Abstract
Corticocortical functional interactions between the primary motor cortex (M1) and secondary motor areas, such as the dorsal (PMd) and ventral (PMv) premotor cortices and the supplementary motor area (SMA) are relevant for residual motor output after subcortical stroke. We hypothesized that the microstructural integrity of the underlying white matter tracts also plays a role in preserved motor output. Using diffusion-tensor imaging we aimed at (i) reconstructing individual probable intrahemispheric connections between M1 and the three secondary areas (PMd, PMv, SMA) and (ii) examining the extent to which the tract-related microstructural integrity correlates with residual motor output. The microstructural integrity of the tract connecting ipsilesional M1 and PMd was significantly associated with motor output (R = 0.78, P = 0.02). The present results support the view that ipsilesional secondary motor areas such as the PMd might support M1 via corticocortical connections to generate motor output after stroke.
Keywords: Fractional anisotropy, Cortical, Diffusion, Recovery, Structural
Highlights
-
•
Cortico-cortical functional interactions in the motor network are relevant for residual motor output after subcortical stroke.
-
•
Microstructural integrity of the underlying white matter tracts might play a significant role in preserved motor functions.
-
•
Diffusion-tensor imaging (DTI) was used in stroke patients to reconstruct individual intrahemispheric connections between M1 and three secondary motor areas (PMd, PMv, SMA).
-
•
The extent to which the tract related microstructural integrity correlates with residual motor function was determined.
-
•
Microstructural integrity of intrahemispheric corticocortical tracts can be determined in stroke patients.
-
•
Data support the view that the microstructural integrity of the tract connecting ipsilesional M1 and PMd is significantly associated with recovered motor output in stroke patients.
1. Introduction
Functional imaging studies (Grefkes et al., 2008; Rehme et al., 2012; Ward et al., 2003a, 2003b) and electrophysiological experiments (Johansen-Berg et al., 2002a; Fridman et al., 2004) have revealed that corticocortical interactions between the primary motor cortex (M1) and secondary motor areas, such as the dorsal (PMd) and ventral (PMv) premotor cortices and the supplementary motor area (SMA) are particularly relevant for motor recovery and residual motor output after subcortical stroke. Connectivity analyses have demonstrated less effective communication between premotor areas and M1 in the affected hemisphere in the early stage after stroke. Subsequent reinstatement of effective coupling was associated with functional improvement (Rehme et al., 2011). Interventional studies have revealed that ipsilesional premotor areas might take over functions that are not controlled by these areas in healthy individuals (Fridman et al., 2004; Ward, 2011).
As the structural integrity of the underlying corticocortical pathways of the motor network is an important basis for neuronal information throughput and relevant for behavior (Schulz et al., 2014), we questioned whether the microstructural integrity of corticocortical white matter tracts might also contribute to motor output after stroke. At the corticospinal level, it has been already shown that the motor output critically relies on the integrity of the corticofugal fibers (Werring et al., 2000; Schaechter et al., 2009; Sterr et al., 2010; Zhu et al., 2010). Tracing (Catsman-Berrevoets and Kuypers, 1976; Dum and Strick, 1991; He et al., 1993; He et al., 1995; Dum and Strick, 1996) and structural imaging studies (Newton et al., 2006; Schulz et al., 2012) have shown that contributions to corticospinal fibers arise not only from M1 but also from secondary motor areas, such as PMd, PMv or SMA. Partly, the integrity of these corticofugal pathways has predicted additional variance in motor output (Newton et al., 2006; Schulz et al., 2012) and treatment gains in chronic stroke patients (Riley et al., 2011).
We hypothesized that such a structure–behavior relationship might also hold true for the structural integrity of specific corticocortical pathways between the primary motor cortex and these three secondary motor areas and motor outcome which has not been investigated so far. Using diffusion-tensor imaging we aimed at (i) reconstructing probable intrahemispheric connections between M1 and both PMd, PMv and SMA and (ii) examining the extent to which tract-related microstructural integrity correlates with preserved motor output in patients with subcortical stroke.
2. Participants and methods
2.1. Participants and clinical data
Ten right-handed patients (mean age 62.4 years, range 30–76, 6 males) with first-ever subcortical strokes (5 in the dominant hemisphere, see Fig. 1 for lesion location and Table 1 for clinical data) in the chronic stage of recovery were recruited from a larger study population of a longitudinal study, focusing on longitudinal changes in intracortical inhibition (Liuzzi et al., 2014). Initial motor deficit included weakness of at least the small hand muscles between 3 and 4 on the Medical Research Council Scale (MRC). In a cross-sectional design (11.6 ± 0.6 months after stroke), the patients were re-evaluated on grip strength, pinch strength and finger tapping speed. For the former assessments, the mean value (in kg) of three consecutive measurements separated by approximately 30 s of rest was calculated. For the latter, patients were seated in front of an electronic keyboard in an upright position with the forearm lying on a table. They were instructed to press a specific key with the paretic index finger as quickly as possible for a total of 10 s. This task was repeated three times with an approximately thirty second rest in between repetitions. Finger tapping was defined as the mean number of taps in the three repetitions. The three behavioral scores were expressed as the ratio (affected hand/unaffected hand, Table 1). Based on them, one composite motor output score (MO) was calculated applying a factor analysis with principal component extraction. Explaining 78.6% of the behavioral variance, this score was used for further analyses (Table 1). Patients gave written informed consent according to the Declaration of Helsinki. The study was approved by the local ethics committee.
Fig. 1.
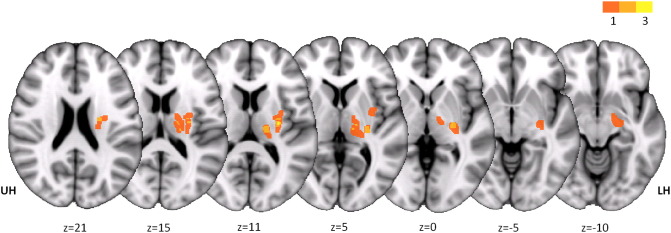
Lesion locations. Subcortical strokes are overlaid on axial MNI T1 slices (z-values in Montreal Neurological Institute (MNI) standard space). Brains with right-sided lesions were flipped over the mid-sagittal plane. Color bar indicates the number of subjects in which voxels are considered part of the lesion. UH unaffected hemisphere, LH lesioned hemisphere.
Table 1.
Clinical data. Age (in years) and sex (M male, F female), stroke location, affected hemisphere (R right, L left). Time in months after stroke. Relative grip and pinch force and finger tapping (FT) speed (ratio affected/unaffected hand, dimensionless) merged to one composite motor output score (MO). PLIC posterior limb of the internal capsule, CR corona radiata, TC thalamocapsular, LC lenticocapsular.
| Patient | Age | Sex | Stroke | Hemisphere | Time | Grip | Pinch | FT | MO |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | F | PLIC | R | 10.1 | 0.55 | 0.77 | 0.76 | −1.89 |
| 2 | 68 | F | CR | R | 8.6 | 0.73 | 0.82 | 0.90 | −0.99 |
| 3 | 67 | M | PLIC | L | 8.8 | 1.51 | 1.42 | 1.44 | 3.30 |
| 4 | 65 | M | CR | L | 12.9 | 0.93 | 1.02 | 0.97 | 0.02 |
| 5 | 30 | F | TC | L | 12.4 | 1.17 | 0.91 | 0.92 | 0.18 |
| 6 | 47 | M | LC | R | 11.6 | 0.96 | 0.79 | 0.87 | −0.64 |
| 7 | 60 | M | CR, LC | R | 13.3 | 0.88 | 0.85 | 0.77 | −1.00 |
| 8 | 76 | F | TC | R | 13.6 | 0.65 | 1.24 | 0.67 | −1.02 |
| 9 | 69 | F | TC, PLIC | L | 12.7 | 1.10 | 1.56 | 1.13 | 1.80 |
| 10 | 70 | M | PLIC | L | 11.7 | 1.03 | 0.96 | 1.01 | 0.23 |
2.2. Brain imaging
A 3 T Trio Siemens MRI scanner (Erlangen, Germany) was used to acquire both diffusion- and T1-weighted images in the stroke patients and a group of nine healthy, age- and gender-matched controls (mean age 65.1 years, range 60–71, Student's T-test P = 0.58; 3 males, Fisher's exact P = 0.37). Probabilistic tractography was carried out using the FSL 4.1 software package (http://www.fmrib.ox.ac.uk/fsl) to reconstruct probable intrahemispheric pathways connecting hand representations of M1 and PMd, PMv and SMA. Individual tracts were used to calculate tract-related and subject-specific mean fractional anisotropy (FA), a surrogate parameter for white matter integrity. FA values were calculated for both the affected and unaffected hemispheres, and proportional FA values (affected/unaffected hemisphere, Schulz et al., 2012) were used for correlation and multiple regression analyses with MO. For imaging details see the online-only data supplement.
2.3. Statistics
One-way and repeated measures (RM) analyses of variance (ANOVA) were used for between and within group comparisons (GROUP) with within-factors TRACT and, for tract volumes and absolute FA values, HEMISPHERE. For group comparisons, four of the control participants were randomly selected and assigned to right (R) versus left (L) tract-related FA proportionality, while L/R proportionality was calculated for the other five. Accordingly, right or left hemispheric absolute FA/tract volumes values were compared with the lesioned (LH) or unaffected hemisphere (UH) in the stroke patients to account for hand dominance. Partial correlation analysis of proportional tract-related FA values and MO were used to infer tract-related structure–behavior relationship. Multiple linear regression analysis was conducted in a stepwise fashion, inclusion/exclusion was determined by F probability of P < 0.05 for inclusion and P > 0.1 for exclusion. Statistical significance was assumed at P-values ≤ 0.05. All results are given as mean ± standard error of the mean (SEM). Statistical analysis was conducted using SPSS 19 software (IBM Corp., NY, US).
3. Results
3.1. Clinical and behavioral data
Clinical and behavioral data are listed in Table 1. For locations of subcortical strokes please see Fig. 1.
A one-way ANOVA revealed a significant effect of the side of the lesion (dominant or non-dominant hemisphere) on MO. Regardless of the initial deficit or amount of recovery over the past year, which was not addressed in the present study, patients with paresis of the dominant hand showed superior motor output (relative to the non-affected hand) compared to patients with paresis of the non-dominant hand (F(1,9) = 10.92, P = 0.01). We used partial correlation analysis to control for this SIDE effect. Moreover, in respect to the broad range of age in the study population, we also controlled for AGE.
3.2. Probabilistic tractography of intrahemispheric premotor–M1 connections
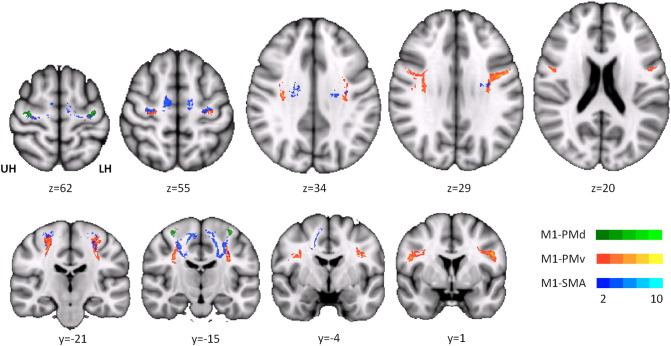
Probable trajectories connecting M1 and premotor areas were successfully obtained in all ten stroke patients. There was good reproducibility of all six tracts across the group, as demonstrated by the trajectory variability map for the stroke patients (Fig. 2). The common course of the intrahemispheric tracts was in good agreement with previous work on intra- and interhemispheric corticocortical connections between primary and secondary motor areas in younger and older healthy participants and their relationship to learning gains (Schulz et al., 2014). Volumes of homologue tracts were similar in the affected and unaffected hemispheres. Comparison with the controls did not reveal any significant group differences. None of the tracts was affected by the initial stroke lesion (for details see figure e2 in the online-only data supplement).
Fig. 2.
Trajectory variability maps for premotor–M1 connections in chronic stroke patients. Probable corticocortical connections between M1 and PMd, PMv and SMA are overlaid on T1 template in MNI standard space (upper row: axial slices with z-values, lower row: coronal slices with y-values). Color bar indicates the number of subjects in which voxels are considered part of the tracts of interest. Note that individual subject-specific binarized tracts of interest were used to calculate tract-related FA and not the group average (for illustration only). Tracts in subjects with right-sided lesions were flipped over the mid-sagittal plane. UH unaffected hemisphere, LH lesioned hemisphere.
3.3. Tract-related white matter integrity and motor output
RM-ANOVA of absolute tract-related FA values with factors HEMISPHERE and TRACT revealed a significant TRACT effect (F[1.11,9.95] = 23.56, P < 0.01). HEMISPHERE (F[1,9] = 0.29, n.s.) and HEMISPHERE × TRACT (F[1.37,12.29] = 0.25, n.s.) were not significant suggesting the absence of a nonspecific reduction of white matter integrity in the affected hemisphere (figure e3 A in the online-only data supplement). Proportional FA values were 1.03 ± 0.05 for M1–PMd, 0.98 ± 0.04 for M1–PMv and 0.97 ± 0.03 for M1–SMA. RM-ANOVA showed that the proportional FA values did not differ between the tracts of interest (F[1.25,11.28] = 0.29, n.s., figure e3 B in the online-only data supplement). Partial correlation analysis correcting for SIDE and AGE revealed a positive correlation between the integrity of the tract (proportional FA values) connecting M1 and PMd and residual motor output in the chronic stage of recovery (R = 0.78, P = 0.02). Contrarily, the microstructural integrity of the tracts connecting M1 with PMv and SMA did not show any significant association with residual motor output (M1–PMv: R = 0.39, P = 0.34, M1–SMA: R = –0.04, P = 0.93).
To estimate the extent to which this measure might explain variance in motor performance apart from SIDE and AGE, we performed a multiple regression analysis in which the tract-related integrity of M1–PMd explained 21% of variance in MO in addition to SIDE (Table 2). Of note, compared to healthy controls, RM-ANOVA did not show any significant differences, neither in regard to the absolute (GROUP F[1,17]= 0.69, n.s.) nor in regard to the proportional tract-related FA values (GROUP F[1,17]= 0.86, n.s.) which were 1.11 for M1–PMd, 0.98 for M1–PMv and 1.01 for M1–SMA. For further details see figure e3 in the online-only data supplement.
Table 2.
Multiple linear regression analysis. Multiple regression analysis revealed that proportional FA in M1–PMd tract explains 21% of variance in motor performance in addition to the side of the lesion.
| Model | Included variables | R | Corr. R2 | F | P | B value | Beta | P |
|---|---|---|---|---|---|---|---|---|
| 1 | SIDE | 0.76 | 0.52 | 10.92 | 0.01 | 2.21 | 0.76 | 0.01 |
| SIDE | 3.86 | 1.33 | < 0.01 | |||||
| 2 | ||||||||
| + FA[Ml-PMd] | 0.89 | 0.73 | 12.84 | 0.01 | 7.63 | 0.73 | 0.04 |
4. Discussion
In the present study, we used diffusion-tensor imaging to reconstruct intrahemispheric corticocortical connections between hand representations within M1 and PMd, PMv and SMA. Proportional microstructural integrity of the tract connecting ipsilesional M1 and PMd was significantly associated with hand motor output in well-recovered chronic stroke patients. By contrast, FA values of tracts connecting M1 and both PMv and SMA did not show significant correlations with residual motor output.
The present result is in good agreement with a number of previous imaging and electrophysiological studies. Task-related enhanced brain activation in secondary motor areas both in the ipsilesional and contralesional hemispheres is a common pattern after stroke, particularly in the early stage of recovery and correlates with motor function and recovery (Ward et al., 2003a; Grefkes et al., 2008; Rehme et al., 2012). For instance, it has been shown that increased brain activity in the ipsilesional PMd was associated with training gains in motor performance in 7 chronic stroke patients (Johansen-Berg et al., 2002a). Resting-state analyses have reported increased functional connectivity between ipsilesional M1 and both premotor cortices (Yin et al., 2012) and frontal brain areas (Park et al., 2011) but have not suggested an association between ipsilesional connectivity and motor performance after stroke (Carter et al., 2010; van Meer et al., 2010). Assessing causality of inter-regional coupling, dynamic causal modeling has revealed a significant reduction of intrinsic positive coupling between ipsilesional premotor cortices and M1 both in the acute (Rehme et al., 2011) and subacute stages after stroke (Grefkes et al., 2008). The subsequent reinstatement of effective connectivity has been related to the amount of recovery over time (Rehme et al., 2011). In well-recovered patients with lesions to the corticospinal outflow originating in M1, transcranial magnetic stimulation has confirmed that particularly the ipsilesional PMd might support M1, potentially by increasing the strength of corticospinal PMd projection (Fridman et al., 2004). In this regard, recent diffusion-tensor imaging studies are of particular interest as they have just extended the understanding of secondary motor areas also for direct corticospinal neural transmission after stroke: Indeed, both residual motor performance (Newton et al., 2006; Schulz et al., 2012) and treatment gains (Riley et al., 2011) in chronic stroke patients did not only rely on the integrity of the corticospinal fibers originating from M1 but also from PMd. The present study now confirmed that such a structure–behavior relationship might also hold true for corticocortical connections between ipsilesional M1 and PMd. The integrity of white matter tracts connecting ipsilesional PMd and M1 positively correlated with preserved motor output in patients with excellent motor recovery. This adds tract-related structural data to the previous functional imaging studies for the understanding of corticocortical premotor–motor interactions and their functional relevance after stroke. Notably, previous whole-brain voxel-wise analyses have already revealed a positive relationship between regional FA within the ipsilesional precentral gyrus and motor function in a similar group of 10 fair to excellent recovered chronic stroke patients (Schaechter et al., 2009). In the context of considerable inter-subject variability in gyral anatomy, regional FA values have been related to major white matter bundles, such as the corticospinal tract and the superior longitudinal fascicle (Schaechter et al., 2009). Here, probabilistic tractography was used to extend these previous findings of structure–behavior relationship by reconstructing individual intrahemispheric premotor–motor pathways. In summary, the present results support the view that ipsilesional secondary motor areas such as the PMd might act in parallel with M1 via both corticocortical and corticospinal connections to generate motor output. To what extent the present results in excellently recovered patients might be also transferred to more affected patients remains to be investigated in future studies. In this context, in more affected patients with a larger lesion load to M1 and PMd, the contralesional PMd was found to be important for motor recovery (Johansen-Berg et al., 2002b; Bestmann et al., 2010).
Surprisingly, we did not find any association between the tract-related integrity of fibers connecting M1 and both PMv and SMA, although various animal (Frost et al., 2003; Dancause et al., 2005) and functional imaging studies (Rehme et al., 2011) have suggested a particular functional role of both areas for motor recovery. For instance, noradrenergic stimulation was found to reduce increased brain activation in ipsilesional PMv and SMA and also resulted in functional improvement. For SMA, dynamic causal modeling has shown that an increase in ipsilesional SMA–M1 coupling positively correlated with motor recovery from the acute stage to the early chronic stage after stroke (Rehme et al., 2011). While that study has correlated the change of coupling parameters and motor function over time, the present study was conducted in a cross-sectional design in the chronic stage of recovery. Potentially, changes of effective and structural SMA–M1 connectivity measures from the acute to the early chronic stage after stroke might be more likely to relate to motor function or improvement over time than persistent tract-related FA values in later stages of recovery. This might explain why the actual analysis has not uncovered a similar structure–behavior relationship for SMA–M1. In this context the integrity of PMd–M1 might exhibit either a particularly persistent functional relevance for residual motor output which can be detected also without the additional information from a longitudinal assessment. Or, on the other hand, this finding may indicate a short lasting status of the motor system in the recovery stage between post-acute and chronic only. Longitudinal studies are needed to address this further. As already mentioned, also the excellent recovery of the present study population might have critically influenced the extent to which the microstructural integrity of the corticocortical pathways connecting M1 and both PMv and SMA contributes to motor output. Future studies in more affected patients are needed to investigate whether in these cases also structural parameters of these connections might gain a functional importance after stroke. Finally, the pathways connecting M1 with PMv and SMA were located in more prominent spatial relation to various crossing association such as the superior longitudinal fascicle or corticofugal fibers in the white matter. It is likely that these fiber populations and their FA values might have influenced the results of the present correlative analyses. For improved simultaneous modeling of other, concurrent fiber tracts which will serve as additional covariates, further studies on larger sample sizes are needed.
There are some critical limitations of the present study. First, the present analysis included chronic stroke patients who were very well recovered. Relative grip force, pinch force and finger tapping did not show a significant asymmetry between the affected and unaffected hands. In line, also an additional post-hoc comparison with a historical healthy control group did not reveal any differences in grip force (see text and figure e4 in the online-only data supplement). Together with the absent group- or hemisphere specific differences in tract-related white matter integrity this underscores the excellent motor recovery of the patients after the stroke in the present sample. In this context, in future investigations similar correlative analyses would be particularly needed to infer to what extent the present findings are normally found in elderly or specifically related to recovery and motor output after stroke. Such an analysis is also needed for a second important reason: Patients with the right dominant hand affected showed superior motor output than patients with the non-dominant hand affected. Partial correlation analyses were used to control for this effect of hand dominance in stroke recovery (Harris and Eng, 2006). The normalization of the affected hand to the unaffected hand has been commonly used in stroke studies to account for inter-subject variability in baseline motor output (Schulz et al., 2012). Controlling for this effect seems to be necessary in studies in which patients with both the dominant and non-dominant hands affected are included. Given this significant effect of side, the present results might also suggest that lateralized M1–PMd connections might generally relate to more lateralized motor performance. A recent study in aged subjects (Schulz et al., 2014), although not designed for this specific question, provides data that does not support this view, as there were no associations between PMd–M1 connections and pure motor behavior. In the absence of behavioral data from the healthy participants in the present study, it cannot be completely ruled out that the present findings relate to rather age-dependent than stroke-recovery-related changes, although, based on previous work (Schulz et al., 2014), unlikely. Future controlled studies are needed to answer these important questions.
Second, probabilistic tractography was used to reconstruct individual fibers between M1 and three secondary motor areas. Despite the visual inspection of the individual trajectories, the courses of the tracts remain probabilistic. Exclusion and waypoint masks were used to guide the tract reconstructions. However, we cannot exclude that the actual courses may differ between the reconstruction and the individual brain anatomy. Particularly, published functional imaging data (Mayka et al., 2006) were used to bias the tract-reconstruction to the hand representations within each motor area as successfully introduced for the reconstruction of the corticospinal tract (Schulz et al., 2012). However, this approach neglects potential cortical reorganization after stroke with gross changes in the cortical representations. Studies which combine individual functional imaging, structural and diffusion data might allow the calculation of individually tailored seed and target regions. Third, mean tract-related FA values were calculated to infer white matter integrity. White matter regions with crossing fibers can influence the FA estimation and result in false-low FA values. Fourth, despite the amount of variance explained in the present regression we are convinced that the microstructural integrity of the pathways of interest does not explain all aspects of the behavior. In a more whole brain perspective, studies with advanced multiple regression analyses are needed to answer how further intra- and interhemispheric connections between motor- and non-motor areas, corticospinal connections and local brain morphology such as cortical thickness are related to residual motor output after stroke.
Acknowledgments
This research was by the German Research Foundation (DFG Ge 844/2-1 to C.G.; SFB 936-C1 to C.G., SFB 936-C4 to F.C.H.). The authors declare that there are no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.11.006.
Appendix A. Supplementary data
Supplementary material.
References
- Bestmann S., Swayne O., Blankenburg F. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS–fMRI. J. Neurosci. 2010;30(36):11926–11937. doi: 10.1523/JNEUROSCI.5642-09.2010. 20826657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.R., Astafiev S.V., Lang C.E. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. 20373348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsman-Berrevoets C.E., Kuypers H.G. Cells of origin of cortical projections to dorsal column nuclei, spinal cord and bulbar medial reticular formation in the rhesus monkey. Neurosci. Lett. 1976;3(5-6):245–252. doi: 10.1016/0304-3940(76)90050-1. 19604894 [DOI] [PubMed] [Google Scholar]
- Dancause N., Barbay S., Frost S.B. Extensive cortical rewiring after brain injury. J. Neurosci. 2005;25(44):10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. 16267224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum R.P., Strick P.L. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 1991;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. 1705965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum R.P., Strick P.L. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J. Neurosci. 1996;16(20):6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. 8815929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E.A., Hanakawa T., Chung M. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(4):747–758. doi: 10.1093/brain/awh082. 14749291 [DOI] [PubMed] [Google Scholar]
- Frost S.B., Barbay S., Friel K.M. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J. Neurophysiol. 2003;89(6):3205–3214. doi: 10.1152/jn.01143.2002. 12783955 [DOI] [PubMed] [Google Scholar]
- Grefkes C., Nowak D.A., Eickhoff S.B. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 2008;63(2):236–246. doi: 10.1002/ana.21228. 17896791 [DOI] [PubMed] [Google Scholar]
- Harris J.E., Eng J.J. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. 2006;20(3):380–389. doi: 10.1177/1545968305284528. 16885424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.Q., Dum R.P., Strick P.L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 1993;13(3):952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. 7680069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.Q., Dum R.P., Strick P.L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J. Neurosci. 1995;15(5 1):3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. 7538558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H., Dawes H., Guy C. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125(12):2731–2742. doi: 10.1093/brain/awf282. 12429600 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Rushworth M.F., Bogdanovic M.D. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. U.S.A. 2002;99(22):14518–14523. doi: 10.1073/pnas.222536799. 12376621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi G., Hörniß V., Lechner P. Development of movement-related intracortical inhibition in acute to chronic subcortical stroke. Neurology. 2014;82(3):198–205. doi: 10.1212/WNL.0000000000000028. 24353337 [DOI] [PubMed] [Google Scholar]
- Mayka M.A., Corcos D.M., Leurgans S.E., Vaillancourt D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31(4):1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. 16571375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton J.M., Ward N.S., Parker G.J. Non-invasive mapping of corticofugal fibres from multiple motor areas — relevance to stroke recovery. Brain. 2006;129(7):1844–1858. doi: 10.1093/brain/awl106. 16702192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Chang W.H., Ohn S.H. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357–1362. doi: 10.1161/STROKEAHA.110.596155. 21441147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Rottschy C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage. 2012;59:2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. 22023742 [DOI] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Wang L.E. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55(3):1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. 21238594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J.D., Le V., Der-Yeghiaian L. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42(2):421–426. doi: 10.1161/STROKEAHA.110.599340. 21164128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter J.D., Fricker Z.P., Perdue K.L. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30(11):3461–3474. doi: 10.1002/hbm.20770. 19370766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Park C.-H., Boudrias M.-H. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke. 2012;43(8):2248–2251. doi: 10.1161/STROKEAHA.112.662619. 22764214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Zimerman M., Timmermann J.E. White matter integrity of motor connections related to training gains in healthy aging. Neurobiol. Aging. 2014;35(6):1404–1411. doi: 10.1016/j.neurobiolaging.2013.11.024. 24387983 [DOI] [PubMed] [Google Scholar]
- Sterr A., Shen S., Szameitat A.J., Herron K.A. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24(5):413–419. doi: 10.1177/1545968309348310. 20516488 [DOI] [PubMed] [Google Scholar]
- Van Meer M.P., van der Marel K., Wang K. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J. Neurosci. 2010;30(11):3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. 20237267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N. Human hand function: the limitations of brain and brawn — clinical and rehabilitation matters. J. Physiol. (Lond.) 2011;589(23):5625–5632. doi: 10.1113/jphysiol.2011.223693. 22063630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N.S., Brown M.M., Thompson A.J., Frackowiak R.S. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(11):2476–2496. doi: 10.1093/brain/awg245. 12937084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N.S., Brown M.M., Thompson A.J., Frackowiak R.S. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126(6):1430–1448. doi: 10.1093/brain/awg145. 12764063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring D.J., Toosy A.T., Clark C.A. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatr. 2000;69(2):269–272. doi: 10.1136/jnnp.69.2.269. 10896709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Song F., Xu D. Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS ONE. 2012;7(12):e52727. doi: 10.1371/journal.pone.0052727. 23285171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.L., Lindenberg R., Alexander M.P., Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41(5):910–915. doi: 10.1161/STROKEAHA.109.577023. 20378864 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.