [Steiger's Z = 2.23–3.65, p < .026]. In the right inferior parietal cortex and left middle temporal cortex, a stronger, more positive correlation between PCL scores and functional connectivity was observed to negative stimuli relative to neutral stimuli [Steiger's Z = 2.14–2.39, p < .032].
Fig. 4.
Emotion-by-PTSD checklist score interaction examining differential functional connectivity between amygdala and left middle temporal cortex and right inferior parietal cortex in 57 trauma-exposed combat veterans. Participants showed greater levels of increased functional connectivity in the left middle temporal cortex and right inferior parietal cortex as a function of increased PTSD symptom severity to both positive and negative stimuli relative to neutral stimuli.
3.4. Supplementary analysis
Given the association between PCL scores and response times, we re-ran the ANCOVA on the BOLD data, but replacing PCL scores with response time as the covariate. The findings related to PCL scores reported above could not be reduced to the relationship of PCL scores with RT. There were regions showing a significant effect of the covariate RT and interactions of RT with task variables but all were within regions of visual and motor cortex not the prefrontal regions implicated in the analysis reported above.
There were interesting results obtained from examining the relationship between average BOLD response for task trials extracted from the region of the right superior/middle frontal gyrus implicated in the PCL score-by-task condition interaction in the main ANCOVA and accuracy (anonymous reviewer's suggestion). BOLD response was not significantly correlated with response latency [r = .124, p = .359]. However, interestingly, BOLD response was significantly inversely associated with response accuracy [r = –.263, p = .048] and with PCL scores [r = .437, p = .001], while PCL scores were associated inversely at trend levels with response accuracy [r = –.248, p = .063]. Critically, PCL scores were seen to mediate the relationship between BOLD response and response accuracy [rpartial = −.178, p = .190 controlling for PCL scores].
4. Discussion
The goal of the current study was to examine amygdala responsiveness to emotional stimuli and the recruitment of top down attention systems as a function of task demands in a population of recently deployed armed service members with sub-threshold PTSD symptoms. There were four main findings. First, greater PTSD symptom severity scores were associated with increased amygdala activation in response to emotional relative to neutral stimuli (though at trend levels for negative stimuli). Second, greater PTSD symptom severity was associated with increased superior/middle frontal cortex response during task conditions relative to passive viewing conditions. Third, greater PTSD symptom severity scores were associated with increased activation in dmFC/dACC, dlPFC, lFC, iPC, temporal cortex, occipital cortex and striatal regions in response to emotional relative to neutral stimuli. Fourth, greater PTSD symptom severity was associated with increased functional connectivity during emotional trials relative to neutral trials in regions including the right inferior parietal cortex and left middle temporal cortex.
Consistent with previous findings (Blair et al., 2013), greater levels of PTSD symptomology were associated with greater amygdala responses to emotional stimuli relative to neutral stimuli. This is consistent with work indicating increased amygdala sensitivity to emotional information in patients with PTSD (Blair et al., 2013; Bremner et al., 2004; El Khoury-Malhame et al., 2011; Felmingham et al., 2010; Rauch et al., 2006; Shin and Liberzon, 2010). It is important to note though that this relationship was seen only at a very lenient threshold. Furthermore, the association between PCL scores and amygdala activation was observed for negative stimuli only at trend levels. While positive distractor stimuli have shown an interference effect in previous work (Vythilingam et al., 2007), such a weak effect of negative distractor stimuli is surprising. Of course, the relative weakness of these findings may reflect the fact that this population did not meet criteria for PTSD. It is plausible that if participants who met criteria for PTSD had been included in the study sample these results would have been stronger. This finding may also represent type II error. It is worth noting the functional connectivity results though. In the PCL score-by-emotion interaction in the connectivity analysis, significantly increased functional connectivity was observed between the right amygdala and bilateral caudate, left anterior insula cortex, left middle temporal cortex, dmFC/dACC, and bilateral iPC. While in most regions, this increased functional connectivity as a function of PTSD symptom severity was also observed only to positive stimuli, increased functional connectivity as a function of PTSD symptom severity to both position and negative emotional conditions was observed in the right inferior parietal cortex and left middle temporal cortex. Thus the current data are somewhat consistent with previous work indicating increased amygdala sensitivity to emotional information in patients with PTSD (Blair et al., 2013; Bremner et al., 2004; El Khoury-Malhame et al., 2011; Felmingham et al., 2010; Rauch et al., 2006; Shin and Liberzon, 2010).
PTSD symptomology was associated with increased activity in the right superior/middle frontal gyrus during task relative to view trials. At first glance, these findings appear inconsistent with three previous studies that found reduced recruitment of frontal cortical regions in PTSD patients relative to healthy controls (New et al., 2009) and as a function of increased PTSD symptom severity (Blair et al., 2013; Pannu Hayes et al., 2009). However, these studies involved patients with PTSD (Blair et al., 2013; New et al., 2009) and individuals endorsing PTSD symptoms over the clinical cut-off on the PCL (Pannu Hayes et al., 2009). In contrast, in the current study, current PTSD was exclusory. Indeed, the current sample did not include any subjects with PCL scores of greater than 50 (often used as a diagnostic cut-off for PTSD; Forbes et al., 2001; Weathers et al., 1993). Interestingly, two of these previous studies also examined the functioning of “trauma controls”; i.e., individuals exposed to traumatic experiences, but without PTSD (Blair et al., 2013; New et al., 2009). Both of these studies found enhanced recruitment of the superior frontal cortex (Blair et al., 2013; New et al., 2009) as a function of task demands (Blair et al., 2013) or reappraisal (New et al., 2009) in the trauma controls relative to patients with PTSD (Blair et al., 2013; New et al., 2009) and healthy comparison individuals (Blair et al., 2013). Blair et al. (2013) and New et al. (2009) suggest that enhanced recruitment of this region, particularly because of a putative role in top down attention, may represent a trait conferring some resilience to PTSD. The sample in the current study thus appears to be similar to these trauma controls. Indeed, all had been exposed to significant combat related stressors and were not, at the time of testing, presenting with PTSD. But it is important to remember that the participants were assessed 8 weeks after returning from deployment. As such, PTSD may not have had sufficient time to develop. More critically though, any explanation based around the participants being trauma controls still does not explain why there should be a significant positive relationship between symptom severity and the recruitment of regions associated with top down attention or why symptom severity should mediate the association between recruitment of these regions and task performance. If a propensity towards greater activation in top down attention regions was a general protective factor, overall greater levels of activation would be expected in a trauma control group, but no specific relationship between activation and PTSD symptom severity would be expected. We hypothesize instead that these data reflect a compensatory response. Part of this compensatory response may reflect “a lower cognitive threshold for needing to recruit middle frontal regions during cognitive inhibition tasks” (anonymous reviewer's suggestion). Increased emotional responsiveness and slower response times were observed as a function of more prominent PTSD symptoms. The increased recruitment of regions associated with top down control may have been necessary, in the face of potentially increased distractibility, to achieve successful task performance. It is important to note however, that further work will be needed to fully test this hypothesis.
The results of the PCL score-by-emotion interactions for both the BOLD response and connectivity data are noteworthy. With respect to the BOLD data, within right dlPFC, lFC, iPC, left dmFC/dACC, bilateral occipital and temporal cortices and bilateral striatal regions, PCL scores showed a stronger positive association with BOLD response for both negative and positive trials relative to neutral trials. In other words, greater PCL scores were associated with greater increases in the BOLD response in these regions during emotional relative to neutral trials. With respect to the connectivity data, PCL scores showed a stronger, more positive correlation between the amygdala seed and dmFC/dACC, bilateral iPC, left caudate/anterior insula cortex, right caudate/lentiform nucleus and left middle temporal cortex for positive (and in the right inferior parietal and left middle temporal cortices for negative) stimuli relative to neutral stimuli. Two interpretations of these data can be considered. First, they might reflect a compensatory response (cf. Blair et al., 2013; New et al., 2009). Lateral frontal cortices, dmFC/dACC and iPC are involved in top down attention (Kastner and Ungerleider, 2000; MacDonald et al., 2000) and successful performance on the affective Stroop task (Blair et al., 2007). Similar to the argument presented above with respect to the PCL score-by-task condition interaction, the increased recruitment of these regions with increasing PCL score may have been necessary to compensate for the increased distractibility of the emotional distracters (as indexed by increased errors and increased RTs for emotional relative to neutral trials). An alternative explanation of the data is also possible. Increased PCL scores were associated with increased responses to emotional relative to neutral stimuli within temporal and occipital regions as well as portions of striatum. In addition, they were associated with increased correlated activity of the amygdala and representational (left middle temporal cortex) and emotional regions (left caudate/anterior insula cortex and caudate/lentiform nucleus). Moreover, these findings were observed as PCL-score-by-emotion interactions rather than PCL-by-emotion-by-task interactions. In other words, the increased associations between BOLD response and correlated activation in these regions with increased PCL-scores reflected responses to emotional stimuli even in view conditions where task performance was unnecessary. It is possible that increased PTSD symptoms are simply associated with increased responsivity to emotional stimuli. Further work is needed to explore these possibilities.
Two important caveats should be considered with respect to the current data. First, PTSD symptoms were assessed using a self-report screening tool. While a cut-off score of 50 on the PCL has been shown effective in distinguishing those likely to have a diagnosis of PTSD from those who likely do not (Forbes et al., 2001; Weathers et al., 1993), it is unclear that the PCL is psychometrically valid for use as a continuous measure of PSTD symptom severity. Second, while participants were all scanned within 8 weeks from returning from deployment, participants were deployed for varying lengths of time and it is not known exactly when the traumatic events took place. Therefore, issues of timing relative to the onset of the symptoms cannot be addressed in the current study.
5. Conclusions
Combat-exposed, but PTSD negative, armed service members showed increased activation in regions associated with emotion processing, object representation and top-down attentional control as a function of increased PTSD symptomology. We suggest that these data may reflect two phenomena. First, these data indicate increased emotional responsiveness by: (i) the positive relationship between PTSD symptom severity and amygdala responsiveness to emotional relative to neutral stimuli; (ii) greater BOLD response as a function of PTSD symptom severity in regions implicated in emotion (striatum) and representation (occipital and temporal cortices) during emotional relative to neutral conditions; and (iii) increased connectivity between the amygdala and regions implicated in emotion (insula/caudate) and representation (middle temporal cortex) as a function of PTSD symptom severity during emotional relative to neutral trials. Second, these data indicate a greater need for the recruitment of regions implicated in top down attention as indicated by (i) greater BOLD response in superior/middle frontal gyrus as a function of PTSD symptom severity in task relative to view conditions; (ii) greater BOLD response in dmFC/dACC, lateral frontal and inferior parietal cortices as a function of PTSD symptom severity in emotional relative to neutral conditions and (iii) greater functional connectivity between the amygdala and right inferior parietal cortex as a function of PTSD symptom severity during emotional relative to neutral conditions. Further work is needed to understand the relationship between emotional responding, top down attention and PTSD symptom severity in sub-clinical samples in order to better understand the development of and resiliency to PTSD.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health under grant number 1-ZIA-MH002860 to James Blair and the Center for Neuroscience and Regenerative Medicine under grant number 300601 8.01 60855510005 to Michael Roy. This work was approved under the Uniformed Services University of the Health Sciences Institutional Review Board protocol 11N-0090. NCT number: NCT01296126.
Footnotes
Any opinions or assertions expressed are solely those of the authors and do not necessarily represent those of Uniformed Services University, the U.S. Army, U.S. Navy, Department of Defense, or the U.S. Government.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.11.012.
Contributor Information
Stuart F. White, Email: stuart.white@nih.gov.
James R. Blair, Email: stuart.white@nih.gov.
Appendix A. Supplementary data
Supplementary material.
Supplementary table.
References
- APA . Diagnostic and Statistical Manual of Mental Disorders. Fifth edition. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. 21349277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Geraci M., Smith B.W., Hollon N., Devido J., Otero M., Pine D.S. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol. Psychiatry. 2012;72:476–482. doi: 10.1016/j.biopsych.2012.04.013. 22592057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Smith B.W., Mitchell D.G., Morton J., Vythilingam M., Pessoa L., Blair R.J. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. 17239620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Vythilingam M., Crowe S.L., McCaffrey D.E., Ng P., Wu C.C., Blair R.J. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol. Med. 2013;43(1):85–95. doi: 10.1017/S0033291712000840. 22571775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E., Vythilingam M., Afzal N., Schmahl C., Elzinga B., Charney D.S. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry. 2004;55(6):612–620. doi: 10.1016/j.biopsych.2003.10.001. 15013830 [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. 23672542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. 7605061 [DOI] [PubMed] [Google Scholar]
- El Khoury-Malhame M., Reynaud E., Soriano A., Michael K., Salgado-Pineda P., Zendjidjian X., Khalfa S. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49(7):1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. 21440563 [DOI] [PubMed] [Google Scholar]
- Esterman M., DeGutis J., Mercado R., Rosenblatt A., Vasterling J.J., Milberg W., McGlinchey R. Stress-related psychological symptoms are associated with increased attentional capture by visually salient distractors. J. Int. Neuropsychol. Soc. 2013;19(7):835–840. doi: 10.1017/S135561771300057X. 23803518 [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Hoeft F., Menon V., Schatzberg A.F. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am. J. Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. 20123913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K., Williams L.M., Kemp A.H., Liddell B., Falconer E., Peduto A., Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J. Abnorm. Psychol. 2010;119(1):241–247. doi: 10.1037/a0017551. 20141261 [DOI] [PubMed] [Google Scholar]
- Forbes D., Creamer M., Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther. 2001;39(8):977–986. doi: 10.1016/s0005-7967(00)00084-x. 11480838 [DOI] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. 21432682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Vanelzakker M.B., Shin L.M. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front. Integr. Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. 23087624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., Castro C.A., Messer S.C., McGurk D., Cotting D.I., Koffman R.L. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N. Engl. J. Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. 15229303 [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav. Rev. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. 19539645 [DOI] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. 10845067 [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., 3rd, Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. 10846167 [DOI] [PubMed] [Google Scholar]
- Miller J.D., Lynam D.R. Psychopathy and the five-factor model of personality: a replication and extension. J. Pers. Assess. 2003;81(2):168–178. doi: 10.1207/S15327752JPA8102_08. 12946923 [DOI] [PubMed] [Google Scholar]
- Mitchell D.G., Luo Q., Mondillo K., Vythilingam M., Finger E.C., Blair R.J. The interference of operant task performance by emotional distracters: an antagonistic relationship between the amygdala and frontoparietal cortices. Neuroimage. 2008;40(2):859–868. doi: 10.1016/j.neuroimage.2007.08.002. 18234519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.G., Nakic M., Fridberg D., Kamel N., Pine D.S., Blair R.J. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. 17161627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.G., Richell R.A., Leonard A., Blair R.J. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. J. Abnorm. Psychol. 2006;115(3):559–566. doi: 10.1037/0021-843X.115.3.559. 16866596 [DOI] [PubMed] [Google Scholar]
- Mylle J., Maes M. Partial posttraumatic stress disorder revisited. J. Affect. Disord. 2004;78(1):37–48. doi: 10.1016/s0165-0327(02)00218-5. 14672795 [DOI] [PubMed] [Google Scholar]
- New A.S., Fan J., Murrough J.W., Liu X., Liebman R.E., Guise K.G., Charney D.S. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. 19589502 [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. (Regul. Ed.) 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. 15866151 [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J., Labar K.S., Petty C.M., McCarthy G., Morey R.A. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172(1):7–15. doi: 10.1016/j.pscychresns.2008.05.005. 19237269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., McKenna M., Gutierrez E., Ungerleider L.G. Neural processing of emotional faces requires attention. Proc. Natl. Acad. Sci. U.S.A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. 12177449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Padmala S., Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. 15993624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. 12946880 [DOI] [PubMed] [Google Scholar]
- Pietrzak R.H., Goldstein M.B., Malley J.C., Johnson D.C., Southwick S.M. Subsyndromal posttraumatic stress disorder is associated with health and psychosocial difficulties in veterans of operations enduring freedom and Iraqi freedom. Depress Anxiety. 2009;26(8):739–744. doi: 10.1002/da.20574. 19496075 [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research — past, present, and future. Biol. Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. 16919525 [DOI] [PubMed] [Google Scholar]
- Regier D.A. Dimensional approaches to psychiatric classification: refining the research agenda for DSM-V: an introduction. Int J Methods Psychiatr Res. 2007;16(Suppl. 1):S1–S5. doi: 10.1002/mpr.209. 17623390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. 23928089 [DOI] [PubMed] [Google Scholar]
- Roy M.J., Costanzo M., Leaman S. Psychophysiologic identification of subthreshold PTSD in combat veterans. Stud. Health Technol. Inform. 2012;181:149–155. 22954846 [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. 19625997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980;87(2):245–251. [Google Scholar]
- Talairach Tournoux. Thieme; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Vythilingam M., Blair K.S., McCaffrey D., Scaramozza M., Jones M., Nakic M., Blair R.J. Biased emotional attention in post-traumatic stress disorder: a help as well as a hindrance? Psychol Med. 2007;37(10):1445–1455. doi: 10.1017/S003329170700092X. 17559703 [DOI] [PubMed] [Google Scholar]
- Weathers F., Litz B., Herman D., Huska J., Keane T. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. Paper Presented at the Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX. 1993 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary table.