Abstract
Despite the impressive literature describing atypical neural activation in visuoperceptual face processing regions in autism, almost nothing is known about whether these perturbations extend to more affective regions in the circuitry and whether they bear any relationship to symptom severity or atypical behavior. Using fMRI, we compared face-, object-, and house-related activation in adolescent males with high-functioning autism (HFA) and typically developing (TD) matched controls. HFA adolescents exhibited hypo-activation throughout the core visuoperceptual regions, particularly in the right hemisphere, as well as in some of the affective/motivational face-processing regions, including the posterior cingulate cortex and right anterior temporal lobe. Conclusions about the relative hyper- or hypo-activation of the amygdala depended on the nature of the contrast that was used to define the activation. Individual differences in symptom severity predicted the magnitude of face activation, particularly in the right fusiform gyrus. Also, among the HFA adolescents, face recognition performance predicted the magnitude of face activation in the right anterior temporal lobe, a region that supports face individuation in TD adults. Our findings reveal a systematic relation between the magnitude of neural dysfunction, severity of autism symptoms, and variation in face recognition behavior in adolescents with autism. In so doing, we uncover brain–behavior relations that underlie one of the most prominent social deficits in autism and help resolve discrepancies in the literature.
Keywords: Fusiform gyrus, Amygdala, Development, Face recognition, fMRI, Individual differences
Abbreviations: TD, typical developing; HFA, high functioning autism; fMRI, functional magnetic iresonance maging; BOLD, blood oxygen level dependent
Highlights
-
•
Adolescents with autism exhibit weak activation in core and extended face regions.
-
•
Fearful and neutral faces as well as objects elicit amygdala activation in TD adolescents.
-
•
Only fearful faces drive amygdala activation in HFA adolescents.
-
•
Individual differences in behavior predict face activation in the anterior temporal lobe.
-
•
Individual differences in symptom severity predict face activation in the fusiform gyrus.
1. Introduction
Although not a diagnostic symptom of autism spectrum disorder (ASD), deficits in face processing represent a model domain in which to understand some of the core behavioral and neural features of autism. For example, many components of face processing (e.g., identity recognition, expression recognition) are developing at the very time that behavioral symptoms of autism are emerging and changing developmentally (infancy through young adulthood), allowing researchers to track aberrant developmental trajectories, and thus identify vulnerable developmental periods. In addition, many of the individual neural regions comprising the broadly distributed circuitry that subserves face recognition abilities (Gobbini and Haxby, 2007) are located within anatomical regions that show pathological structural growth patterns during infancy, toddlerhood, and adolescence in autism. These regions include the temporal and frontal lobes as well as the amygdala (Schumann et al., 2010), suggesting that they may be particularly vulnerable throughout the developmental course of the disorder. Finally, given that faces are the pre-eminent social stimulus from which we extract multiple kinds of social information that guide behavior, they provide a useful index of atypical neural organization of social-information processing across a spectrum of social–emotional disorders (e.g., Evans et al., 2008; Kucharska-Pietura et al., 2005; Marsh and Blair, 2008). Therefore, understanding the profile of atypical neural activation during face processing in autism, particularly during vulnerable developmental periods, is a fruitful approach to studying a core feature of autism; that is, disruption of the social brain and social information processing more generally.
The central goal of the current project was to evaluate the nature and extent of disruption in the social brain during face processing in autism, particularly during adolescence. We focus specifically on adolescence (i.e., the second decade of life) as this is a developmental period of emerging vulnerability for individuals with autism in terms of face processing behavior (O'Hearn et al., 2010) and neural circuitry (Dalton et al., 2005; Scherf et al., 2010; Wang et al., 2004). Also, an estimated one-third of children with autism experience deterioration in functioning during adolescence, which is associated with concomitant neurological complications (Gillberg and Steffenburg, 1987; Kanne et al., 2011), a substantial increase in social withdrawal (Anderson et al., 2011), and a potential heightened risk for developing comorbid depression and anxiety (Brereton et al., 2006; Kuusikko et al., 2008; Mayes et al., 2011; McPheeters et al., 2011).
In this work, we include a particular focus on the functional profile of activation within the fusiform face area (FFA; Kanwisher et al., 1997) of the temporal lobe and the amygdala, two critical regions supporting multiple aspects of face processing (i.e., identity recognition, affective processing, trait attribution). Our focus on atypical activation within the FFA and amygdala in autism stems from contradictions within the existing literature that have made it difficult to ascertain a profile of atypical functional activation and organization among these regions even in adulthood autism. Importantly, while the amygdala is central for processing affective information about faces, it is only one of several other critical regions that make up the extended face network (Gobbini and Haxby, 2007). Surprisingly, little is known about the neural profile of these extended regions in autism, which might be especially disrupted given the known social and affective impairments in autism.
1.1. Discrepancies concerning atypical face-related activation in autism
The FFA in the fusiform gyrus (FG) together with a lateral region in the inferior occipital cortex [“occipital face area” (OFA); Gauthier et al., 2000] and the posterior superior temporal sulcus (STS; Hoffman and Haxby, 2000) comprise the “core regions” in the broadly distributed neural circuitry supporting face processing (Gobbini and Haxby, 2007; Haxby et al., 2000). Although these core regions are strongly implicated in supporting the visuoperceptual and cognitive analysis of faces, they also receive strong inputs from the extended regions, which are implicated in the more social and emotional aspects of face processing (Said et al., 2010, 2011). The extended face processing regions include the amygdala, insula, and medial prefrontal cortex, regions in the anterior paracingulate cortex, and the anterior temporal lobe (Gobbini and Haxby, 2007). These extended regions process more changeable aspects of faces, such as facial expressions and associating “person knowledge” with faces, including personal traits, attitudes, mental states, and intentions. The overwhelming majority of studies investigating the neural basis of face processing in autism have focused on understanding whether face-related activation in the FFA and the amygdala is atypical.
1.1.1. Fusiform face area
Many studies report hypo-activation in the FFA in individuals with autism during unfamiliar face processing (Dalton et al., 2005; Domes et al., 2013; Grelotti et al., 2005; Humphreys et al., 2008; Kleinhans et al., 2011; Malisza et al., 2011; Pelphrey et al., 2007; Pierce et al., 2001; Pierce and Redcay, 2008; Pinkham et al., 2008; Richey et al., 2014; Sato et al., 2012; Schultz et al., 2000; Wang et al., 2004). For example, we previously reported that during passive viewing of movies of faces, hypo-activation is evident in the FFA as well as other core (i.e., perceptual) regions of the face-processing network in adults (Humphreys et al., 2008) and adolescents (Scherf et al., 2010) with high-functioning autism (HFA). However, there are several studies that fail to find atypical activation within the fusiform gyrus (Bird et al., 2006; Dapretto et al., 2006; Hadjikhani et al., 2004, 2007; Kleinshans et al., 2008) in autism. For example, in contrast to our previous finding, Hadjikhani et al., who used a passive viewing task of static face photographs but asked participants to fixate a red fixation cross positioned on the bridge of the nose of the face images, failed to find differences in face-related activation in the FG of adults with autism (Hadjikhani et al., 2007). It would seem that encouraging participants with autism to fixate the face improves signal in the FFA; however, a similar a study of adults with autism using the same procedure reported face-related hypo-activation in the FG (Humphreys et al., 2008). One important difference between these two studies is that the participants in the studies varied in the magnitude of their symptom severity with the participants in the study by Hadjikhani and colleagues consisting of almost an equal distribution of autism, and Asperger's/PDD participants whereas the study by Humphrey and colleagues only included participants with autism.
A review of this literature suggests that the pattern of mixed findings of face-related activation in the fusiform gyrus is not likely to be related to differences in task demands (e.g., passive viewing versus face matching) or the specific contrast used to define the face activation (e.g., affective faces versus neutral faces, faces versus objects, faces versus shapes). Patterns of both hypo- and comparable face-related activation in the FFA have been observed under the full range of these conditions. The pattern of mixed findings is also not likely to be related to the familiarity of the face stimuli since findings of both hypo- and comparable face-related activation have been observed when the face stimuli are familiar to participants (hypo-active, Dalton et al., 2005; comparable, Pierce et al., 2004; Pierce and Redcay, 2008). Instead, the studies appear to differ in terms of the relative severity of the autism participants. Specifically, all the studies reporting comparable face-related activation in people with autism, particularly in the FFA, have included a large proportion of participants with Asperger's Syndrome and PDD-NOS, who are less severely impacted symptomatically than those with an autism diagnosis. In contrast, the studies reporting hypo-activation in the FFA have largely included participants with a diagnosis of autism who are more severely affected by the disorder.
Based on these findings, we suggest that the discrepancies in the existing literature, particularly with respect to face-related activation in the fusiform gyrus, may actually reflect a systematic relation between the magnitude of activation and the severity of autism symptoms and/or variation in face recognition behavior. Importantly, this hypothesis has not been systematically examined. Understanding the potential relation between symptom severity, face recognition behavior, and FFA activation in response to faces may provide a critical step in reconciling the notable discrepancies about the development of the social brain in autism.
1.1.2. Amygdala
Findings about atypical amygdala activation during face processing in autism are equally discrepant. Given the social impairments of autism and the reported difficulties in processing emotional expressions (Adolphs et al., 2001; Dawson et al., 2005), amygdala activation is likely to be atypical, particularly in response to affective faces. However, the nature of this atypicality is controversial and the existing results conflict, with many reporting hypo-activation (Ashwin et al., 2007; Bookheimer et al., 2008; Corbett et al., 2009; Critchley et al., 2000; Grelotti et al., 2005; Hadjikhani et al., 2007; Iidaka et al., 2012; Pelphrey et al., 2007; Pierce et al., 2001), some reporting hyper-activation (Dalton et al., 2005; Monk et al., 2010; Swartz et al., 2013; Tottenham et al., 2014; Weng et al., 2011), and still others reporting comparable activation (Pierce et al., 2004; Wang et al., 2004) in the amygdala compared to typically developing (TD) individuals.
Our review of this literature suggests that, instead of symptom severity, the discrepancy in findings about amygdala activation in autism may be related to methodological differences in the way neural activation is defined, particularly with respect to the comparison baseline condition. For example, studies reporting amygdala hyper-activation in autism generally contrast affective faces (e.g., sad, happy) with fixation (e.g., Dalton et al., 2005; Tottenham et al., 2014; Weng et al., 2011). Under these conditions, hyper-activation compared to controls could result from either higher magnitude responses to the faces and/or lower responses to the fixation, which could both contribute to a larger difference score (i.e., hyper-activation) across these two conditions. In contrast, studies reporting amygdala hypo-activation in autism have employed a variety of contrasts in which affective or neural faces are compared with other visual objects, shapes, or scrambled images (e.g., Bookheimer et al., 2008; Corbett et al., 2009; Pierce et al., 2001). In this case, the reduced responsivity of the amygdala in autism compared to controls could result from either lower magnitude responses to faces and/or higher magnitude responses to the other visual categories, resulting in a lower difference score (i.e., hypo-activation) across these two conditions. Given this pattern of findings, it is difficult to assess whether aberrant activation in the amygdala in autism is largely indicative of atypical processing of faces specifically (as might be concluded from the work contrasting faces with fixation baseline), or whether there is a broader atypicality in amygdala function that affects the processing of a wide array of visual objects (as might be concluded by the work contrasting faces with more complex comparison images). Careful investigation of the profile of amygdala activation in response to faces (both affective and neutral) as well as to a wide range of other visual stimuli will help address this question.
1.2. Current study
In this study, we aimed to identify disruptions in neural activation through the core and extended regions supporting face processing (and social-information processing more generally) in adolescents with autism and to explore individual differences as reflected in the relationship between variations in behavior and/or symptom severity and face-related activation within these regions. We studied high functioning adolescents (HFA) with autism (ages 10–17 years) and age-matched typically developing (TD) adolescents. We measured brain activation using fMRI while participants performed a recognition task with both affective and neutral faces as well as a range of other visual stimuli, including common objects, houses, and scrambled images. This enabled us to map and compare face-related activation in both core and extended face processing regions across the groups to determine the extent to which atypical activation exists in the full network of regions. We also interrogated the profile of amygdala activation across the entire range of stimuli in order to evaluate the claim that faces, and not other visual objects, specifically elicit atypical activation in the amygdala. We assayed the behavioral profile of face recognition abilities for upright and inverted faces outside the scanner. The face inversion effect (i.e., more accurate recognition for upright compared to inverted faces, Yin, 1969) is a hallmark of typical face perception and the magnitude of the face inversion effect has been used as a measure of individual difference in face processing studies previously (Russell et al., 2009). Finally, we correlated the magnitude of face-related activation throughout the brain, and separately within our a priori regions of interest, with autism symptom severity, levels of adaptive social functioning, and face recognition behavior. Because of our sensitivity to the developmental course of the disorder and age as a proxy measure of that continuum, we also included age as an independent factor in all the regression analyses between neural activation and behavior/symptom severity measures.
2. Materials and methods
2.1. Participants
The participants included 20 male HFA adolescents (range 10–17 years) and 12 age-matched TD adolescents (range 11–17 years). The mean age did not differ across groups, F(1,30) = 0.07, p = ns. The mean IQ was in the average range for both groups (see Table 1 for demographic and IQ information, as determined using the Wechsler Abbreviated Scale of Intelligence). The TD group had higher Verbal IQ scores, F(1,30) = 5.3, p < .025, which contributed to slightly higher Full Scale IQs, F(1,30) = 3.6, p = .07.
Table 1.
Demographic characteristics of participants.
| Autism |
Typical |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sbj | Age | Hand | FSIQ | VIQ | PIQ | ADOS | Sbj | Age | Hand | FSIQ | VIQ | PIQ |
| 1 | 13 | R | 106 | 115 | 96 | 14 | 1 | 14 | R | 97 | 98 | 96 |
| 2 | 13 | R | 99 | 98 | 99 | 17 | ||||||
| 3 | 17 | L | 108 | 105 | 109 | 17 | 2 | 17 | L | 137 | 138 | 127 |
| 4 | 16 | L | 111 | 101 | 120 | 13 | ||||||
| 5 | 12 | R | 123 | 123 | 116 | 16 | 3 | 11 | R | 124 | 129 | 112 |
| 6 | 11 | R | 113 | 102 | 124 | 15 | ||||||
| 7 | 14 | L | 125 | 110 | 134 | 18 | 4 | 11 | L | 131 | 119 | 123 |
| 8 | 12 | L | 127 | 108 | 142 | 12 | ||||||
| 9 | 14 | R | 97 | 86 | 108 | 19 | 5 | 14 | R | 108 | 100 | 114 |
| 10 | 14 | R | 100 | 102 | 98 | 12 | ||||||
| 11 | 17 | R | 100 | 97 | 103 | 16 | 6 | 17 | R | 106 | 103 | 107 |
| 12 | 17 | R | 100 | 95 | 104 | 10 | ||||||
| 13 | 12 | R | 98 | 98 | 98 | 11 | 7 | 11 | R | 98 | 98 | 96 |
| 14 | 13 | R | 92 | 85 | 102 | 13 | ||||||
| 15 | 17 | R | 116 | 109 | 119 | 12 | 8 | 17 | R | 105 | 108 | 101 |
| 16 | 17 | R | 105 | 98 | 111 | 15 | ||||||
| 17 | 13 | R | 123 | 119 | 119 | 11 | 9 | 14 | R | 119 | 119 | 115 |
| 18 | 16 | R | 97 | 86 | 109 | 19 | 10 | 15 | R | 112 | 108 | 114 |
| 19 | 10 | R | 129 | 120 | 132 | 15 | 11 | 11 | R | 134 | 128 | 133 |
| 20 | 13 | R | 100 | 105 | 103 | 13 | 12 | 14 | R | 104 | 145 | 125 |
| Means: | 14.1 | 108.5 | 103.2 | 112.3 | 14.4 | 13.8 | 117.6 | 116.1* | 113.6 | |||
Note: Pairs of participants with autism are yoked to each other as well as to a typically developing adolescent on handedness, age, sex, and FSIQ (as much as possible). The shading in the table identifies these yoked participants. The groups differed in VIQ (p < .05).
The diagnosis of autism was established using the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994), the Autism Diagnostic Observation Schedule-G (ADOS) (Lord et al., 2001), and expert clinical diagnosis (Minshew, 1996). The HFA adolescents were medically healthy; had no identifiable genetic, metabolic, or infectious etiology for their disorder; and, were free of birth or traumatic brain injury, seizures, attention deficit disorder, and depression. HFA participants were not asked to withhold medication prior to testing.
TD participants were included if they were medically healthy, free of regular medication usage, and had good peer relationships as determined by parent, self-report, and staff observations during the screening procedures. TD participants were excluded if they or their first-degree relatives had a history of autism, neurological or psychiatric illness, acquired brain injury, learning disabilities, developmental delay, school problems, substance abuse, or medical disorders with central nervous system implications. A single episode of depression in a parent during a stressful episode was not considered grounds for exclusion providing no other family members reported depressive episodes.
Both HFA and TD adolescents were recruited to be part of a longitudinal study investigating the effects of visuoperceptual training. In this ongoing study, pairs of participants with autism are yoked to a single TD participant with each triad of participants systematically matched on age, sex, and FSIQ. This explains the relatively smaller sample size of the TD compared to HFA adolescents described in this project. The data reported here are from the pre-training assessment. Written informed consent was obtained from participants' guardians, and written assent from the participants themselves, using procedures approved by the Internal Review Boards of the University of Pittsburgh and Carnegie Mellon University.
2.2. Measures
2.2.1. Social skills surveys
Parents completed two scales of social functioning about their adolescent, the Social Responsiveness Scale (SRS: Constantino et al., 2003) and the Vineland Adaptive Behavioral Scales, Second Edition (VABS-II: Sparrow et al., 2005). The SRS is a questionnaire that measures the severity of autism spectrum symptoms as they occur in natural social settings; higher scores reflect more severe symptoms. The VBAS-II is a standardized caregiver interview that measures communication, social, daily living and motor skills; higher skills reflect more adaptive functioning. The VBAS-II social score was not collected for one HFA participant.
2.2.2. Cambridge face memory task (CFMT)
The CFMT (Duchaine and Nakayama, 2006) was used to measure face recognition behavior outside the scanner. This task has been used previously with TD children and with adolescents with autism (O'Hearn et al., 2010). Participants performed separate blocks for upright and inverted faces. As in our previous work (Scherf et al., 2008), participants always performed the upright version first to maximize the possibility that participants with autism would initially approach the task in an ecologically valid way prior to having to confront the less naturally occurring inverted faces. One HFA participant did not complete the inverted block of CFMT.
2.2.3. MRI acquisition
All participants were placed in a mock MR scanner for approximately 20 min and practiced versions of the tasks that were administered in the full scan. This procedure acclimates participants to the scanner environment and minimizes motion artifact and anxiety. High-resolution structural images and functional images were then acquired in a single session.
Participants were scanned using a Siemens 3 T Verio MRI scanner, equipped with a 32-channel adult head coil, at Carnegie Mellon. Anatomical images were acquired using a 3D-MPRAGE pulse sequence with 176 T1-weighted AC-PC aligned sagittal slices (TR/TE/TI = 1700, 2.48, 900 ms; voxel size = 1 mm3, FOV = 256 × 256, iPAT = 2). Functional EPI images were acquired in 36 AC–PC aligned slices, covering most of the brain and all the occipital and temporal lobes (TR/TE = 2000, 25 ms, FOV = 192, matrix 64 × 64, flip angle = 79°, voxel size = 3 mm3, iPAT = 2).
2.2.3.1. fMRI localizer task
This task was designed to elicit activation in response to several visual categories and to actively engage recognition behavior. Functional images were acquired across two runs of a 1-back localizer task, which included blocks of neutral faces, fearful faces, common objects, vehicles, houses, novel objects (i.e., Greebles: Gauthier and Tarr, 1997), and scrambled images (Fig. 1). Faces were selected from the NimStim (Tottenham et al., 2009) and Karolinska (Lundqvist et al., 1998) databases. Images of houses and vehicles were downloaded from the Internet. Common objects were selected from the Face-Place database (http://www.tarrlab.org). Scrambled images were created in Adobe Photoshop by scrambling pixels in the images of the common objects.
Fig. 1.
Examples of gray-scale version of stimuli from each visual category represented in the fMRI localizer task.
Each run lasted a total of 9 min and 12 s and began with a 20-s block of fixation and a 12-s block of patterns. Thereafter, blocks of stimuli were presented in a randomized order followed by intervening blocks of fixation (6 s). Within a block, 12 stimuli were each presented for 800 ms, followed by a 200 ms fixation. The order of the images was randomized within each block for each participant. Participants were required to indicate, by button press, when they detected a repeated image. There were two repeats in each of the stimulus blocks, the position of which was counterbalanced across blocks. In each run, there were four blocks of each stimulus category such that in the final analysis when the two runs were combined, each participant observed 8 blocks of each stimulus category.
2.3. Data analyses
2.3.1. fMRI data
The neuroimaging data were analyzed using Brain Voyager QX v2.3 (Brain Innovation, Masstricht, The Netherlands). Preprocessing of functional images included 3D-motion correction, slice scan time correction, filtering low frequencies, and re-sampling the voxels to 1 mm3. Runs in which participants exhibited spikes in motion of more than 2.9 mm in any of the six motion directions on any image were excluded from the analyses. A single run was excluded for each of two HFA and one TD participant. The average motion (between each time point) in each group on both runs of the task was less than 1 mm in all six dimensions and did not differ between groups (p > .10).
For each participant, the time series images for each brain volume were analyzed for category differences in a fixed-factor GLM. Each category was defined as a separate predictor and modeled with a box-car function adjusted for the delay in hemodynamic response. Following the recommendations of Weiner and Grill-Spector (2012), the functional data were not spatially smoothed. The time series images were then spatially normalized into Talairach space, which is common practice in autism neuroimaging research, particularly in the study of adolescents and adults when brain volumes are comparable to those of TD adolescents and adults (Redcay and Courchesne, 2005). Although participants viewed multiple visual categories in the Localizer task, here we focus on differences in the topography of face-, common-object, and house-related activation with respect to activation elicited by scrambled images.
2.3.1.1. Region of interest analyses
Functional ROIs were defined for each individual subject for the region of interest analyses. For each participant, the time series images were submitted to a fixed-effects GLM in which category was a fixed factor. As in our previous work, we defined the measures of category-selectivity with respect to all other categories (Scherf et al., 2007, 2010, 2012). Note that these definitions are extremely conservative in that they identify many fewer voxels as compared to a contrast that would define each visual category against a fixation (or scrambled image) baseline. Critically, these contrasts identify non-overlapping sets of voxels in all participants, indicating that they identify the most selective of voxels for each visual category. For example, face selectivity was defined with the following balanced contrast: {[3 * (neutral faces) + 3 * (fearful faces)] − [2 * (common objects) + 2 * (houses) + 2 * (scrambled images)]}.1 Similarly, object selectivity was defined as {[4 * (common objects)] − [(houses) + (neutral faces) + (fearful faces) + (scrambled images)]}; and house selectivity as {[4 * (houses)] − [(common objects) + (neutral faces) + (fearful faces) + (scrambled images)]}. The resulting individual maps were corrected for false positive activation using the False Discovery Rate procedure (Genovese et al., 2002) with a q < .01, which is appropriate for identifying individual-level regions of interest (ROI).
The right and left FFA were defined as the most anterior cluster of contiguous significant voxels in the fusiform gyrus generated from each participant's face-activation map. Unfortunately, the amygdalae were not definable as functional ROIs consistently across the individual participant face-activation maps. As a result, given our a priori hypotheses about group differences in activation in the amygdala, we defined right and left hemisphere amygdala ROIs by creating a 6 mm sphere around functionally defined Talairach coordinates from previous work (Blasi et al., 2009). The left amygdala ROI was centered at (−19, −5, −17) and the right centered at (22, −1, −17).
Within each of these ROIs, we conducted an ROI-based GLM on the time series data for each individual participant to generate the resulting beta weights for each visual category. The beta weights were submitted to repeated-measures ANOVAs with the factors of visual category (5) and group (2) separately for the right and left ROIs. Estimates of face-selectivity were also determined for each ROI by computing a balanced difference score in the beta weights (e.g., faces − objects). In addition, the FFA ROIs were quantified in terms of the size (number of significantly active voxels).
2.3.1.2. Whole-brain group comparison
Category selectivity was determined separately for each group (HFA, TD) by submitting the time-series images from each participant within the group to a random-effects GLM with category as a fixed factor and participant as a random factor. The contrasts used to define face-, object-, and house-related activation at the group level were the same as those for the individual level ROIs (e.g., faces vs houses, objects, scrambled). However, given the addition of between-subjects variance in these maps, we used a Monte Carlo simulation to correct the group maps for multiple comparisons (p < .05) separately for the TD (16 contiguous voxels at a t-value ≥ 2.7) and HFA (12 contiguous voxels at a t-value ≥ 2.5) participants, given the different number of participants in the two groups.
To compare group differences in category-selectivity, the full set of time series data from all participants was submitted to a mixed-model ANOVA including Group and Category as fixed factors and Subject as a random factor.2 We specifically evaluated Group × Category interactions in each voxel in a whole brain analysis based on the contrasts of interest. For example, to compare group differences in face-selective activation, we coded the following interaction: TD (faces > other) > HFA (faces > other). To correct the resulting interaction maps for false positive activations, we used a Monte Carlo simulation (p < .05 required a minimum of 33 contiguous voxels at a t-value ≥ 2.0).
2.3.1.3. Correlation analyses
To examine associations between patterns of brain activation and participant characteristics, we evaluated correlations between CFMT accuracy, raw SRS scores, and VBAS-Social scores with the individually defined ROI metrics (e.g., magnitude of activation, size of ROI) as well as in whole brain analyses. The various ROI metrics were submitted to separate step-wise regressions with age as the first factor and the relevant measure of interest (e.g., raw SRS score) as the second factor. This enabled us to determine whether age and the relevant measure of interest independently accounted for variation in each of these ROI metrics.
Whole brain ANCOVAs were computed in the HFA individuals to identify voxels in which there was significant co-variance between category-selective activation and age, raw SRS scores, VBAS-Social scores, and CFMT accuracy. These analyses generated separate whole-brain correlational maps that were thresholded at a corrected r-value of p < .01 using a Monte Carlo simulation to determine the number of contiguous voxels (8 with r > .56). ROIs that survived this threshold were defined. To illustrate the nature of the relation between the scores and activation in each of these ROIs, we generated beta weights for all visual categories (faces, objects, houses, scrambled images) by computing a separate GLM within each ROI for each participant. Using these beta weights, a difference score was computed that reflected the original balanced category-selective contrast (e.g., faces > other), which was then plotted against the specific measure of interest. As described for the ROI-based correlations, we submitted these difference scores to a step-wise regression with age as the first factor and the relevant measure of interest as the second factor in order to determine the independent contributions of these factors to variation in the profile of face selectivity in each region.
3. Results
3.1. Social skills surveys
The SRS and VABS-II Social scores for the two groups are plotted in Fig. 2. For both measures, there was unequal variance across the groups (p < .005). HFA adolescents had significantly higher SRS scores, t(26.8) = 13.4, p < .001, indicating more severe autism-like symptoms, as well as significantly lower VABS-II Social scores, t(20.6) = 6.2, p < .001, reflecting lower adaptive functioning than the TD participants. Separate regressions of age on the SRS and VABS-II scores failed to reveal age-related changes in these measures in either group.
Fig. 2.

Distribution of raw (a) social responsiveness scores (SRS) and (b) Vineland Adaptive Behavioral Scale — II social (VABS) scores for the high-functioning adolescents with autism (HFA) and typically developing (TD) adolescents separately. Higher scores on the SRS indicate more severe autism-like symptoms whereas higher scores on the VABS indicate higher levels of adaptive functioning. On both measures, the groups were significantly different from each other (p < .001).
3.2. Cambridge face memory task
The HFA adolescents were less accurate and failed to show an inversion effect in the CFMT (Fig. 3a). A repeated-measures ANOVA including the within-subject factor of orientation and the between-subject factor of group, revealed a main effect of group, F(1,29) = 6.1, p < .025, indicating that the HFA adolescents (M = 41.6%) performed worse than the TD adolescents (M = 48.0%) across both the upright and inverted versions of the task. The low performance in both groups is still above the chance rate of 33% and is comparable to the performance reported of similarly aged TD and ASD participants on this same task (O'Hearn et al., 2010).3
Fig. 3.
Behavioral data outside (a) and inside (b) the scanner plotted as a function of group. HFA adolescents were less accurate than TD adolescents in the Cambridge face memory task (CFMT) and failed to show an inversion effect (i.e., upright > inverted). During the 1-back working memory task in the scanner (b), HFA and TD participants performed similarly and were both less accurate when recognizing neutral faces compared to fearful faces, objects, or houses, but not scrambled objects.
There was also a main effect of orientation, F(1,29) = 4.7, p < .05 (upright M = 46.9%; inverted M = 42.5%), but this was qualified by an orientation × group interaction, F(1,29) = 6.2, p < .025. Paired-samples t-tests conducted separately for each group revealed an orientation effect (i.e., upright > inverted) in the TD group, t(11) = 2.5, p < .05, but not in the HFA group, t(18) = 0.3, p = ns. Separate regressions of age on the upright CFMT scores failed to reveal age-related changes in this measure in either group.
3.2.1. fMRI localizer task
3.2.1.1. Behavioral data
As evident from Fig. 3b, there were no group differences in accuracy or reaction time (RT) when participants performed the 1-back memory task in the scanner. A repeated-measures ANOVA with visual category as the within-subjects factor and group as the between-subjects factor revealed neither a main effect of group, F(1,30) = 0.6, p = ns, nor a group × category interaction, F(1,30) = .985, p = ns. There was, however, a main effect of visual category, F(1,30) = 8.1, p < .005, with reduced accuracy for neutral faces compared to fearful faces, common objects, and houses (all Bonferroni corrected p < .01), but not scrambled images (p = ns). There were no significant effects in the analysis of the RT data. Therefore, group differences in the BOLD response to these different categories of visual objects cannot be attributed to performance differences in the 1-back memory task during scanning.
3.2.1.2. fMRI data
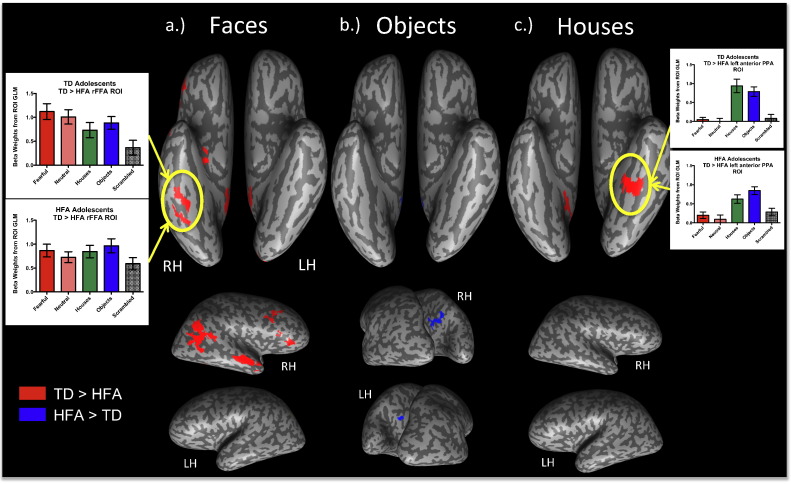
Fig. 4a–b shows the category-selective activation for each group for faces (red), places (green) and common objects (blue).
Fig. 4.
Topographic organization of ventral visual pathway in typically developing adolescents (a) and those with high functioning autism (b). Group maps were projected onto a representative inflated brain and thresholded at a corrected at p < .025. The graphs represent the mean beta weights (extracted separately for each individual and averaged within groups) for each visual category in each group level right FFA ROI.
3.3. Group maps
3.3.1. Face-related activation
TD adolescents exhibited extensive activation in both core (i.e., right FFA, bilateral occipital face area (OFA), right STS) and extended (i.e., bilateral amygdala, PCC, and vmPFC) regions (Table 2). Although HFA adolescents exhibited some activation in a subset of the core face-processing regions (i.e., bilateral FFA), they did not exhibit face-related activation in the OFA or STS core regions, or in the anterior temporal lobe, PCC, or vmPFC (Table 2). Statistical comparison of the HFA and TD face-related group maps revealed significant hypo-activation in multiple core regions in the HFA adolescents, including the bilateral OFA, right STS, and right (but not left) FFA, as well as in extended regions, including the right ATL, PCC and vmPFC (Fig. 5a). In addition, there were several other regions that were hypoactive in the HFA adolescents during face processing, including parietal, medial temporal, as well as prefrontal regions (Table 3).
Table 2.
Regions of face-, object, and house-related activation identified in TD and HFA adolescent group maps.
| TD |
HFA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | ROI | Size | BA | X | Y | Z | Size | BA | X | Y | Z |
| Faces | |||||||||||
| Core regions | rFFA | 1520 | 37 | 38 | −45 | −21 | 613 | 37 | 38 | −40 | −20 |
| lFFA | 711 | 37 | −41 | −45 | −20 | ||||||
| rOFA | 186 | 19 | 47 | −68 | 8 | ||||||
| lOFA | |||||||||||
| rSTS | 3340 | 21 | 51 | −44 | 10 | ||||||
| lSTS | |||||||||||
| Extended regions | rATL | ||||||||||
| lATL | |||||||||||
| rAmyg | 1276 | 28 | 18 | −7 | −11 | 438 | 28 | 20 | −4 | −41 | |
| lAmyg | |||||||||||
| vmPFC | 734 | 32/10 | −2 | 44 | −9 | ||||||
| PCC | 2154 | 29/30 | 3 | −47 | 20 | ||||||
| Houses | rPPA | 1474 | 37 | 22 | −40 | −11 | 2188 | 37 | 23 | −42 | −11 |
| lPPA | 1366 | 37 | −25 | −43 | −12 | 1574 | 37 | −27 | −45 | −9 | |
| Objects | rLO | 3390 | 19 | 44 | −62 | −13 | 837 | 19 | 43 | −64 | −14 |
| lLO | 3263 | 19 | −47 | −64 | −13 | 4740 | 19 | −46 | −65 | −12 | |
Note: these regions were generated from the corrected group level activation maps for each group separately. The face-related activation was corrected at p < .05, so as to provide maximal opportunity to observe such activation among the HFA adolescents, while the house- and object-related activation was corrected at p < .001.
Fig. 5.
Group differences in category-selective activation for faces (a), common objects (b), and houses (c). Regions in which the HFA adolescents exhibited LESS activation than the TD controls are represented in red, and regions in which they exhibited MORE activation are represented in blue in each map. The maps are all corrected at p < .05. Note the pronounced differences in face-related activation in both core and extended regions, in which HFA adolescents exhibited less activation (a). In contrast, the HFA adolescents exhibited MORE object-related activation (b) in the precuneus of both hemispheres than the TD adolescents. TD adolescents also exhibited stronger house-related activation than HFA adolescents in the left parahippocampal gyrus, which is evident in the magnitude of the beta weights for each group.
Table 3.
Regions in which TD adolescents exhibited greater face-related activation than did HFA adolescents.
| Right hemisphere |
Left hemisphere |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Size | BA | X | Y | Z | Size | BA | X | Y | Z |
| Core regions | ||||||||||
| FFA | 1453 | 36 | 36 | −45 | −19 | |||||
| OFA | 262 | 37 | 59 | −52 | −1 | |||||
| pSTS | 3288 | 22, 21 | 49 | −52 | 11 | |||||
| Extended regions | ||||||||||
| rATL | 1341 | 38 | 51 | 9 | −21 | |||||
| raSTS | 1529 | 21 | 50 | −9 | −12 | |||||
| PCC | 3995 | 30, 23 | 4 | −56 | 23 | |||||
| Other regions | ||||||||||
| Cuneus | 1025 | 17 | −12 | −94 | 3 | |||||
| PCu | 786 | 31 | 7 | −46 | 36 | |||||
| Angular gyrus | 926 | 39 | 46 | −60 | 24 | |||||
| dmPFC | 1815 | 10 | 3 | 56 | 21 | |||||
| vlPFC | 1984 | 47 | 52 | 24 | 4 | |||||
Note: these regions were generated from the voxelwise analysis using the mixed-factors ANOVA in which the following Group × Category interaction was evaluated: TD (faces > other) > HFA (faces > other). The maps were corrected for false positive activations at p < .05.
3.3.2. Object-related activation
Both the HFA and TD groups exhibited extensive and comparable activation of the ventral visual processing stream bilaterally during common object processing (see Figs. 4b and 5b) except that the HFA adolescents exhibited stronger object-related activation bilaterally in the precuneus than the TD adolescents (Fig. 5b). Additional comparisons using a more lenient contrast for determining object-related activation (objects versus scrambled images) revealed no group differences in object-related activation.
3.3.3. House-related activation
Fig. 4 reflects that both groups exhibited strong activation bilaterally in the PPA during house blocks. However, the HFA adolescents exhibited weaker activation in the left PPA than TD adolescents during house blocks (Fig. 5c).
3.4. ROI analyses
3.4.1. FFA
The right FFA was of comparable size in the two groups. A one-tailed independent-samples t-test failed to reveal a significant difference between groups in the number of voxels within the individually defined right FFA ROI, t(30) = 0.7, p = ns. However, the groups tended to differ with respect to the magnitude of face selectivity of the activation in these individually defined rFFA ROIs, t(30) = 1.5, p = .07, one-tailed. This finding replicates our previous findings in a new sample of HFA and TD adolescents (Scherf et al., 2010).
3.4.2. Amygdala
The magnitude of activation (i.e., beta weights from ROI-based GLMs) for each visual category for each group is illustrated separately for the right and left amygdala ROIs in Fig. 6. In the right amygdala, there was no main effect of group, F(1, 30) = 0.0, p = ns; however, there was a main effect of visual category, F(4, 120) = 11.7, p < .000, as well as a group × category interaction, F(4, 120) = 4.1, p < .005. Bonferroni corrected post-hoc tests revealed that, for both groups, fearful faces elicited greater activation than houses and scrambled images (p < .001) and tended to elicit more activation than neutral faces (p = .051). Additionally, neutral faces and objects also elicited more activation in the right amygdala than scrambled images (p < .05). Separate repeated-measures ANOVAs within each group revealed that there were main effects of visual category in both the TD, F(4, 44) = 13.4, p < .001, and the HFA, F(4, 76) = 3.9, p < .01, adolescents. However, the Bonferroni corrected post-hoc tests revealed that the groups differed with respect to the categories that elicited the strongest activation in the right amygdala. Specifically, in the TD adolescents, fearful and neutral faces as well as objects elicited stronger activation than scrambled images (p < .05); however, these categories were not different from one another. In contrast, in the HFA adolescents, the only significant difference was between fearful faces and houses (p < .05); none of the visual categories was different from the scrambled images as was evident in the TD adolescents. Interestingly, the negative response to scrambled images among the TD adolescents in the right amygdala was 12 times larger (M = −.201) than the modestly positive activation to scrambled images (M = .016) in the HFA adolescents. Independent samples t-tests comparing activation on each visual category between groups confirmed that the scrambled images condition was the only one for which the HFA and TD adolescents differed, t(30) = 2.0, p < .05. This pattern of differences in response to the “baseline” condition is highly relevant for considering discrepancies in the current literature about the hyper- or hypo-active signal in the amygdala in autism.
Fig. 6.
Activation to each visual category in the amygdala by group. Adolescents with autism did not exhibit hyper- or hypo-activation to faces, houses, or objects compared to the typically developing adolescents in either the right or left amygdala. However, HFA adolescents did not show the same magnitude of negative activation in the right amygdala in response to the scrambled images that was present in the TD adolescents (p < .05). In both groups, fearful faces elicited more activation in the right amygdala than houses (p < .000) but not more than objects (p = ns). In the left amygdala, both groups exhibited stronger activation to the fearful faces than to the scrambled images (p < .000), but there were no other significant differences between visual categories.
In the left amygdala, there was no main effect of group, F(1, 30) = 1.7, p = ns, and no interaction between group and visual category, F(4, 120) = 1.9, p = ns. However, there was a main effect of visual category, F(4, 120) = 4.5, p < .005. Across both groups, fearful faces elicited stronger activation than scrambled images (p < .001), and tended to elicit stronger activation than neutral faces (p = .081), but no other categories were different from one another. Independent samples t-tests failed to reveal group differences in the magnitude of activation to any of the visual categories in the left amygdala.
3.5. Brain–age correlations
There were no regions in either the HFA or TD adolescents in which there was a significant correlation between age and face-, object-, or house-related activation.
3.5.1. Brain–behavior correlations
3.5.1.1. Social functioning measures
The whole-brain correlation analyses between SRS score and face-related activation in the HFA adolescents revealed that the right FFA was negatively correlated with SRS scores (Fig. 7a): participants with higher SRS scores had consistently lower magnitude face-related activation in the right FFA. The stepwise regression including the predictors of age and raw SRS score on the beta weight difference scores generated for each participant in this ROI was significant, F(2, 17) = 9.4, p < .005, r2 = .53; however, only raw SRS score was a significant independent predictor of face-related activation within this ROI (p < .001), age was not significant. The locus of the right FFA identified in this analysis (31, −42, −14) overlapped with the same right FFA region that was identified in the group level contrasts of face-related activation (37, −47, −20) during face processing. Similarly, the magnitude of the face-related activation in the individually defined right FFA was significantly negatively related to the raw SRS score among the HFA participants (Fig. 7b). The stepwise regression including the predictors of age and raw SRS score on the beta weight difference scores generated for each participant in their individually defined right FFA was significant, F(2, 15) = 6.0, p < .025, r2 = .44; however, only raw SRS score was a significant independent predictor of face-related activation within this ROI (p < .005); age was not significant (see Fig. 7b). Participants with higher SRS scores had lower face-selective activation in their individually defined right FFA ROI. However, the size of these individually defined ROIs was not related to SRS scores, F(1, 18) = 2.2, p = ns, r2 = .11, nor was the age of the participants (p = ns).
Fig. 7.
Correlations between symptom severity as measured on the Social Responsiveness Scale (SRS) and magnitude of face-related activation in the HFA adolescents using a whole-brain voxelwise analysis (a) and individually defined right FFA (b). The whole-brain analysis was thresholded at a corrected p < .01. The only region to survive this threshold was the right FFA, in which higher SRS scores (i.e., more symptoms) were negatively related to the magnitude of face-related activation (more object-like activation in the anterior portion of the fusiform gyrus). For illustration purposes, the relation between the magnitude of activation and raw SRS scores is plotted for each HFA adolescent within this right FFA region from the whole-brain correlation. The stepwise regression with age and raw SRS score revealed that only SRS score was related to the magnitude of selectivity in the right fusiform gyrus (p < .001). In (b), the magnitude of face-related activation within each individually defined right FFA among the HFA adolescents (represented in a separate color for each HFA participant on the single inflated brain) was significantly related to raw SRS scores (p < .005), even after controlling for age (p = ns). In other words, the more severe the autism symptoms, the lower magnitude face-related activation was present in the right FFA of these adolescents.
Fig. 8.
Comparison of activation in the right FFA across analyses. This image shows the extent of overlap in the right FFA regions that were identified in the TD group map of face activation (red), the HFA group map of face activation (green), the TD > HFA face activation analysis (magenta), and in the whole-brain correlation with the SRS (blue) among the HFA adolescents. There is extensive overlap in the FFA regions of interest identified in each of these analyses, suggesting that the right FFA may be a particularly vulnerable region in individuals developing with autism.
In contrast, the level of adaptive function in the HFA group was not significantly related to the level of face-related activation anywhere in the brain. There were no regions in the TD adolescents in which either SRS or Vineland scores correlated with face-related activation.
3.5.1.2. CFMT
The whole-brain correlational analyses between CFMT performance and face-related activation among the HFA adolescents revealed that activation in the right ATL (31, −3, −23) was positively correlated with performance. Importantly, the stepwise regression analyses of the beta weights extracted from the individual participant GLMs in this ROI with the predictors of age and upright face recognition accuracy was significant, F(2,17) = 13.4, p < .001, r2 = .61. However, only upright CFMT performance was an independent predictor of face-related activation in this ROI (p < .001), age was not (p = ns). The stepwise regression with age and inverted face accuracy was not significant, F(2,16) = 1.0, p = ns, r2 = .12 (Fig. 9). There were no regions in which face-related activation was related to performance on the CFMT in the TD adolescents.
Fig. 9.
Correlation between behavioral performance on the upright CFMT and face-related activation in the HFA adolescents. The correlation map was thresholded at r = .056 with a cluster correction of 8 voxels, which corresponds to a corrected p < .025. The only region to survive this threshold was the right anterior temporal lobe, in which higher CFMT scores (i.e., better performance) were positively related to the magnitude of face-related activation. In the graph, the relation between the magnitude of activation and upright CFMT scores is plotted for each HFA adolescent within this right anterior temporal lobe region from the whole-brain correlation. The performance on the inverted version of the CFMT is also plotted against the beta weights from this ATL region, which shows no relation between signal in the ATL and performance on the inverted version of the task.
4. Discussion
The central goals of this investigation were to evaluate face-related activation in adolescents with HFA in both core and extended regions of the broader face-processing network, with particular focus on the fusiform gyrus and the amygdala, and to explore a potential relation between the magnitude of this face-related activation and autism symptom severity, levels of adaptive social functioning, and variations in behavioral face recognition performance.
4.1. Face recognition behavior is impaired in adolescents with autism
Using a classic task of unfamiliar face recognition, we replicated and extended previous findings that adolescents with autism are impaired in upright face recognition abilities compared to age- and IQ-matched TD adolescents. In addition, to our knowledge, we are the first to use the CFMT to evaluate the magnitude of the face inversion effect (FIE: Yin, 1969) in adolescents with autism. The FIE is often taken as a marker of typical face perception; however, findings of the presence and magnitude of an FIE in autism are mixed. A recent review suggests that people with ASD do not demonstrate qualitative differences in the FIE (Weigelt et al., 2012). Here, we report that adolescents with autism do not exhibit an FIE when tested with the CFMT, which is in contrast to our own previous findings (Scherf et al., 2008). We suggest that these findings can be explained by the relative difficulty of the CFMT. This is a much harder task than has been used to test the FIE in the vast majority of previous studies. There is empirical evidence of a developmental progression in performance on the upright version of the task that continues into early adulthood in TD individuals, but this progression plateaus in HFA individuals in adolescence (O'Hearn et al., 2010). The TD adolescents outperformed the HFA adolescents in the upright condition of this task, but the groups were indistinguishable in their performance on the inverted condition. Therefore, we suggest that the FIE may only be observable in autism under conditions when upright face recognition is optimized.
4.1.1. Pervasive, though not ubiquitous, hypo-activation in the face processing network
Using a paradigm that was designed to elicit activation in both core (i.e., visuoperceptual and cognitive) and extended (i.e., motivational and affective) regions of the face processing system, we determined that HFA adolescents exhibit hypo-activation in the majority, but not all, regions compared to TD controls. Specifically, although HFA adolescents, as a group, exhibited face-related activation in the pre-eminent FFA in both hemispheres; activation in the right, but not the left, FFA was significantly hypo-active compared to the TD adolescents (Fig. 5a). Also, face-related activation in the right and left OFA and in the right posterior STS were hypoactive in the HFA group as well. Importantly, this hypo-activation was only evident during face processing. HFA adolescents exhibited comparable activation to TDs bilaterally in the LOC and hyper-activation in the precuneus during object-recognition, and comparable activation in the PPA during house-recognition. These findings were evident even though the sample size of HFA adolescents was nearly twice the size of the TD adolescents. It is important to note that the smaller TD sample size compared to the HFA group size is not ideal, but does not likely challenge the pattern of results reported here given that the group comparison is at most risk for Type II error (false negative). In spite of the fact that we have a smaller number of control participants, we still had enough power to observe strong group differences in favor of the controls. In other words, there is more power and consistency in the face-related activation of 12 TD controls than among 20 HFA adolescents. This is due, in part, to the powerful signal-to-noise ratio that is generated from the blocked fMRI design and the fact that we collected two independent runs of the experiment from each participant to boost signal even more.
These findings largely replicate our own and other previous findings in adolescents with autism (Dalton et al., 2005; Grelotti et al., 2005; Pierce and Redcay, 2008; Scherf et al., 2010) with one exception. Here, we find that HFA adolescents exhibited strong, consistent face-related activation in the left FFA that was not present among the TD adolescents (Table 2). Our finding that adolescents with HFA recruit the left FFA during face recognition task is particularly useful for understanding that some parts of the face-processing network are preserved and even highly functional in autism. One possible explanation for the left FFA activation in the HFA adolescents relates to findings of hemispheric asymmetries in the kinds of information encoded by the fusiform gyri. There is growing consensus that the right fusiform is more specialized for holistic processing, while the left fusiform is more implicated for part-based processing (Meng et al., 2012; Rossion et al., 2000). Thus, the reliance on the left FFA during face processing in the HFA adolescents may reflect the use of a more part-based representation to process face identity. This interpretation is consistent with findings that individuals with autism have biased visuoperceptual systems that emphasize feature-based processing of local details in visual scenes (Behrmann et al., 2006).
With respect to extended regions, as a group, the HFA adolescents only activated the left amygdala. They did not exhibit activation in the right amygdala, PCC, anterior STS, right or left ATL, or vmPFC. In contrast, TD adolescents exhibited activation in the right amygdala, PCC, and vmPFC. Note that the TD adolescents did not show group level activation in the ATL in either hemisphere, suggesting that these regions may continue to develop through adolescence. However, when pitted against each other directly, the HFA adolescents exhibited hypo-activation in the right ATL, the right anterior STS, and the PCC, as well as in several other regions compared to the TD adolescents (Table 3). There were no regions in which the HFA adolescents exhibited greater activation than the TD adolescents during face processing.
Importantly, there were no group differences in the profile of activation of the left amygdala for any of the stimulus categories. Both groups exhibited the strongest magnitude response to fearful faces and a negative response to scrambled images. In contrast, in the right amygdala, there were differences between the groups in the profile of activation, but these differences were not specific to faces. In the right hemisphere, the only reliably different response in amygdala activation was to scrambled images. The TD adolescents exhibited a strong negative response to scrambled images, whereas there was no such negative response in the HFA adolescents. There were no other group differences in response to either fearful or neutral faces, houses, or common objects. These findings show how a contrast between fearful or neutral faces and scrambled images would lead to a conclusion that HFA adolescents exhibited hypo-activation in the right amygdala, as has been reported in previous studies that used scrambled images as a contrast to face stimuli (Ashwin et al., 2007; Hadjikhani et al., 2007; Kleinshans et al., 2008). However, a contrast between fearful and neutral faces or between fearful or neutral faces and objects would lead to a conclusion of comparable amygdala activation across the groups, which is consistent with findings from one previous study (Weng et al., 2011). In our data, there was no contrast that reflected hyper-activation to faces in the amygdala among HFA adolescents. This finding stands in contrast with several previous findings of relative hyper-activation in the amygdala during affective face processing in autism.
There are multiple potential explanations for the absence of hyper-activation of the amygdala during face processing. Importantly, many of our autism participants have been in several previous research studies, including those employing functional neuroimaging. As a result, most of our HFA adolescents were experienced and especially comfortable being in the fMRI scanner, which may have significantly reduced anxiety and thus amygdala activation. We suggest that this is an important consideration for other studies reporting hyper-activation in the amygdala in individuals with autism; it may reflect more generalized anxiety about the scanner environment compared to typically developing individuals.
Alternatively, one might suggest that our participants were avoiding looking at the eye region of the faces, thereby reducing amygdala activation. A recent study reported hyper-activation in amygdala responses from an autism group viewing neutral faces, particularly when they were directed to look at the eye region of the face (Swartz et al., 2013). This prediction would be consistent with the hypothesis that there is decreased motivation to attend to (i.e., look at) social stimuli, like faces (Dawson et al., 2002; Grelotti et al., 2002), which leads to hypo-activation in the fusiform gyrus (Dalton et al., 2005). Together, these findings might suggest that the adolescents in our sample were not looking at the eye region of the faces to the same extent as were the TD adolescents and that this aversion to the eye region led to the hypo-activation throughout the core and extended regions of the face processing network. We did not collect eye-tracking data, which limits our ability to investigate this possibility. However, both groups performed comparably on the 1-back recognition task for faces, and all other visual objects, in the scanner. This suggests that the adolescents with autism attended to the faces sufficiently to support near ceiling performance on the recognition task while the hypo-active BOLD signal was being acquired. Also, it should be noted that the relation between purported atypicalities in the locus of fixations during face processing and cortical activation patterns in children with autism is controversial (see Boraston and Blakemore, 2007). For example, one study of young adolescents with autism found no differences from TD controls in fixation patterns when observing facial expressions, despite finding impressive differences in the patterns of neural activation under these same conditions (Dapretto et al., 2006). Also, at least one study in adults with autism found similar patterns of face-related hypoactivation in the FG when participants were required to fixate a central dot overlaid on the center of each stimulus and under free viewing conditions (Humphreys et al., 2008).
4.2. Hypo-activation related to symptom severity and face recognition behavior
We also report novel evidence that the magnitude of hypo-activation in the right FFA among the HFA adolescents is selectively related to the severity of autism symptoms. Specifically, individuals with more severe autism symptoms (i.e., higher SRS scores) exhibited less face-related activation in the right FFA and no other region. In other words, there was a negative relation between the magnitude of SRS scores and face-activation. The illustrative plot of the beta weights from these analyses suggest that the most severely affected adolescents with autism exhibited more object- than face-related activation in the right fusiform gyrus. This finding is consistent with the notion that individuals with autism may treat faces more like common objects with respect to the visuoperceptual strategy that they employ for recognition (Mottron et al., 2006). It is also consistent with several other studies, which report that typical face-processing regions are actually object-selective in autism (Humphreys et al., 2008; Scherf et al., 2010; Schultz et al., 2000).
Importantly, there were no regions in which object- or house-related activation correlated with symptom severity or levels of social functioning. These highly selective results suggest that the right FFA is particularly vulnerable in autism and that activation in this region may be related to the success with which individuals with autism interact with the social world. Although these findings do not indicate a causal direction of the effect (i.e., impaired FFA activation leads to social deficits or vice versa), there may be a bidirectional influence between face-processing and symptom severity and/or social functioning in autism. The individual differences approach that we employed in this work to understand brain-behavior correspondences in autism may help reconcile discrepancies in the literature concerning hypo-activation in the FFA and suggest that studies failing to report such hypo-activation are likely to have a sample of individuals with less severe symptoms.
We did not find a similar relation between face-related activation in the FFA (or any other region) in the TD adolescents and either their autism-like behaviors or their levels of adaptive functioning. This null result may be related to the limited range of individual differences on these measures among the TD adolescents and the small number of participants. It is possible that TD adolescents with higher numbers of autism-like traits (as measured by the Autism Quotient; Baron-Cohen et al., 2001) might show a similar relation between face-related activation and the severity of these traits. This kind of finding would help determine whether the relation between the neural profile of activation for faces and autism symptoms/traits is specifically vulnerable in and characteristic of autism or whether it reflects a broader relation between social information processing of human faces and levels of social functioning in the population more broadly.
In spite of the association between face-activation and symptom severity in the right FFA, we did not find a relation between variation in face-recognition behavioral performance and the magnitude of face-selective activation in the fusiform gyrus among the HFA adolescents. This null result is consistent with recent findings of adults with ASD (Jiang et al., 2013). These same authors also reported that, using a novel analysis of voxelwise correlations and an fMRI-adaptation paradigm to probe the sparseness of face-related representations within the FFA, adults with autism who exhibit particularly poor face recognition skills have less sparse (and therefore less selective) neural representations for faces in the FFA (Jiang et al., 2013). In other words, the whole-brain correlational analysis using a category-selective definition of face-related activation (as determined by the faces–other visual categories contrast) may not have been sensitive enough to detect the brain–behavior relation in the FFA that has been detected in adults with autism.
However, in spite of this limitation within the FFA, we did find a brain–behavior relation in the right ATL, a region implicated in supporting face individuation (Kriegeskorte et al., 2007). Specifically, HFA adolescents who scored higher on the upright version of the CFMT outside the scanner exhibited stronger face-related activation in the right ATL during the face-recognition task in the scanner. This finding suggests that there may be substantial heterogeneity in activation patterns that might be used to predict and/or identify which individuals could benefit the most from targeted cognitive remediation (e.g., face training). Given that the ATL is also associated with linking biographic information about faces to perceptual representations (Haxby et al., 2000), HFA adolescents who showed stronger activation in this region might benefit from strategies such as linking names to faces or encoding a semantic detail about the face (e.g., looks like my teacher). The behavioral recognition data alone could not have provided this insight.
We did not observe a similar relation between face-recognition behavior and face-related activation in the right ATL (or any other region) among the TD adolescents. The small number of TD participants (n = 12) likely underpowered the whole-brain correlational analyses of individual differences in this group.4 At the same time, reports of brain–behavior correlations within the face-processing system are actually quite limited, with some reporting positive correlations between the volume of the right FFA with recognition behavior (Golarai et al., 2007, 2010) and others reporting positive correlations between the magnitude of behavioral and neural responses to face inversion within the right FFA (Alyward et al., 2005; Passarotti et al., 2007) in samples that combine adolescents and adults. These correlations could be driven by developmental changes in both face recognition behavior and neural organization within the FFA and/or by individual differences in these characteristics across the age range. Future work investigating the developmental emergence of these brain–behavior relations separate from individual differences in these relations among typically developing individuals will be critical for interpreting our findings of individual differences among HFA adolescents.
5. Conclusion
In conclusion, our findings identify the right FFA as a particularly vulnerable node in the broadly distributed face-processing network in autism, particularly during adolescence when this region is maturing among typically developing adolescents. Importantly, we show that it is not the only atypical node, indicating that the extent of impairment in the functional organization of neural regions supporting face processing in autism is much broader than previously reported. Interestingly, conclusions about the relative hyper- or hypo-activation of the amygdala depended on the nature of the contrast that was used to define the activation. We suggest that our findings reflect a systematic relation between the magnitude of neural dysfunction, severity of autism symptoms, and variation in face recognition behavior, which provides new insight about reconciling discrepancies in the existing literature. By elucidating brain–behavior relations that underlie one of the most prominent social deficits in autism, this research helps resolve discrepancies in the literature concerning hypo-activation of the social brain in autism, and points to a specific vulnerability in the development of the fusiform gyrus.
Funding
The research reported in this paper was supported by Pennsylvania Department of Health SAP grant 4100047862 (M.B., K.S.S., N.M.), NICHD/NIDCD P01/U19 HD35469-07 (M.B., PI-Nancy Minshew), and a grant from the Simons Foundation 298640 to Marlene Behrmann (PI: D. Heeger).
Acknowledgements
We thank Dr. Kwan-Jin Jung, Scott Kurdilla, and Debbie Viszlay from the Scientific Imaging & Brain Research Center at Carnegie Mellon University, as well as Justine Record and Ryan Egan, for their help in acquiring the imaging data. We are also grateful to our study families for making this research possible.
Footnotes
Because some of the existing work investigating the functional topography of the ventral visual pathway had used a faces vs objects contrast to define face-related activation (e.g., Kanwisher et al., 1997), we also conducted all the group and ROI-based analyses using this contrast as well. The pattern of results remained the same with this contrast.
Recent empirical work has shown that the hemodynamic response in autism is similar to that found in typically developing children (Feczko et al., 2012), which provides support for the approach of comparing groups maps to measure significant differences in activation between the groups.
Even TD adults only tend to perform at about 76% correct on the CFMT across the three blocks (Duchaine and Nakayama, 2006; O'Hearn et al., 2010).
Note that this sample size was not underpowered with respect to the ability to identify significant group differences in activation patterns between the TD and HFA groups in the core and extended face processing regions.
References
- Adolphs R., Sears L., Piven J. Abnormal processing of social information from faces in autism. J. Cogn. Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. 11244548 [DOI] [PubMed] [Google Scholar]
- Anderson D.K., Maye M.P., Lord C. Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. Am. J. Intellect. Dev. Disabil. 2011;116(5):381–397. doi: 10.1352/1944-7558-116.5.381. 21905806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E.H., Park J.E., Field K.M., Parsons A.C., Richards T.L., Cramer S.C., Meltzoff A.N. Brain activation during face perception: evidence of a developmental change. J. Cogn. Neurosci. 2005;17(2):308–319. doi: 10.1162/0898929053124884. 15811242 [DOI] [PubMed] [Google Scholar]
- Ashwin C., Baron-Cohen S., Wheelwright S., O’Riordan M., Bullmore E.T. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. 16806312 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. 11439754 [DOI] [PubMed] [Google Scholar]
- Behrmann M., Avidan G., Leonard G.L., Kimchi R., Luna B., Humphreys K., Minshew N. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44(1):110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. 15907952 [DOI] [PubMed] [Google Scholar]
- Bird G., Catmur C., Silani G., Frith C., Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31(4):1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. 16616862 [DOI] [PubMed] [Google Scholar]
- Blasi G., Hariri A.R., Alce G., Taurisano P., Sambataro F., Das S., Mattay V.S. Preferential amygdala reactivity to the negative assessment of neutral faces. Biological Psychiatry. 2009;66(9):847–853. doi: 10.1016/j.biopsych.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S.Y., Wang A.T., Scott A., Sigman M., Dapretto M. Frontal contributions to face processing differences in autism: evidence from fmri of inverted face processing. J. Int. Neuropsychol. Soc. 2008;14(6):922–932. doi: 10.1017/S135561770808140X. 18954473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraston Z., Blakemore S.J. The Application of eye-tracking technology in the study of autism. J. Physiol. (Lond.) 2007;581(3):893–898. doi: 10.1113/jphysiol.2007.133587. 17430985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton A.V., Tonge B.J., Einfeld S.L. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. J. Autism Dev. Disord. 2006;36(7):863–870. doi: 10.1007/s10803-006-0125-y. 16897401 [DOI] [PubMed] [Google Scholar]
- Constantino J.N., Davis S.A., Todd R.D., Schindler M.K., Gross M.M., Brophy S.L. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev. Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. 12959421 [DOI] [PubMed] [Google Scholar]
- Corbett B.A., Carmean V., Ravizza S., Wendelken C., Henry M.L., Carter C., Rivera S.M. A functional and structural study of emotion and face processing in children with autism. Psychiatry Res. 2009;173(3):196–205. doi: 10.1016/j.pscychresns.2008.08.005. 19665877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Daly E.M., Bullmore E.T., Williams S.C., Van Amelsvoort T., Robertson D.M., Rowe A., Phillips M., McAlonan G., Howlin P. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(11):2203–2212. doi: 10.1093/brain/123.11.2203. 11050021 [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., Schaefer H.S., Gernsbacher M.A., Goldsmith H.H., Alexander A.L., Davidson R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. 15750588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. 16327784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Carver L., Meltzoff A.N., Panagiotides H., McPartland J., Webb S.J. Neural correlates of face and object recognition in oung children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. 12038546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Webb S.J., McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Kumbier E., Grossmann A., Hauenstein K., Herpertz S.C. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol. Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. 23510581 [DOI] [PubMed] [Google Scholar]
- Duchaine B., Nakayama K. The Cambridge face memory test: results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. 16169565 [DOI] [PubMed] [Google Scholar]
- Evans K.C., Wright C.I., Wedig M.M., Gold A.L., Pollack M.H., Rauch S.L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety. 2008;25(6):496–505. doi: 10.1002/da.20347. 17595018 [DOI] [PubMed] [Google Scholar]
- Feczko E., Miezin F.M., Constantino J.N., Schlaggar B.L., Petersen S.E., Pruett J.R., Jr. The hemodynamic response in children with simplex autism. Dev. Cogn. Neurosci. 2012;2(4):396–408. doi: 10.1016/j.dcn.2012.06.001. 22795455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I., Tarr M.J. Becoming a “greeble” expert: exploring mechanisms for face recognition. Vision Res. 1997;37(12):1673–1682. doi: 10.1016/s0042-6989(96)00286-6. 9231232 [DOI] [PubMed] [Google Scholar]
- Gauthier I., Tarr M.J., Moylan J., Skudlarski P., Gore J.C., Anderson A.W. The fusiform “face area” is part of a network that processes faces at the individual level. J. Cogn. Neurosci. 2000;12(3):495–504. doi: 10.1162/089892900562165. 10931774 [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery Rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. 11906227 [DOI] [PubMed] [Google Scholar]
- Gillberg C., Steffenburg S. Outcome and prognostic factors in infantile autism and similar conditions: a population-based study of 46 cases followed through puberty. J. Autism Dev. Disord. 1987;17(2):273–287. doi: 10.1007/BF01495061. 3610999 [DOI] [PubMed] [Google Scholar]
- Gobbini M.I., Haxby J.V. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. 16797608 [DOI] [PubMed] [Google Scholar]
- Golarai G., Liberman A., Yoon J.M., Grill-Spector K. Differential development of the ventral visual cortex extends through adolescence. Front. Hum. Neurosci. 2010;3:80. doi: 10.3389/neuro.09.080.2009. 20204140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G., Ghahremani D.G., Whitfield-Gabrieli S., Reiss A., Eberhardt J.L., Gabrieli J.D., Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci. 2007;10(4):512–522. doi: 10.1038/nn1865. 17351637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti D.J., Klin A.J., Gauthier I., Skudlarski P., Cohen D.J., Gore J.C., Volkmar F.R., Schultz R.T. FMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. 15707614 [DOI] [PubMed] [Google Scholar]
- Grelotti D.J., Gauthier I., Schultz R.T. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev. Psychobiol. 2002;40(3):213–225. doi: 10.1002/dev.10028. 11891634 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Joseph R.M., Snyder J., Chabris C.F., Clark J., Steele S., McGrath L., Vangel M., Aharon I., Feczko E., Harris G.J., Tager-Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. 15219586 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Joseph R.M., Snyder J., Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum. Brain Mapp. 2007;28(5):441–449. doi: 10.1002/hbm.20283. 17133386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. (Regul. Ed.) 2000;4(6):223–232. doi: 10.1016/s1364-6613(00)01482-0. 10827445 [DOI] [PubMed] [Google Scholar]
- Hoffman E.A., Haxby J.V. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat. Neurosci. 2000;3(1):80–84. doi: 10.1038/71152. 10607399 [DOI] [PubMed] [Google Scholar]
- Humphreys K., Hasson U., Avidan G., Minshew N., Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Res. 2008;1(1):52–63. doi: 10.1002/aur.1. 19360650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T., Miyakoshi M., Harada T., Nakai T. White matter connectivity between superior temporal sulcus and amygdala is associated with autistic trait in healthy humans. Neurosci. Lett. 2012;510(2):154–158. doi: 10.1016/j.neulet.2012.01.029. 22285821 [DOI] [PubMed] [Google Scholar]
- Jiang X., Bollich A., Cox P., Hyder E., James J., Gowani S.A., Hadjikhani N., Blanz V., Manoach D.S., Barton J.J.S., Gaillard W.D., Riesenhuber M. A quantitative link between face discrimination deficits and neuronal selectivity for faces in autism. NeuroImage: Clinical. 2013;2:320–331. doi: 10.1016/j.nicl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne S.M., Gerber A.J., Quirmbach L.M., Sparrow S.S., Cicchetti D.V., Saulnier C.A. The role of adaptive behavior in autism spectrum disorders: implications for functional outcome. J. Autism Dev. Disord. 2011;41(8):1007–1018. doi: 10.1007/s10803-010-1126-4. 21042872 [DOI] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. 9151747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–1012. doi: 10.1093/brain/awm334. 18234695 [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Johnson L.C., Weaver K.E., Greenson J., Dawson G., Aylward E. FMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54(1):697–704. doi: 10.1016/j.neuroimage.2010.07.037. 20656041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Formisano E., Sorger B., Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc. Natl. Acad. Sci. U.S.A. 2007;104(51):20600–20605. doi: 10.1073/pnas.0705654104. 18077383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska-Pietura K., David A.S., Masiak M. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. 16319404 [DOI] [PubMed] [Google Scholar]
- Kuusikko S., Pollock-Wurman R., Jussila K., Carter A.S., Mattila M.L., Ebeling H., Moilanen I. Social anxiety in high-functioning children and adolescents with autism and asperger syndrome. J Autism Dev Disord. 2008;38(9):1697–1709. doi: 10.1007/s10803-008-0555-9. 18324461 [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. 7814313 [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P.C., Risi S. Autism Diagnostic Observation Schedule (ADOS) Western Psychological Services; Los Angeles, CA: 2001. [Google Scholar]
- Lundqvist D., Flykt A., Öhman A. The Karolinska Directed Emotional Faces. Karolinska Institute; Stockholm, Sweden: 1998. [Google Scholar]
- Malisza K.L., Clancy C., Shiloff D., Holden J., Jones C., Paulson K., Yu D.C., Summers R., Chudley A.E. Functional magnetic resonance imaging of facial information processing in children with autistic disorder, attention deficit hyperactivity disorder and typically developing controls. Int. J. Adolesc. Med. Health. 2011;23(3):269–277. doi: 10.1515/ijamh.2011.055. 22191195 [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Blair R.J. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci. Biobehav. Rev. 2008;32(3):454–465. doi: 10.1016/j.neubiorev.2007.08.003. 17915324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes S.D., Calhoun S.L., Murray M.J., Ahuja M., Smith L.A. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Res. Autism Spec. Disord. 2011;5:474–485. [Google Scholar]
- McPheeters M.L., Davis A., Navarre J.R., Scott T.A. Family report of ASD concomitant with depression or anxiety among US children. J. Autism Dev. Disord. 2011;41(5):646–653. doi: 10.1007/s10803-010-1085-9. 20697792 [DOI] [PubMed] [Google Scholar]
- Meng M., Cherian T., Singal G., Sinha P. Lateralization of face processing in the human brain. Proc. Biol. Sci. 2012;279(1735):2052–2061. doi: 10.1098/rspb.2011.1784. 22217726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew N. Autism. In: Berg B.O., editor. Principles of Child Neurology. McGraw-Hill; New York: 1996. pp. 1713–1729. [Google Scholar]
- Monk C.S., Weng S.J., Wiggins J.L., Kurapati N., Louro H.M., Carrasco M., Maslowsky J., Risi S., Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35(2):105–114. doi: 10.1503/jpn.090085. 20184808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L., Dawson M., Soulières I., Hubert B., Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. 16453071 [DOI] [PubMed] [Google Scholar]
- O’Hearn K., Schroer E., Minshew N., Luna B. Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 2010;48(13):3955–3960. doi: 10.1016/j.neuropsychologia.2010.08.024. 20813119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Smith J., DeLano M., Huang J. Developmental differences in the neural bases of the face inversion effect show progressive tuning of face-selective regions to the upright orientation. Neuroimage. 2007;34(4):1708–1722. doi: 10.1016/j.neuroimage.2006.07.045. 17188904 [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P., McCarthy G., LaBar K.S. Perception of dynamic changes in facial affect and identity in autism. Soc. Cogn. Affect. Neurosci. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. 18174910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K., Haist F., Sedaghat F., Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127(12):2703–2716. doi: 10.1093/brain/awh289. 15319275 [DOI] [PubMed] [Google Scholar]
- Pierce K., Müller R.A., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. 11571222 [DOI] [PubMed] [Google Scholar]
- Pierce K., Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol. Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. 18621359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Hopfinger J.B., Pelphrey K.A., Piven J., Penn D.L. Neural bases for impaired social cognition in schizophrenia and autism Spectrum disorders. Schizophr. Res. 2008;99(1-3):164–175. doi: 10.1016/j.schres.2007.10.024. 18053686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry. 2005;64(1):589–598. doi: 10.1016/j.biopsych.2005.03.026. 15935993 [DOI] [PubMed] [Google Scholar]
- Richey J.A., Rittenberg A., Hughes L., Damiano C.R., Sabatino A., Miller S., Hanna E., Bodfish J.W., Dichter G.S. Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2014;9:367–377. doi: 10.1093/scan/nss146. 23223206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B., Dricot L., Devolder A., Bodart J.M., Crommelinck M., De Gelder B., Zoontjes R. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J Cogn Neurosci. 2000;12(5):793–802. doi: 10.1162/089892900562606. 11054921 [DOI] [PubMed] [Google Scholar]
- Russell R., Duchaine B., Nakayama K. Super−recognizers: People with extraordinary face recognition ability. Psychonomic Bulletin & Review. 2009;16(2):252–257. doi: 10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said C.P., Dotsch R., Todorov A. The amygdala and FFA track both social and non-social face dimensions. Neuropsychologia. 2010;48(12):3596–3605. doi: 10.1016/j.neuropsychologia.2010.08.009. 20727365 [DOI] [PubMed] [Google Scholar]
- Said C.P., Haxby J.V., Todorov A. Brain systems for assessing the affective value of faces. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2011;366(1571):1660–1670. doi: 10.1098/rstb.2010.0351. 21536552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W., Toichi M., Uono S., Kochiyama T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neurosci. 2012;13:99. doi: 10.1186/1471-2202-13-99. 22889284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Humphreys K., Luna B. Visual category-selectivity for faces, places, and objects emerges along different developmental trajectories. Dev Sci. 2007;10(4):F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. 17552930 [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Dahl R.E. Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev Cogn Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. 22483070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Minshew N., Luna B. Atypical development of face and Greeble recognition in autism. J Child Psychol Psychiatry. 2008;49(8):838–847. doi: 10.1111/j.1469-7610.2008.01903.x. 18422548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Luna B., Minshew N., Behrmann M. Location, location, location: alterations in the functional topography of face-but not object- or place-related cortex in adolescents with autism. Front. Hum. Neurosci. 2010;4:26. doi: 10.3389/fnhum.2010.00026. 20631857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R.T., Gauthier I., Klin A., Fulbright R.K., Anderson A.W., Volkmar F., Skudlarski P., Lacadie C., Cohen D.J., Gore J.C. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and asperger syndrome. Arch. Gen. Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. 10768694 [DOI] [PubMed] [Google Scholar]
- Schumann C.M., Bloss C.S., Barnes C.C., Wideman G.M., Carper R.A., Akshoomoff N., Pierce K., Hagler D., Schork N., Lord C., Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. 20335478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S.S., Balla D.A., Cicchetti D.V. Vineland-II Adaptive Behavior Scales. AGS Publishing; 2005. [Google Scholar]
- Swartz J.R., Wiggins J.L., Carrasco M., Lord C., Monk C.S. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. 23265636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The Nimstim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. 19564050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hertzig M.E., Gillespie-Lynch K., Gilhooly T., Millner A.J., Casey B.J. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc. Cogn. Affect. Neurosci. 2014;9:106–117. doi: 10.1093/scan/nst050. 23596190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Dapretto M., Hariri A.R., Sigman M., Bookheimer S.Y. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. 15187809 [DOI] [PubMed] [Google Scholar]
- Weigelt S., Koldewyn K., Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci. Biobehav. Rev. 2012;36(3):1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. 22212588 [DOI] [PubMed] [Google Scholar]
- Weiner K.S., Grill-Spector K. The improbable simplicity of the fusiform face area. Trends Cogn. Sci. (Regul. Ed.) 2012;16(5):251–254. doi: 10.1016/j.tics.2012.03.003. 22481071 [DOI] [PubMed] [Google Scholar]
- Weng S.J., Carrasco M., Swartz J.R., Wiggins J.L., Kurapati N., Liberzon I., Risi S., Lord C., Monk C.S. Neural activation to emotional faces in adolescents with autism spectrum disorders. J. Child Psychol. Psychiatry. 2011;52(3):296–305. doi: 10.1111/j.1469-7610.2010.02317.x. 21039484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R.K. Looking at upside-down faces. J. Exp. Psychol. Learn. 1969;81:141. [Google Scholar]