Abstract
Background:
Pulmonary arterial hypertension secondary to untreated left-to-right shunt defects leads to increased pulmonary blood flow, endothelial dysfunction, increased pulmonary vascular resistance, vascular remodelling, neointimal and plexiform lesions. Some recent studies have shown that inflammation has an important role in the pathophysiology of pulmonary arterial hypertension.
Aims:
The aim of this study is to evaluate serum pentraxin 3 and high sensitive (hs)-C reactive protein (hs-CRP) levels in children with severe pulmonary arterial hypertension (PAH) secondary to untreated congenital heart defects and evaluate the role of inflammation in pulmonary hypertension.
Study Design:
Cross sectional study.
Methods:
After ethics committee approval and receiving consent from parents, there were 31 children were selected for the study with severe PAH, mostly with a left-to-right shunt, who had been assessed by cardiac catheterisation and were taking specific pulmonary vasodilators. The control group consisted of 39 age and gender matched healthy children. After recording data about all the patients including age, gender, weight, haemodynamic studies and vasodilator testing, a physical examination was done for all subjects. Blood was taken from patients and the control group using peripheral veins to analyse serum Pentraxin 3, N-terminal pro-Brain Natriuretic Peptide (NT-ProBNP) and hs-CRP levels. Serum Pentraxin-3 levels were measured by enzyme linked immunosorbent assay (ELISA) and expressed as ng/mL. Serum hs-CRP levels were measured with an immunonephelometric method and expressed as mg/dL. The serum concentration of NT-proBNP was determined by a chemiluminescent immunumetric assay and expressed as pg/mL.
Results:
Serum Pentraxin- 3 levels were determined to be 1.28±2.12 (0.12–11.43) in the PAH group (group 1) and 0.40±0.72 (0.07–3.45) in group 2. There was a statistically significant difference between the two groups (p<0.01). Serum hs-CRP levels were measured as 2.92±2.12 (0.32–14.7) mg/dL in group 1 and 0.35±0.16 (0.07–3.45) mg/dL in group 2. The hs-CRP level was increased in the PAH group to a significant degree (p<0.01).
Conclusion:
Our study showed that pentraxin 3 and hs-CRP levels were increased significantly in the PAH group. We consider that inflammation plays an important role in severe pulmonary hypertension and progressive pulmonary arterial hypertension in children with PAH.
Keywords: hs-CRP, inflammation, pentraxin 3, pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is defined as a mean pulmonary arterial pressure of >25 mmHg (1). Although there have been developments in diagnostic tools such as echocardiography and treatment modalities, PAH secondary to undiagnosed or untreated congenital heart diseases, especially left-to-right shunt defects, remains an important cause of pulmonary arterial hypertension in childhood. The pathophysiology of PAH is multifactorial, complex and incompletely understood (2–4). PAH secondary left-to-right shunt defects is related to increased pulmonary blood flow leading to endothelial dysfunction, increased pulmonary vascular resistance, vascular remodelling and luminal obstruction due to in situ thrombosis and neointimal and plexiform lesions (5–7). Some recent studies have shown that inflammation has an important role in the pathophysiology of PAH (8). C-reactive protein (CRP) is a classic short pentraxin that is produced in liver secondary to systemic inflammation. Pentraxin 3 is one of the long pentraxins and is synthesized by local vascular cells, such as smooth muscle cells, endothelium and fibroblasts, as well as innate immune cells at sites of inflammation. Pentraxin 3 plays a key role in the regulation of cell proliferation and angiogenesis (9, 10).
The aim of this study was to evaluate serum pentraxin 3 and high sensitive-CRP (hs-CRP) levels in children with severe pulmonary arterial hypertension secondary to untreated congenital heart defects, and determine the role of inflammation in pulmonary hypertension.
MATERIAL AND METHODS
After ethics committee approval and consent from parents, 31 children with severe PAH from two centres were selected for the study who mostly had a left-to-right shunt and had been assessed with cardiac catheterisation, required diagnostic catheterisation and were taking specific pulmonary vasodilators. The control group consisted of 39 age- and gender-matched healthy children. After recording data about all the patients including age, gender, weight, echocardiographic assessment, haemodynamic studies and vasodilator testing, all subjects received a physical examination. Patients and control subjects who had an infection, fever or history of infection in the previous month or other chronic disease were excluded from the study.
Medical history, age, weight, height, and body measurements were obtained from all observed children. All cardiac haemodynamic studies which had been performed previously according to standard procedures were as follows: intravenous fluids and electrolyte therapy were given to all patients and oral ingestion had been stopped three hours before cardiac catheterisation. For patients under three years of age, we selected oxygen consumption as 10–14 mLO2/kg for patients weighing 2–5 kg, and 7–11 mLO2/kg for patients weighing 5–8 kg. For the remaining patients, oxygen consumption values were estimated according to the formulas of the LaFarge and Miettinen method.7 Based on the results obtained during cardiac catheterisation and the Fick principle, the pulmonary and systemic blood flows, pulmonary vascular resistance and systemic vascular resistance were calculated. Additionally, the ratio of pulmonary to systemic flow, and the ratio of pulmonary to systemic resistance were calculated using PedCath 3 Software (Scientific Software Solutions Inc, Charlottesville, USA). Pulmonary vascular resistance and systemic vascular resistance were expressed as mmHg X min/L. If patients had severe pulmonary artery hypertension (elevation of pulmonary artery pressure to near systemic levels, or pulmonary vascular resistance >8 Wood unit·m2), a vasoreactivity test was performed using 25 ng/kg/minute nebulised prostacycline with oxygen over a ten minute period.
Blood (2 cc) was also taken from the patients and control group using a peripheral vein for analysis of the serum Pentraxin 3, ProBNP and hs-CRP levels. After collection, the blood samples were centrifuged at 3500 rpm to separate the serum, which then was stored in Eppendorf tubes and stored in deep freeze (−70 degrees Celsius). Serum Pentraxin 3 levels were measured by enzyme linked immunosorbent assay (ELISA) using Quantikine® ELISA (Human Pentraxin 3/TSG-14 Immunoassay, R & D systems, Inc, Minneapolis, USA). Values were expressed as ng/mL. Serum hs-CRP levels were measured with an immunonephelometric method (hsCRP, Siemens, Erlangen, Germany) and expressed as mg/dl. The serum level of NT-proBNP was measured by a chemiluminescent immunumetric assay (IMMULITE 2000 NT proBNP, Siemens Erlangen, Germany) and expressed as pg/mL.
Statistical analysis
All analysis were performed using the Statistical Package for the Social Sciences (SPSS) (SPSS 16.0 Chicago, IL, USA). Normality for continuous variables in groups was determined by the Shapiro Wilk test. The variables did not show normal distribution (p>0.05). The Mann-Whitney U test was used for comparisons of variables between the studied groups.
RESULTS
A total of 31 children with severe pulmonary hypertension (15 females, 16 males) and 39 healthy children (19 females, 20 males) were selected for study. The mean age of the PAH group (group 1) was 9.2 years ±0.39 (1–18 years); the mean age of the grup of healthy children (group 2) was 9.64 years ±4.73 (3–18 years). There was no statistically significant difference between the two groups according to age and gender (p>0.05). The mean weight of group 1 was 26.01 kg ±15.85 (5.2–69) and the mean weight of group 2 was 36.37±19.9 (5.6–80). Group 1 had a statistically significant lower weight than group 2 (p<0.05).
Fourteen patients in group 1 had a ventricular septal defect (VSD) and PAH, 4 patients had primary PAH (2 had small muscular VSD, 1 had a secundum atrial septal defect (ASD)), 2 patients had patent ductus arteriosus (PDA) and PAH, 6 patients had a complete atrioventricular septal defect (AVSD) and PAH, 3 patients were diagnosed as postoperative residual severe PAH (they were operated for VSD), 1 patient had transposition of great arteries (d-TGA) and PAH, 1 patient had a double outlet right ventricle (DORV),VSD and PAH and 1 patient had a secundum ASD-abnormal systemic artery supply to the left lower lobe of the lung. Eleven patients in group 1 also had Down syndrome. The demographic data on the PAH and control groups are shown in Table 1.
TABLE 1.
Serum NT-pro BNP, Pentraxin-3 and hs CRP levels in the PAH and control groups
| PAH group n:31 | Control group n:39 | p | |
|---|---|---|---|
| Age (years) | 9.2±0.39 (1–18) | 9.64±4.73 (3–18) | >0.05 |
| Weight (kg) | 26.01 kg ±15.85 (5.2–69) | 36.37±19.9 (5.6–80) | <0.05 |
| NT-proBNP (pg/mL) | 1047.2±2032.4 (60–9555) | 76.04±59.3 (10–294) | <0.01 |
| Pentraxin -3 (ng/mL) | 1.28±2.12 (0.12–11.43) | 0.40±0.72 (0.07–3.45) | <0.01 |
| hs-CRP (mg/dL) | 2.92±2.12 (0.32–14.72) | 0.35±0.16 (0.32–1.28) | <0.01 |
PAH: pulmonary arterial hypertension; hs-CRP: highly sensitive C-reactive protein; NT-proBNP: n terminal brain natriuretic peptide
On the haemodynamic evaluation, the mean pulmonary artery pressure was measured as 69.1±16.51 (47–94) mmHg and the mean aortic pressure was 75.41±12.83 (43–91) mmHg in the PAH group. The shunt ratio was 1.25±0.57 (0.7–2.3). Mean pulmonary resistance (Rp) was 13.48±9.97 (2.87–26) Uxm2, systemic resistance (Rs) was 18.96±22.72 (4.05–26) Uxm2, Rp/Rs was 0.94±0.66 (0.23–2.25). Vasoreactivity testing was performed on 30 patients due to severe pulmonary hypertension with nebulised prostacycline (Ventavis®, Bayer Group, Germany). Among these patients, vasoreactivity was found to negative in 20, and 10 were found to have partially reversible pulmonary arterial hypertension. One patient was followed-up with Iloprost and 26 patients with Bosentan treatment, while 4 patients required combination treatment (Bosentan and Iloprost). Diagnostic and haemodynamic data on the PAH group are shown in Table 2.
TABLE 2.
Diagnostic and haemodynamic data from the PAH group
| Diagnosis | VSD: 14 |
| Primary PAH: 4 | |
| AVSD: 6 | |
| PDA: 2 | |
| Postoperative residual PAH: 3 | |
| DORV: 1 | |
| d-TGA: 1 | |
| ASD: 1 | |
| Abnormal systemic artery: 1 | |
| Qp/Qs | 1.25±0.57 (0.7–2.3) |
| Mean PAP (mmHg) | 69.1±16.51 (47–94) |
| Mean Aortic Pressure (mmHg) | 75.41±12.83 (43–91) |
| Rp (U × m2) | 13.48±9.97 (2.87–26) |
| Rs (U× m2) | 18.96±22.72 (4.05–26) |
| Rp/Rs | 0.94±0.66 (0.23–2.25) |
| Vasoreactivity testing | Negative in 20 patients |
| Partially reversible in 10 patients | |
| Treatment | Bosentan for 26 patients |
| Nebulised Iloprost for 1 patient | |
| Combination therapy for 4 patients |
VSD: ventricular septal defect; PAH: pulmonary arterial hypertension; AVSD: atrioventricular septal defect; PDA: patent ductus arteriosus; DORV: double outlet right ventricle; ASD: atrial septal defect; PAP: pulmonary artery pressure; Rp: pulmonary resistance; Rs: systemic resistance
Serum NT-proBNP levels were measured in the pulmonary hypertension group as 1047.2±2032.4 (60–9555) pg/mL and 76.04±59.3 (10–294) pg/mL in group 2. There was a statistically significant difference between the two groups (p<0.01).
Serum Pentraxin- 3 levels were measured as 1.28±2.12 (0.12–11.43) ng/mL (median 0.49) in the pulmonary hypertension group (group 1) and 0.40±0.72 (0.07–3.45) ng/mL (median 0.16) in group 2 (Figure 1). There was a statistically significant difference between the two groups (p<0.01). Serum hs-CRP levels were measured as 2.92±2.12 (0.32–14.72) mg/dL in group 1 and 0.35±0.16 (0.32–1.28) mg/dL in the control group (Figure 2). The CRP level was significantly increased in the PAH group (p<0.01). The results for serum pentraxin 3, hs-CRP and NT-proBNP are shown in Table 1.
FIG. 1.
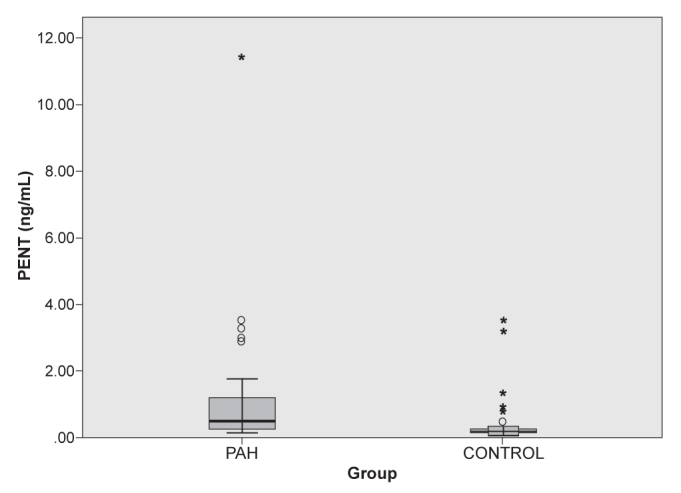
Serum Pentraxin 3 levels were increased in the PAH group
FIG. 2.
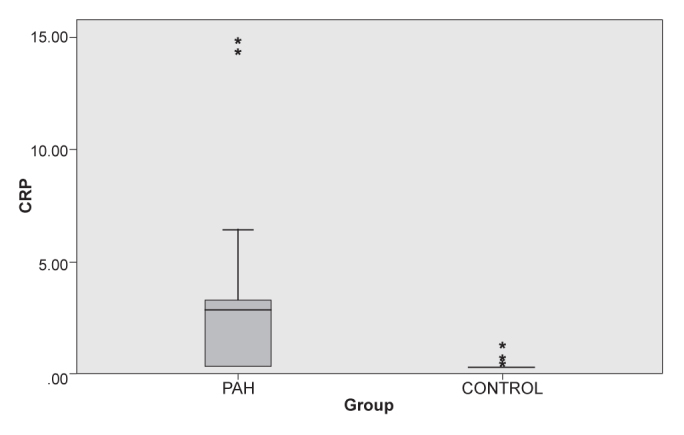
Serum hs-CRP levels were increased in the PAH group
DISCUSSION
Pulmonary arterial hypertension (PAH) is a severe condition characterised by vascular proliferation and remodelling of small pulmonary arteries. PAH is defined as mean pulmonary artery pressure greater than 25 mmHg and pulmonary vascular resistance greater than 3 Wood units × m2 at rest (11). Pulmonary arterial hypertension may be related to increased blood flow (hyperkinetic pulmonary arterial hypertension) or pulmonary venous obstruction. PAH is an important cause of morbidity and mortality. Large registries of paediatric pulmonary hypertension show that the most common causes of PAH are idiopathic PAH, PAH related to congenital heart disease, postoperative PAH and paediatric lung disease (12–14).
Our cases were selected from two paediatric cardiology centres, both of them located in the southern part of our country. Most of our patients have severe PAH related to late diagnosed congenital heart disease or postoperative PAH.
Although there has been diagnostic and therapeutic progress in PAH, undiagnosed and/or late diagnosed congenital heart disease and postoperative PAH are still important causes of severe PAH or Eisenmenger syndrome in developing countries. The pathophysiology of PAH is complex and includes various factors. Pulmonary hypertension as a complication in children with congenital heart disease is either secondary to increased pulmonary blood flow or to increased post-capillary pressure.
Pulmonary vascular endothelium has a central role in PAH (15).
Pulmonary vasoconstriction is mediated largely by endothelial dysfunction secondary to increased blood flow or pressure in congenital heart disease. Endogenous vasodilators such as nitric oxide and prostacycline are decreased while endogenous vasoconstrictors such as endothelin-1 (ET-1) and serotonin are increased in patients with pulmonary hypertension (16, 17).
The pathology of PAH has been well defined by Edwards, and Rabinowich (6, 7). Intimal and medial hypertrophy, vascular remodelling, and neovasculogenesis lead to vascular occlusion and reduced pulmonary flow. Current PAH-specific therapies are targeted to re-establish pulmonary vasodilatation and reduce smooth muscle proliferation and hypertrophy in pulmonary arteries (6, 7). Inflammation may be an important contributing factor to the progression of severe PAH. Inflammation is considered to play an important role of some types of pulmonary arterial hypertension such as connective tissue disease and human immune deficiency virus related pulmonary arterial hypertension. The pathological changes in pulmonary vasculature associated with severe pulmonary arterial hypertension or Eisenmenger syndrome include medial thickening and plexiform lesions resembling idiopathic pulmonary arterial hypertension (18). Plexiform lesions are rich in macrophages, T- and B-lymphocytes, and dendritic cells (19).
Markers of inflammation, including interleukin-1b, interleukin -6 and P selectin are elevated in idiopathic PAH. Tumour necrosis factor-α, CRP and interleukin-6 are also elevated in idiopathic PAH, PAH associated with chronic thromboembolic disease and connective tissue associated PAH (20, 21). Ramakrishnan et al. (22) showed that interferon-ɣ and hs-CRP levels are significantly elevated in Eisenmenger syndrome. They also found that elevated hs-CRP was associated with older age and shorter 6 min walking distance, but the levels of inflammatory markers were not predictive of clinical events (22). Our study showed that serum hs-CRP levels were significantly elevated in children with severe PAH (p<0.01). Pentraxins are a family of evolutionarily conserved proteins and divided into short and long pentraxins according to their structure. CRP is a classic short pentraxin and produced in liver secondary to inflammation (8).
Pentraxin 3 is one of the long pentraxins, synthesized by smooth muscle cells, endothelial cells, fibroblasts and other immune cells at sites of inflammation. Pentraxin 3 plays a key role in the regulation of cell proliferation and angiogenesis (9, 10).
Tamura et al. (23) proposed human pentraxin 3 as a novel biomaker for the diagnosis of pulmonary hypertension. They compared Pentraxin 3 levels were significantly elevated in 50 PAH patients, 27 with idiopathic PAH, 17 with PAH associated with connective tissue disease and six with congenital heart disease (23). Most of our patients selected for this study were patients with severe pulmonary arterial hypertension related to congenital heart disease. We found that pentraxin 3 levels were significantly increased in the PAH group. We consider that inflammation has an important role in severe paediatric pulmonary hypertension related to congenital heart disease, like connective tissue related PAH.
Our study shows that pentraxin 3 and hsCRP levels were significantly increased in the PAH group. We consider that inflammation has an important role in progression of severe pulmonary hypertension in paediatric PAH.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of the İnönü University Faculty of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - C.K., O.B., S.Ç.; Design - C.K., O.B., S.Ç.; Supervision - C.K., S.Ç., D.Ş.; Resource - C.K., S.Ç., Ç.T.; Materials - C.K., S.Ç., O.B., D.Ş.; Data Collection&/or Processing - C.K., S.Ç., D.Ş., O.B.; Analysis&/or Interpretation - S.Y.; Literature Search - C.K., O.B.; Writing - C.K.,O.B.; Critical Reviews - C.K., O.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Ivy D. Advances in pediatric pulmonary arterial hypertension. Curr Opin Cardiol. 2012;27:70–81. doi: 10.1097/HCO.0b013e32835018cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moledina S, Hislop AA, Foster H, Schulze-Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: A national cohort study. Heart. 2010;96:1401–6. doi: 10.1136/hrt.2009.182378. [DOI] [PubMed] [Google Scholar]
- 3.Fraisse A, Jais X, Schleich JM, di Filippo S, Maragnes P, Beghetti M, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis. 2010;103:66–74. doi: 10.1016/j.acvd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Beghetti M, Tissot C. Pulmonary hypertension in congenital shunts. Rev Esp Cardiol. 2010;63:1179–93. doi: 10.1016/s1885-5857(10)70232-2. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovitch M, Keane JF, Norwood WI, Castaneda AR, Reid L. Vascular structure in lung biopsy tissue correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation. 1984;69:655–67. doi: 10.1161/01.cir.69.4.655. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovitch M, Keane JF, Fellows KE, Castaneda AR, Reid L. Quantitative analysis of the pulmonary wedge angiogram in congenital heart defects: correlation with hemodynamic data and morphometric findings in lung biopsy tissue. Circulation. 1981;63:152–64. doi: 10.1161/01.cir.63.1.152. [DOI] [PubMed] [Google Scholar]
- 7.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease. Circulation. 1958;18:533–47. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- 8.Maciocia PM. Inflammatory signaling in pulmonary hypertension:the controversial role of CRP, and the search for new therapies. Cardiovasc Ther. 2010;28:1–4. doi: 10.1111/j.1755-5922.2009.00128.x. [DOI] [PubMed] [Google Scholar]
- 9.Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, et al. Production of the soluble pattern recognition receptor ptx3 by myleoid, but not plasmacytoid, dendtritic cells. Eur J Immunol. 2003;33:2886–93. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Garlanda C, Doni A, Botazzi B. Pentraxins in innate immunity: From c-reactive protein to the long pentraxin ptx3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 11.Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr. 2011;23:298–304. doi: 10.1097/MOP.0b013e3283464a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haworth SG, Beghetti M. Assessment of endpoints in the pediatric population: congenital heart disease and idiopathic pulmonary arterial hypertension. Curr Opin Pulm Med. 2010;16(Suppl 1):S35–41. doi: 10.1097/01.mcp.0000370209.45756.a1. [DOI] [PubMed] [Google Scholar]
- 13.van Loon RL, Roofthooft MT, van Osch-Gevers M, Delhaas T, Strengers JL, Blom NA, et al. Clinical characterization of pediatric pulmonary hypertension: complex presentation and diagnosis. J Pediatr. 2009;155:176–82. doi: 10.1016/j.jpeds.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Fasnacht MS, Tolsa JF, Beghetti M. The Swiss registry for pulmonary arterial hypertension: The paediatric experience. Swiss Med Wkly. 2007;137:510–3. doi: 10.4414/smw.2007.11895. [DOI] [PubMed] [Google Scholar]
- 15.Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87:440–6. doi: 10.1161/01.cir.87.2.440. [DOI] [PubMed] [Google Scholar]
- 16.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 17.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 18.Price LC, Wort SJ, Perros F, Dormüller P, Huertas A, Montani D, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012;41:210–21. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 19.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: A perspective. Eur Respir J. 2005;26:1110–8. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 20.Price LC, Wort SJ, Perros F, Dormüller P, Huertas A, Montani D, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–21. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 21.Tuder RM, Voelkel NF. Pulmonary hypertension and inflammation. J Lab Clin Med. 1998;132:16–24. doi: 10.1016/s0022-2143(98)90020-8. [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishnan S, Kukreti BB, Salahudin S, Pendharkar A, Karthikeyan G, Bhargava B, et al. Inflammatory markers are elevated are in Eisenmenger syndrome. Pediatr Cardiol. 2013;34:1791–6. doi: 10.1007/s00246-013-0715-3. [DOI] [PubMed] [Google Scholar]
- 23.Tamura Y, Ono T, Kuwana M, Inoue K, Takei M, Yamamoto T, et al. Human Pentraxin 3 (PTX3) as novel biomarker for the diagnosis of pulmonary hypertension. PLOS One. 2012;7:1–5. doi: 10.1371/journal.pone.0045834. [DOI] [PMC free article] [PubMed] [Google Scholar]


