Abstract
ADHD is characterized by increased intra-individual variability in response times during the performance of cognitive tasks. However, little is known about developmental changes in intra-individual variability, and how these changes relate to cognitive performance. Twenty subjects with ADHD aged 7–24 years and 20 age-matched, typically developing controls participated in an fMRI-scan while they performed a go-no-go task. We fit an ex-Gaussian distribution on the response distribution to objectively separate extremely slow responses, related to lapses of attention, from variability on fast responses. We assessed developmental changes in these intra-individual variability measures, and investigated their relation to no-go performance. Results show that the ex-Gaussian measures were better predictors of no-go performance than traditional measures of reaction time. Furthermore, we found between-group differences in the change in ex-Gaussian parameters with age, and their relation to task performance: subjects with ADHD showed age-related decreases in their variability on fast responses (sigma), but not in lapses of attention (tau), whereas control subjects showed a decrease in both measures of variability. For control subjects, but not subjects with ADHD, this age-related reduction in variability was predictive of task performance. This group difference was reflected in neural activation: for typically developing subjects, the age-related decrease in intra-individual variability on fast responses (sigma) predicted activity in the dorsal anterior cingulate gyrus (dACG), whereas for subjects with ADHD, activity in this region was related to improved no-go performance with age, but not to intra-individual variability. These data show that using more sophisticated measures of intra-individual variability allows the capturing of the dynamics of task performance and associated neural changes not permitted by more traditional measures.
Keywords: Development, ADHD, Functional MRI, ICA, Response variability, Ex-Gaussian
Highlights
-
•
We fit an ex-Gaussian distribution on the response distribution to separate variability on fast and slow responses.
-
•
We assessed how ex-Gaussian measures of variability and their relation to no-go performance changed over development.
-
•
Our subjects were individually matched for age.
-
•
The ex-Gaussian measures explained a larger proportion of variance and were better predictors of task performance.
-
•
Variability on fast responses was differentially related to task performance and activity in the dACG in each group.
1. Introduction
ADHD is a common developmental disorder that affects approximately 5% of all children. An estimated 50% of affected children outgrow the disorder (Lara et al., 2009; Schweren et al., 2013), suggesting that development itself may be a factor in the etiology of ADHD. It is becoming increasingly clear that ADHD is heterogeneous, with differences between affected individuals in cognitive deficits and the underlying neurobiology (Durston et al., 2011; Sonuga-Barke, 2005a). However, one surprisingly consistent finding is that individuals with ADHD show more intra-individual variability in the timing of responses during neurocognitive tasks than controls (Alderson et al., 2007; Castellanos et al., 2005; Klein et al., 2006; Simmonds et al., 2007; Wodka et al., 2007). Intra-individual variability reflects temporal variation within an individual's performance on a cognitive task. This temporal variation is reflected in the shape of the response time distribution. One approach to understand how between-subject differences affect intra-individual variability is to explicitly investigate their effect on parameters that describe the response time distribution.
Most often, response time (RT) variability is computed on the basis of the mean and standard deviations of reaction times across a task, resulting in a single point estimate of variability across the task. The use of such RT measures assumes that the underlying response distribution is normal (Gaussian). However recent studies have shown that this assumption does not hold true in ADHD, due to infrequent extremely slow responses (in the absence of extremely fast responses) (Buzy et al., 2009; Epstein et al., 2009; Hervey et al., 2006; Leth-Steensen et al., 2000). These extremely slow responses have been linked to attentional lapses, where the subject is momentarily distracted from performing the task, and to which individuals with ADHD may be more prone than controls. By separating extremely slow responses from the distribution of faster responses in the analyses, one is able to estimate both more accurately. One way to achieve this is to use the ex-Gaussian distribution model. This distribution is a convolution of a normal (Gaussian) distribution, with mean mu (μ) and standard deviation sigma (σ) and an exponential distribution with mean tau (τ). Tau represents the positive skew in the data, or the variability of slow responses, whereas mu and sigma approach the mean and standard deviation of the distribution of fast responses.
The shape of the response time distribution is very sensitive to differences between subjects such as in age and task performance (Heathcote et al., 1991; Schmiedek et al., 2007; Tse et al., 2010). During childhood and adolescence there is a marked decrease in intra-individual variability (Li et al., 2009, 2004; MacDonald et al., 2009; Williams et al., 2007, 2005). The rate at which response variability decreases over age differs between response variability on fast responses and slow responses (McAuley et al., 2006; Williams et al., 2005). This suggests that there may be separable neural mechanisms that give rise to variability on fast and slow responses. This idea is supported by studies that have specifically investigated variability in slow responses, or attentional lapses, as captured by the ex-Gaussian parameter tau. Attentional lapses have been linked to interference from the default mode network (DMN), a subset of regions which show coherent activity, suppression of which appears instrumental in successful task performance (Christakou et al., 2013; Fassbender et al., 2009; Liddle et al., 2011; Sonuga-Barke, 2005a; Weissman et al., 2006). Response variability on fast responses on the other hand, as captured by sigma, has been associated with inefficiencies in neural processing related to altered neurotransmitter modulation, and decreased white matter integration between higher order control regions, and as such is tightly linked to between-subject differences in top down control functions (Bellgrove et al., 2004; Bunce et al., 2007; MacDonald et al., 2009; Stuss et al., 2003; West et al., 2002).
In this study we set out to investigate the relation between age-related changes in intra-individual variability and performance on a cognitive control task, in subjects with ADHD and typically developing subjects, aged 7–24 years old. There is evidence that response variability in ADHD may be related to an increase in attentional lapses, as captured by tau (Buzy et al., 2009; Hervey et al., 2006; Leth-Steensen et al., 2000; Vaurio et al., 2009). At the same time, neuroimaging studies of children with ADHD have suggested that cortical maturation may be delayed in ADHD, which might result in increased sigma compared to typically children (Castellanos et al., 2002; Seidman et al., 2005; Shaw et al., 2013; Shaw and Rabin, 2009; Tamnes et al., 2012). Given these findings, we hypothesized that tau and sigma would both show different developmental trajectories in ADHD. In addition we hypothesized that in both groups sigma would be stronger predictor of no-go task performance than tau. To address the neural underpinnings of any developmental changes, we collected fMRI scans during task performance for all participants.
2. Materials and methods
2.1. Ethics statement
This study was conducted in agreement with the Declaration of Helsinki (Edinburgh Amendments). It was conducted in accordance to the requirements of ICH Good Clinical Practice and the recommendations of the World Health Organization (WHO). The medical–ethical review board of the University Medical Center Utrecht approved the study and its procedures.
2.2. Participants
Twenty-two subjects with ADHD aged 7–24 years, and 22 individually age-matched typically developing controls participated in this study. Participants were recruited through the Department of Psychiatry at the University Medical Center Utrecht in the Netherlands (ADHD) and through the local community and schools (controls).
Subjects with ADHD were required to have received a clinical ADHD diagnosis from our department and additionally to meet DSM-IV criteria for ADHD, as assessed by standardized diagnostic interview (DISC-IV) (Shaffer et al., 2000) or MINI (for subjects older than 16) (Sheehan et al., 1998) at the time of the study. Ten subjects with ADHD met the criteria for ADHD combined type, seven for ADHD inattentive subtype and five for the hyperactive/ impulsive subtype. Three subjects with ADHD met the criteria for ODD. Twelve subjects with ADHD were on stimulant medication; all discontinued medication for at least 24 h prior to the scan. No subjects with ADHD took additional psychoactive medication. Inclusion criteria for control subjects included having no past or current neurological disorder or psychiatric diagnosis, as confirmed on DISC-IV or MINI, other than a single phobia. Two control subjects met the criteria for single phobia, as did two subjects with ADHD. Signed informed consent was obtained from adult subjects and from parents for subjects aged younger than 18 years. Children and adolescents signed for assent. IQ was assessed for all participants using the Wechsler Intelligence Scale for Children, third edition (WISC-III, ages 7–15 years) or Wechsler Adult Intelligence Scale, third Edition (WAIS-III, from age 16 years). All participants had a total IQ score > 75. Sample characteristics are listed in Table 1.
Table 1.
Subject characteristics for the 40 subjects included in the fMRI analyses.
| NC (n = 20) | ADHD (n = 20) | P | |
|---|---|---|---|
| Gender (M/F) | 14/6 | 17/3 | χ2(1,40) = 1.3; p = |
| Age (years) | 15.1 (5.0) 7–24 | 15.6 (4.4) 8–23 | t(38) = 2.9; p = 0.79 |
| TIQ | 116.7 (17.1) 84–152 | 101.6 (14.9) 75–129 | t(38) = –.27; p = 0.006⁎ |
| Hand preference (R/L/ambidexter) | 20/0/0 | 18/0/2 | χ2(1,40) = 2.1; p = 0. |
| ADHD subtype (combined/hyperactive/inattentive) | 0/0 | 11/2/7 | − |
p< 0.05
2.3. Go no-go paradigm
All subjects participated in an fMRI-session during which they performed a parametric go no-go paradigm, as described previously (Durston et al., 2002a; Durston et al., 2002b; Durston et al., 2006, 2003). The instructions were to press a button in response to visually presented stimuli as quickly as possible, but to avoid responding to a rare non-target. The task consisted of 5 runs, which lasted 3 min and 56 s each. Each run contained a total of 57 trials, with 75% go-trials, resulting in a total of 70 no-go trials, including 20 of each type (with 1, 3, or 5 preceding go trials, representing increasing levels of task-difficulty) per subject. Previous work with this task has shown that subjects with and without ADHD make more errors on trials that are preceded by more go-trials (Durston et al., 2002; Durston et al., 2002a; Durston et al., 2006, 2003). Foil trials (no-go trials after 2 or 4 go trials) were also included, to prevent subjects learning the pattern. The order of presentation of the different types of no-go trials was pseudorandomized. In order to make the task more interesting for children, characters from the Pokemon cartoon series were used as stimuli. Stimulus duration was 500 ms and the interstimulus interval was 3500 ms (total trial length 4000 ms). Stimuli were projected using a through-projection screen and slide projector. Responses were collected using an MRI compatible air pressure button box. The fMRI task was followed by the T1 acquisition, which lasted 10 min, during which subjects watched a DVD of their choice.
2.4. Behavioral data analysis
Behavioral data were analyzed using the SPSS statistical package (SPSS for Mac, release 19, 2011. Chicago: SPSS Inc.). We calculated accuracy on go-trials and accuracy on no-go trials as the proportion correct responses, both across the whole task and for the different parametric conditions separately, and mean reaction time (RT) for correct responses on go-trials. The traditional measure of within subject variability, the intra-individual coefficient of variation (ICV), was calculated as the standard deviation of the RT on go trials, divided by the mean RT. We obtained ex-Gaussian parameters (μ, σ and τ) for each subject by fitting the ex-Gaussian distribution to the RT data from correct go trials with maximum likelihood estimation, using the Simplex routine (Nelder et al., 1965) implemented in Matlab (https://github.com/bramzandbelt/exgauss). To check whether the ex-Gaussian fit was successful, we inspected the ex-Gaussian Probability Density Function (PDF), against the RT distribution for each individual subject. We used the Mahlanobis distance (D2) to identify subjects with extreme outlier responses on all outcome measures. For each group we calculated the Pearson correlation between age and all outcome measures. To test for differences between groups in the relation between age and outcome variables, we ran a regression analysis using a dummy variable for group: this explicitly tests whether the relationship between response variability measures and no-go accuracy, as described by the regression slope, differs between groups. To investigate how groups differed in the relationship between age and task performance we used stepwise regression to determine the best model for each group separately.
2.5. fMRI acquisition
Before MRI acquisition all participants participated in a practice session in a mock MRI-scanner, located in the NICHE Laboratory at the Department of Psychiatry at the University Medical Center Utrecht. The purpose of this session was to acquaint subjects with the scanner environment, the fMRI paradigm and the researchers present during the fMRI-scan, in order to reduce potential anxiety (Durston et al., 2009). Only in the case of a successful mock-session did participants take part in the actual MRI session. Data were acquired on a 3.0 T Philips Achieva MR scanner (Philips Medical Systems, Best, the Netherlands). A total of 595 functional images were acquired during the task (5 runs of 119 images) with 2D EPI-SENSE (TR = 2000 ms, TE = 35 ms, flip angle = 70°, matrix = 68 × 66, voxel size 3 × 3.5 × 3.5, 35 slices, FOV 240 × 119 × 240 mm). Following the task, a high-resolution (0.75 × 0.75 × 0.8 mm) coronal three-dimensional fast-field echo T1-weighted image was acquired in order to allow for spatial normalization and visualization (TR = 10 ms, TE = 4.6 ms flip angle = 8°, matrix = 304× 299, FOV 24 cm. 200 slices, FOV 240 × 240 × 116 mm).
2.6. fMRI image preprocessing
FMRI-data were preprocessed using SPM5 (Wellcome Dept of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk) Preprocessing consisted of the following steps: (1) rigid body correction for inter-frame head motion within and across runs, unwarping of the images to remove any residual variance caused by (task-related) movement. Estimated movement parameters were individually inspected to ensure that movement did not exceed 3 mm or the size of one voxel. None of the 40 subjects included in the final analysis had more than 3 mm motion. (2) Co-registration of functional and anatomical images. (3) Segmentation of anatomical scans into gray and white matter, and (4) normalization of both anatomical and functional scans to Montreal Neurological Institute (MNI) template, using the segmentation parameters obtained in step 3. We chose to normalize to one common template across the age range, as studies have shown that this is a feasible approach for the age range included in this study (Burgund et al., 2002; Kang et al., 2003). Following normalization, all images were visually inspected to check whether normalization had been successful. (6) Spatial smoothing using a 6-mm full-width at half-maximum Gaussian kernel. For each subject, average scan–scan rotation and translation from the realignment parameters were calculated to evaluate potential age effects on subject motion.
2.7.1. fMRI data analysis: Independent Component Analysis (ICA)
In this study we used Independent Component Analysis (ICA) to analyze our data. ICA decomposes functional imaging data into spatially independent, but temporally coherent brain networks, each with their own time course (Calhoun et al., 2006, 2009, 2002; McKeown et al., 1998). ICA has several advantages over GLM methods: 1) Decomposing the brain into networks greatly reduces the number of multiple comparisons, and because of this ICA is more sensitive than GLM analysis which is performed on each voxel separately (Congdon et al., 2010; McKeown et al., 2003) 2) The networks identified by ICA can occur concurrently within the same voxel: one voxel can be involved in multiple (temporally) different responses. For this reason, ICA can detect activity that might be hidden in traditional GLM analysis (Beldzik et al., 2013; Xu et al., 2013) (Beldzik et al., 2013; Xu et al., 2013). 3) Lastly, ICA circumvents a common problem in developmental imaging, which is that group differences in task performance can affect the estimation of the fMRI results, either through reduced power as a result of fewer correct trials, or through group differences in the strategy applied to solve a task. Because ICA is model free, and thus independent of task performance, its results are components that are present across all subjects.
ICA analysis consisted of three steps. First, individual and group fMRI data were decomposed into spatially and temporally independent and coherent networks. Second, we identified networks that were related to the task. Third, we investigated how inter-individual differences in response variability were reflected in inter-individual differences in network activation. These steps are described in detail below.
2.7.2. ICA network identification
We wanted to minimize the effects of subject motion and other artifacts by using ICA to identify and remove artifacts from the data. To this end, ICA analysis was run for each subject individually, before entering the group ICA (GICA). For each subject, components were estimated using the Infomax algorithm (Bell and Sejnowski, 1995) available in the Group ICA of fMRI toolbox (GIFT, http://icatb.sourceforge.net, version 2.e) implemented in Matlab. This estimation was repeated 20 times in Icasso (http://cis.hut.fi/projects/ica/icasso) to get an estimate of reliability (Himberg et al., 2004). All components had a cluster quality index greater than .8, indicating a highly stable ICA decomposition. The average dimensionality of the data was estimated by the modified minimum description length (MDL) algorithm criteria (Li et al., 2007) to account for correlated samples. This was followed by back reconstruction (GICA 3) where each individual's functional networks were reconstructed from the raw data using the ICA mixing matrix, resulting in subject-specific maps and time series. Each participant's components were then scaled to reflect percent signal change. At the individual subject level, a systematic process was used to identify components to be retained for analysis: (1) each spatial map was inspected for the presence of obvious artifacts (e.g., edges, ventricles, scanner artifacts); (2) the temporal association of each subject's components with their movement parameters was used to exclude components reflecting signal variation due to motion. Components were discarded if the partial correlation between component and motion parameters, corrected for correlation with task events, exceeded p ≤ 0.001 (corrected for multiple comparisons). All components that were determined to be artifacts according to these two criteria were removed from the raw data, and modified data were then written and re-analyzed at group level.
For the group analysis, one group ICA was run on data from all 40 participants, after two outliers and their individually matched controls were removed. Components were estimated using the Infomax algorithm, repeated 20 times in Icasso. All components had a cluster quality index greater than .8, indicating a highly stable ICA decomposition. Subject-specific spatial maps and temporal components were estimated using GICA3. Time-series were Z-scaled. The spatial overlap between the back reconstructed components (the spatial maps) and a priory probability maps as provided in SPM5 was calculated, using multiple regression. Components that showed a spatial overlap greater than r2 ≥ 0.025 with white matter or CSF were discarded. Coding for subject status (ADHD vs. controls) was done after the ICA analysis.
2.7.3. Identification of task related networks
We were interested in those components involved in the task. For each subject, we regressed the back-reconstructed component time courses onto the task model. This approach is analogous to standard GLM fMRI, except that component time courses rather than voxel time courses are used. So, the beta values from this regression represent the degree to which the component was modulated by the task event. Components with a significant beta weight with either go or no-go events following a T-test were selected (p ≤ 0.05 Bonferroni corrected). We then ran an ANOVA on the beta weights to investigate whether any of those components were more closely related to movement than task events (p ≤ 0.05 Bonferroni corrected). As we were also interested in Default Mode intrusions (i.e., attentional lapses), we further selected components that had a spatial overlap greater than r2 ≥ 0.025 with two templates for the DMN (vDMN and dDMN) from a spatial atlas (Shirer et al., 2012).
2.7.4. Relation between group membership and activation within networks
We then investigated how between subject differences were related to the recruitment of the selected networks. To investigate age related differences within each group, both groups were split into a younger and an older group (median split at 15 years). We included group status (younger ADHD, older ADHD, younger controls and older controls), ex-Gaussian measures (mu, sigma and tau), and task performance (no-go accuracy) as predictors, as well the interaction terms between group and performance measures. This categorical operationalization of age allowed us to formally compare the neural activity related to the interaction between age and behavioral measures, both within and between groups. This is important given that the effect of age may differ between the groups, and this would have been obscured if age had been entered as a dimensional (continuous) variable. An additional analysis including age as a continuous analysis was also run. As ADHD subjects discontinued any stimulant medication 24 h before the MRI, medication withdrawal effects may have affected any age-related findings within this group. Therefore we tested whether the older and younger ADHD groups differed in the number of subjects that discontinued medication, as well as for main and interaction effects of medication on the outcome variables. We used a multivariate model selection strategy as implemented in the MANCOVAN toolbox in GIFT (see Allen et al., 2011). This analysis is analogous to a standard ANOVA F-test, and performs backwards model selection by testing whether each predictor (group, measures of task performance) explains variability in the multivariate fMRI results, using a multivariate analysis of covariance. The multivariate selection process is important to select covariates that show an overall effect within networks, but the multivariate output is difficult to interpret. Therefore the toolbox runs a standard univariate analysis of variance (ANOVA) on the reduced model, to test for specific covariate effects. During the univariate analysis the relationship between predictors and variables is calculated as the partial correlation coefficient, adjusted for correlation between predictors. Outcome measures are spatial maps (SMs), indicating activity within components that is thus uniquely related to predictors. Before entering the MANCOVAN these spatial maps were thresholded to select voxels most representative for the component. Thresholding was based on the distribution of voxelwise T-statistics with cutoff t > μ + 4σ. This selects voxels with T-values that fit a normal-gamma–gamma distribution (NGG), as opposed to a normal distribution that is characteristic of noise (Allen et al., 2011). The univariate results were corrected using False Discovery Rate (FDR, Genovese et al., 2002).
3. Results
3.1. Traditional measures of task performance
Both groups were equally able to perform the task across all levels of difficulty, as indexed by their performance: mean accuracy on go-trials was 99% (sd = 0.02) in the control group and 99% (sd = 0.03) in the ADHD group (t38 = .72, p = .44); mean no-go accuracy (proportion correct) was 88% for control subjects and 84% for subjects with ADHD (t38 = .21, p = 0.32). Response time on go-trials did not differ between groups (mRT; t38 = .9, p = 0.47; sdRT t38 = .8, p = .98), and neither did within-subject variation (ICV; t38 = 1.3, p = .79) (Table 2). Groups did not differ in the effect of age on traditional measures of task performance: for both groups both RT measures (mean and sd) decreased with age, and no-go accuracy increased. Within the ADHD group, there were no effects of ADHD subtype on task performance.
Table 2.
Task performance for the 40 subjects included in the fMRI analyses.
| NC (n = 20) | ADHD (n = 20) | P | |
|---|---|---|---|
| Mean RT go trials (ms) | 616.5 (77.1) 458–764 | 632.4 (66.7) 455–743 | 0.47 |
| sdRT go trials (ms) | 139.1 (59) 72.3–328.6 | 139.6 (74.2) 74.3–336.4 | 0.98 |
| Accuracy no-go trials | 0.88 (0.1) 0.6-1 | 0.84 (0.12) 0.46-1 | 0.32 |
| Accuracy no-go 1/3/5 trials | .88 (.1)/.88 (.14)/86 (.1) | .9 (.12)/.82 (.15)/.82 (.15) | 0.83/0.12/0.28 |
| Accuracy go trials | 0.99 (0.02) 0.94-1 | 0.99 (0.03) 0.9-1 | 0.44 |
| ICV | 0.23 (0.08) 0.1–0.5 | 0.22 (0.1) 0.1–0.6 | 0.79 |
| Mu | 504.8 (57) 376–599 | 527 (53) 397–595 | 0.21 |
| Sigma | 67.2 (17.8) 35–110 | 62.7 (13.4) 43–94 | 0.38 |
| Tau | 101.9 (41.7) 47–198 | 100.6 (38.9) 57–214 | 0.92 |
3.2. Ex-Gaussian measures of task performance
Overall, the ex-Gaussian measures of task performance did not differ between groups (mu t38 = 1.3, p = .21; sigma t38 = .9, p = .38; tau, t38 = .1, p = .92). However, there was a between-group difference in the relationship these measures had with age: for controls, both the variability related to extremely slow responses (tau) and variability on fast responses (sigma) decreased with age (r = –.45; p < 0.05, and r = –.55; p < 0.01 respectively). For subjects with ADHD, tau was not related to age, but mu (mean response time) and sigma decreased with age (r = –.58; p < 0.01 and r = .5; p < 0.03 respectively) (Table S1).
From the stepwise regression, the single best predictor of no-go performance for controls was sigma (F19 (11) p < 0.005). As such, controls with lower variability in reaction time on faster responses had better no-go performance. For subjects with ADHD, there was no predictor for overall no-go performance. The best predictor of performance on easier no-go trials (following one go-trial) was average response time (mu), where higher average response time predicted better performance on no-go trials. Age best predicted performance on more difficult no-go trials (following 5 go-trials) for subjects with ADHD (Table 3).
Table 3.
Results of stepwise regression, using backwards selection to select the variables with best predictive value for no-go performance for each group (criterion F-probability < 0.1). Results are given for each no-go condition (no-go 1/3/5: no-go trials preceded by 1.3 or 5 go trials respectively) separately from the total, since the occurrence of extremely slow responses captured by tau is influenced by the number of preceding trials.
| Predictors in control group | p value | R2 | Predictors in ADHD group | p value | R2 | |
|---|---|---|---|---|---|---|
| No-go 1 | Sigma | F19 (9.5) p < 0.006 | 0.35 | Mu | F19 (5.9) p < 0.025 | 0.25 |
| No-go 3 | − | n.s. | − | n.s. | ||
| No-go 5 | Sigma and tau | F19 (−8.5) p < 0.003 | 0.5 | Age | F19 (6) p < 0.025 | 0.25 |
| No-go overall | Sigma | F19 (11) p < 0.005 | 0.37 | − | n.s |
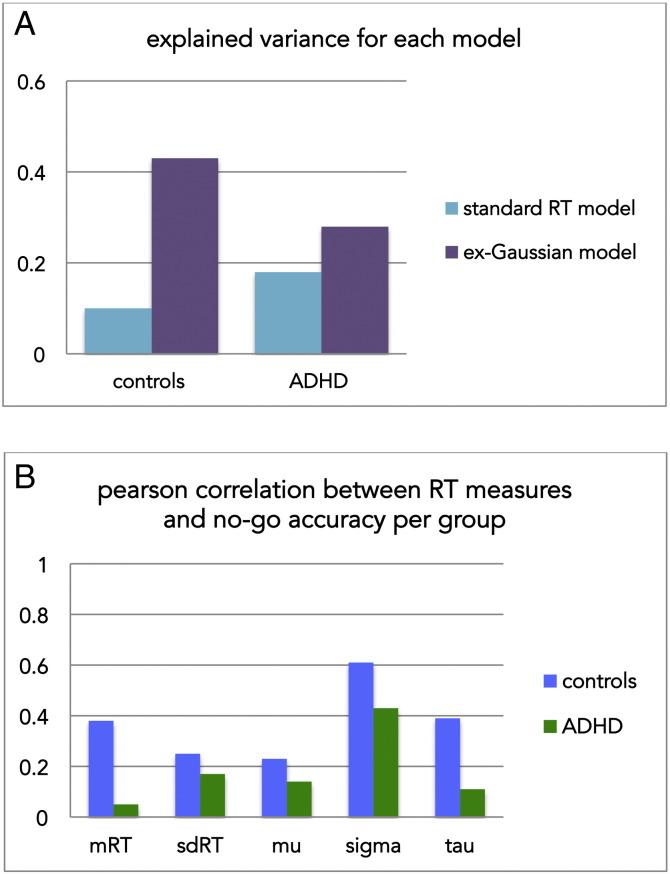
Importantly, in both groups the ex-Gaussian parameters were a much better predictor of no-go performance than the mean and standard deviation of the reaction times, as was reflected by the explained variance (R2 = .1 vs. R2 = .43 respectively for NC; R2 = .18 vs. R2 = .28 for the ADHD group; see Fig. 1).
Fig. 1.
Groups differ in the relationship between no-go accuracy and response times. This difference is better described by the ex- Gaussian parameters mu, sigma and tau that describe the response time distribution, than the standard RT model, as shown in panel A, which shows explained variance (R2) for each set of predictors. Panel B shows the Pearson correlation between RT measures and no-go accuracy separately for each group.
There were more subjects who discontinued medication in the younger ADHD group than in the older group (7 and 3 respectively; χ2 (1, N = 20) = 5.05, p = 0.025). However medication status did not mediate any of the effects of age on either the ex-Gaussian parameters or no-go performance within the ADHD group, and was therefore not included in further analyses.
3.3.1. Selection of networks
For individual subjects, an average of 36 components were estimated with ICA. On average, eight components were excluded following inspection for artifacts (e.g., edges, ventricles, scanner artifacts). These cleaned data were the basis for the group ICA. Translation and rotation parameters did not differ between groups (translation T38(.2) p > .86, rotation (T38 = .13) P > .9) and were not affected by age (translation r = .19, p = > 0.24; rotation r = .11, p = > 0.5), suggesting that motion correction did not introduce any artificial age effects.
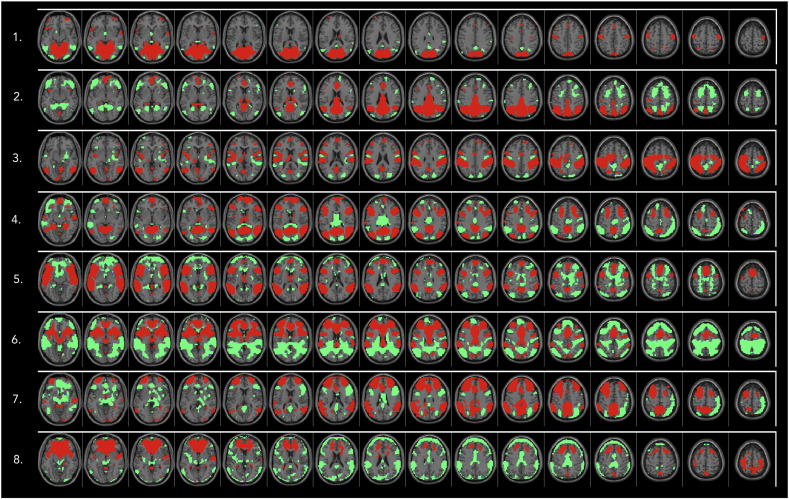
Over the whole group, 34 components were estimated. Ten components showed a significant spatial overlap with white matter or CSF and were discarded. Eight components had a significant relation (beta value) with go or no-go events in the temporal regression, indicating that their time-courses showed high synchrony with the task events. Of those components, one was more closely related to the movement parameters than the task events (ANOVA) and was therefore excluded. One component was added as it had a significant spatial correlation with the DMN template, resulting in a set of eight components that were carried forward to the MANCOVA analysis. Fig. 2 shows an overview of all selected components.
Fig. 2.
T-maps of the ICA components entered in the MANCOVAN analysis. Thresholded at T > 6 (red) and T < –6 (green).
3.3.2. Between-subject task performance and network activity
In line with the behavioral results, group status by itself (when age was not considered) did not predict activity within the selected networks. Between-subject differences in no-go accuracy, ex-Gaussian measures or standard RT measures did not predict activation within the selected networks, nor did the interaction between group and these task measures.
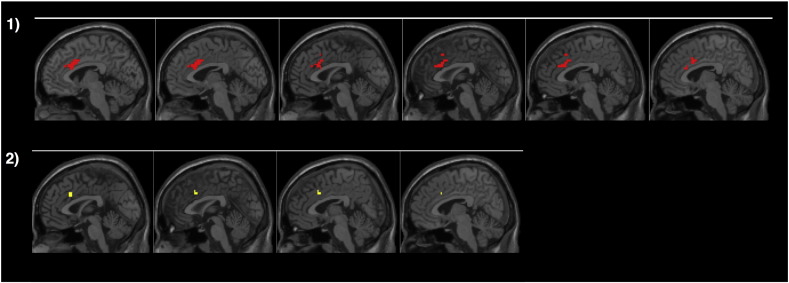
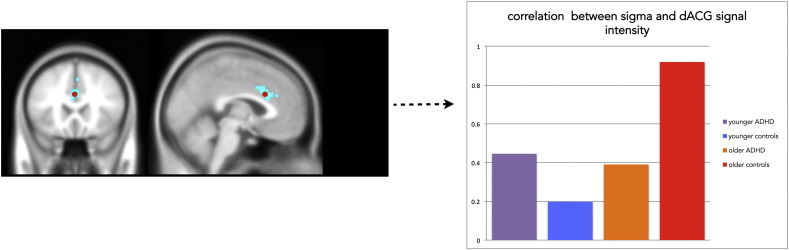
However, groups differed in activation related to the effect of age on task performance: for the control group, a decrease in sigma with age, reflecting lower response variability on fast responses in older participants, was related to increased activity in dorsal ACG. In ADHD, increased activity in this same region was related to a decrease in the accuracy on no-go trials, but not to sigma (Fig. 3). Fig. 4 shows the changes in neural activity in this region of dACG related to sigma for both groups. Tau was not related to changes in neural activity within or between groups.
Fig. 3.
Activity related to sigma and task performance on the no-go 5 condition with increasing age in control subjects (panel 1) and ADHD subjects (panel 2). Panel 1: increased activation in the dACG with decreasing sigma over age in the control group, p < 0.05 (FDR corrected). Panel 2: results for the ADHD group, showing activation related to improved no-go 5 task accuracy over age p < 0.05 (FDR corrected).
Fig. 4.
dACG region is uniquely sensitive to developmental changes in variability on quick responses (sigma) in the control group, but not in subjects with ADHD: the plot shows Spearman's correlations between sigma and signal intensity in the dACG region for younger subjects (age 7–15 years) and older subjects (15–24 years), for both control subjects and subjects with ADHD. The graph shows data from a 5 mm sphere at (0, 20, 29) within the significant cluster visible in top panel. For the purposes of visualization, correlation values are absolute.
In the analysis including age as a continuous measure, there were no interactive effects of age and group on brain activity, see Fig. S1.
4. Discussion
In this study we investigated ex-Gaussian measures of response variability, representing both on-task variability in performance (sigma) and attentional lapses (tau). We set out to study developmental differences in these measures and their relationship to task performance between subjects with ADHD and typically developing subjects. We found that the ex-Gaussian measures of intra-individual response variability were better predictors of task performance than standard RT measures: the proportion of variance explained by these measures was two to four times as great as the proportion explained by traditional measures. Furthermore, variability on fast responses was differentially related to task performance and activity in the dorsal anterior cingulate gyrus (dACG) in each group.
The ex-Gaussian parameters of intra-individual variability were able to capture a developmental difference between the ADHD group and the control group that was not reflected in the more traditional measures of reaction time (mean and sd). Moreover, these developmental differences were differentially related to task performance in each group: controls showed a steady decrease in response variability (both sigma and tau) with age, which predicted no-go accuracy. Subjects with ADHD, on the other hand, showed a reduction in the variability of quick responses (sigma) over age, but not of attentional lapses (tau), and neither of these measures was predictive of no-go accuracy.
In this study, subjects were individually matched for age, and there were no between-group differences in task performance or response variability. Therefore, this result suggests that within each group, for subjects of similar age, successful task performance may be mediated through different neural processes. We investigated these neural underpinnings using fMRI: we divided the groups into two age groups (based on a median split at 15 years). Results showed that the difference in interaction between sigma and task performance within each group was related to a differential pattern of activation in dorsal anterior cingulate gyrus (dACG): for younger subjects, there was no difference between subjects with ADHD and controls in the relationship between activity in this region and sigma. However, for older subjects, there was a clear difference: older control subjects had increased dACG activity, related to the reduction in response variability (sigma), whereas older subjects with ADHD showed no such relationship.
Therefore, these results suggest that in typically developing subjects, and specifically in the adolescents (15–24 years old), improvements in cognitive control with age are linked to both a reduction of response variability, and active involvement of the dorsal ACG. In ADHD, a similar improvement in task performance with age was related to greater activity in dorsal ACG, but this was not related to a reduction in response variability.
These findings are consistent with previous work on the role of the dACG in various functions including conflict monitoring (Botvinick et al., 2004; Carter, 1998) error monitoring (Gehring and Fencsik, 2001; Gehring and Knight, 2000; Holroyd et al., 1998; Lorist et al., 2005), response selection (Awh and Gehring, 1999; Milham et al., 2001; Paus et al., 1993; Paus, 2001) and top down control more generally, as part of a larger frontoparietal control network (Chambers et al., 2009; Corbetta and Shulman, 2002; Kerns et al., 2004; Petersen and Laprell, 1999; Posner et al., 1997). The ACG is relatively late to mature compared to other brain regions, with a protracted development continuing into early adulthood (Gogtay et al., 2004; Sowell et al., 2003, 2001), which is characterized by an increase in connectivity with nearby prefrontal regions (Kelly et al., 2009). Functionally, the development of ACG is associated with improvements in motor and cognitive control during childhood and adolescence (Davidson et al., 2006; Ridderinkhof and Van Der Molen, 1997; Rueda et al., 2005, 2004).
As for the behavioral development of cognitive control, there is evidence that developmental improvements in this ability are not always reflected by improved accuracy, but are sometimes more evident in decreases in reaction time and variability of RT, perhaps reflecting a more efficient speed/accuracy trade-off (Bellgrove et al., 2004; Davidson et al., 2006; Luna et al., 2010; Simmonds et al., 2007). These findings support the notion that greater activity in dACG in our older controls, associated with reduced response variability during the go no-go task, may reflect increased top down control in order to maintain task performance.
In ADHD, decreased dACG activity has consistently been linked to deficits in cognitive control: in fact, hypoactivation in ACG is one of the most common findings in neuroimaging studies of ADHD (Bush, 2011; Dickstein et al., 2006). ADHD has also been suggested to represent a delay in neural maturation (e.g., Shaw et al., 2011). Furthermore, other studies have suggested that lower response variability in ADHD may be associated with prefrontal compensatory activation (Suskauer et al., 2008). As such, ACG has been implicated in both developmental improvements in cognitive control and related to deficits in cognitive control in ADHD. Taken together with our findings, these data suggest that the developmental trajectory of ACG activity and its relation to successful cognitive control may differ between subjects with ADHD and typically developing controls.
We hypothesized that we would find developmental differences between subjects with ADHD and typically developing controls in terms of attentional lapses (tau). Attentional lapses (reflected by tau) were present in both groups; however, they persisted over age in ADHD, whereas the number of attentional lapses decreased with age for typically developing controls. Increased numbers of attentional lapses have been suggested to be a marker of ADHD, in keeping with the idea of increased numbers of DMN intrusions in this disorder (Sonuga-Barke, 2005b). This study suggests that a difference in the number of attentional lapses (tau) between diagnostic groups depends on two factors: first, the age of the sample being tested, and second, on task difficulty. Our findings suggest that an age-related difference between groups in the effect of attentional lapses on task performance was mediated by task difficulty: in the control group, but not the ADHD group, a reduction of the number of attentional lapses with age was a predictor for task performance, but only in the more difficult (no-go 5) condition. Tentative support for the notion of age-dependent differences comes from Leth-Steensen and colleagues (2000). They compared children with ADHD to two typically developing control groups, one younger group (average age 7 years) and one age matched control group (9–13 years). They found a difference in tau when comparing the age-matched groups, but no difference in tau between subjects with ADHD and the younger controls. This suggests that increased tau in ADHD may in fact reflect a developmental delay, in keeping with our result (Leth-Steensen et al., 2000).
Despite behavioral differences in tau, we did not find any differences in neural activity related to this measure between subjects with ADHD and typically developing controls. This could be related to a difference in statistical power between the two analyses, as age was used as a continuous measure in the behavioral analysis and as a categorical on in the imaging analysis. However, even at reduced statistical thresholds, we found no evidence of age-related differences between groups. One explanation could be that the analysis to detect between-group differences in neural activity specifically tested for activation differences within networks. There is increasing evidence that attentional lapses, as captured by tau, are related to more global changes in the interaction between networks (e.g., interference from the default mode network) (Christakou et al., 2013; Fassbender et al., 2009; Sun et al., 2012) and may be mediated by differences in the frequencies of neural oscillation (Adamo et al., 2014; Feige et al., 2013; Helps et al., 2008; Sato et al., 2012) which would not be detected in an fMRI analysis.
This study used Independent Component Analysis (ICA) to investigate neural networks involved in cognitive control, as opposed to the General Linear model (GLM) which is the most predominant method in the analysis of fMRI data. ICA has many advantages over the GLM, but an important advantage in the light of this study is that performance differences between subjects do not affect the estimation of independent components, whereas in the GLM, task performance (the number of successful trials) directly affects the power of the estimated effects. In GLM analyses, activation differences may therefore equally reflect this difference in power, rather than a true difference in activation. The networks resulting from ICA in this study, which were the basis of the between subjects analysis (MANCOVAN), were present across both groups and unaffected by performance. We believe that ICA is a good method to use in developmental imaging studies, where between-subject differences in both performance and neural activation are considerable.
There are some methodological choices that should be taken into account when interpreting our results. First, the splitting of the two diagnostic groups into two age groups resulted in a relatively low number of subjects per age group, especially given the age range. We chose to do this, as we did not want to make the assumption that age-effects would be the same in the diagnostic groups. However, this choice did of necessity reduce our power to detect differences between the groups. As such, the neural activation results should be interpreted with some caution, certainly until they are replicated in a larger sample. Second, the ex-Gaussian parameters represent one of many ways to describe the RT distribution. We chose to use ex-Gaussian parameters over formal models of RT distribution, in order to directly show the difference with standard mean-centered RT measures, as the sum of the ex-Gaussian parameters approaches the mean centered distribution. However, there may be other sets of parameters that can capture developmental differences between groups (Matzke and Wagenmakers, 2009). As such, findings of this study should be validated using other theoretical accounts of the response time distribution (for overview, see (Van Zandt, 2000)).
Third is the issue of multi-colinearity, which is always problematic in investigating the development of functions with shared neural underpinnings. Our regression model relied on the process of backwards elimination and was linear, and as such not sensitive to changing values of predictors due to multi-colinearity.
A last consideration is that the majority of our ADHD subjects were treated with methylphenidate. They discontinued medication for a minimum of 24 h before participating. However, the long-term effects of methylphenidate on neural development are not clear (Schweren et al., 2013).
5. Conclusions
In this study, we investigated developmental changes in response variability in subjects with ADHD and typically developing subjects using parameters from the ex-Gaussian distribution. This permitted us to separate attentional lapses from intra-individual variability on fast responses. Results showed that ex-Gaussian measures were better predictors of developmental improvements in task performance than traditional measures of reaction time. Furthermore, they were differentially related to task performance and neural activity in dACG in each group: in typically developing controls, improvements in task performance with age were related to reduced response variability on fast responses, and increased recruitment of dACG. For subjects with ADHD, a similar improvement in task performance with age was related to recruitment of dACG, but not to reductions in response variability.
The following are the supplementary data related to this article.
Correlations between age and task performance for subjects with ADHD and controls. Significant correlations (p < 0.05) are indicated with an asterisk (*).
When age is operationalized as a continuous variable, we find widespread posterior decreases in activity with age, p < 0.01 uncorrected. Yellow: controls > ADHD group; blue: decreases with age; red increases with age. This analysis yielded no interactive effects between group and age.
Acknowledgements
The authors would like to thank all participants and their parents for their time and effort, and an anonymous reviewer for helpful suggestions. This work was supported by Dutch Science Foundation (NWO) VIDI 016.076.384 and VICI 016.115.602 to Sarah Durston.
References
- Adamo N., Huo L., Adelsberg S., Petkova E., Castellanos F.X., Di Martino A. Response time intra-subject variability: commonalities between children with autism spectrum disorders and children with ADHD. Eur. Child Adolesc. Psychiatry. 2014;23(2):69–79. doi: 10.1007/s00787-013-0428-4. 23716135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson R.M., Rapport M.D., Kofler M.J. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J. Abnorm. Child Psychol. 2007;35(5):745–758. doi: 10.1007/s10802-007-9131-6. 17668315 [DOI] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A.M., Caprihan A., Turner J.A., Eichele T., Adelsheim S., Bryan A.D., Bustillo J., Clark V.P., Feldstein Ewing S.W., Filbey F., Ford C.C., Hutchison K., Jung R.E., Kiehl K.A., Kodituwakku P., Komesu Y.M., Mayer A.R., Pearlson G.D., Phillips J.P., Sadek J.R., Stevens M., Teuscher U., Thoma R.J., Calhoun V.D. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. 21442040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E., Gehring W.J. The anterior cingulate cortex lends a hand in response selection. Nat. Neurosci. 1999;2(10):853–854. doi: 10.1038/13145. 10491598 [DOI] [PubMed] [Google Scholar]
- Beldzik E., Domagalik A., Daselaar S., Fafrowicz M., Froncisz W., Oginska H., Marek T. Contributive sources analysis: a measure of neural networks' contribution to brain activations. Neuroimage. 2013;76:304–312. doi: 10.1016/j.neuroimage.2013.03.014. 23523811 [DOI] [PubMed] [Google Scholar]
- Bell A.J., Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. 7584893 [DOI] [PubMed] [Google Scholar]
- Bellgrove M.A., Hester R., Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. 15381021 [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. 15556023 [DOI] [PubMed] [Google Scholar]
- Bunce D., Anstey K.J., Christensen H., Dear K., Wen W., Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60–64 years. Neuropsychologia. 2007;45(9):2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. 17382358 [DOI] [PubMed] [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. 12482076 [DOI] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. 21489409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzy W.M., Medoff D.R., Schweitzer J.B. Intra-individual variability among children with ADHD on a working memory task: an ex-Gaussian approach. Child Neuropsychol. 2009;15(5):441–459. doi: 10.1080/09297040802646991. 19184779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., van Zijl P.C., Pekar J.J. Independent component analysis of fMRI data in the complex domain. Magn. Reson. Med. 2002;48(1):180–192. doi: 10.1002/mrm.10202. 12111945 [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(Suppl 1):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. 19059344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Calhoun V.D. Unmixing fMRI with independent component analysis. I. E.E.E. Eng. Med. Biol. Mag. 2006;25(2):79–90. doi: 10.1109/memb.2006.1607672. 16568940 [DOI] [PubMed] [Google Scholar]
- Carter C.S. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. 9563953 [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S., Blumenthal J.D., James R.S., Ebens C.L., Walter J.M., Zijdenbos A., Evans A.C., Giedd J.N., Rapoport J.L. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. 12365958 [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J., Scheres A., Di Martino A., Hyde C. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol. Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. 15950016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.D., Garavan H., Bellgrove M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009;33(5):631–646. doi: 10.1016/j.neubiorev.2008.08.016. 18835296 [DOI] [PubMed] [Google Scholar]
- Christakou A., Murphy C.M., Chantiluke K., Cubillo A.I., Smith A.B., Giampietro V., Daly E., Ecker C., Robertson D., Murphy D.G., Rubia K. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol. Psychiatry. 2013;18(2):236–244. doi: 10.1038/mp.2011.185. 22290121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E., Mumford J.A., Cohen J.R., Galvan A., Aron A.R., Xue G., Miller E., Poldrack R.A. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53(2):653–663. doi: 10.1016/j.neuroimage.2010.06.062. 20600962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. 11994752 [DOI] [PubMed] [Google Scholar]
- Davidson M.C., Amso D., Anderson L.C., Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. 16580701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. 17073984 [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. 16445387 [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Worden M.S., Yang Y., Casey B.J. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16(2):449–453. doi: 10.1006/nimg.2002.1074. 12030830 [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Yang Y., Ulug A.M., Zimmerman R.D., Casey B.J. A neural basis for the development of inhibitory control. Dev. Sci. 2002;5:F9–F16. [Google Scholar]
- Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.M., Yang Y., Ulug A.M., Casey B.J. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. 12742674 [DOI] [PubMed] [Google Scholar]
- Durston S., Van Belle J., De Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):1178–1184. doi: 10.1016/j.biopsych.2010.07.037. 20965496 [DOI] [PubMed] [Google Scholar]
- Epstein J.N., Delbello M.P., Adler C.M., Altaye M., Kramer M., Mills N.P., Strakowski S.M., Holland S. Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task. Neuropediatrics. 2009;40(1):1–5. doi: 10.1055/s-0029-1220686. 19639521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. 19281801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B., Biscaldi M., Saville C.W., Kluckert C., Bender S., Ebner-Priemer U., Hennighausen K., Rauh R., Fleischhaker C., Klein C. On the temporal characteristics of performance variability in attention deficit hyperactivity disorder (ADHD) PLOS One. 2013;8(10):e69674. doi: 10.1371/journal.pone.0069674. 24204553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Fencsik D.E. Functions of the medial frontal cortex in the processing of conflict and errors. J. Neurosci. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. 11717376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. 10769394 [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. 11906227 [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N. Proc. Natl Acad. Sci, U.S.A. 2004. Dynamic mapping of human cortical development during childhood through early adulthood.15148381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote A., Popiel S.J., Mewhort D.J. Analysis of response time distributions: An example using the Stroop task. Psychol. Bull. 1991;109(2):340–347. [Google Scholar]
- Helps S., James C., Debener S., Karl A., Sonuga-Barke E.J. Very low frequency EEG oscillations and the resting brain in young adults: a preliminary study of localisation, stability and association with symptoms of inattention. J. Neural Transm. 2008;115:279–285. doi: 10.1007/s00702-007-0825-2. 17994187 [DOI] [PubMed] [Google Scholar]
- Hervey A.S., Epstein J.N., Curry J.F., Tonev S., Eugene Arnold L., Keith Conners C., Hinshaw S.P., Swanson J.M., Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12(2):125–140. doi: 10.1080/09297040500499081. 16754533 [DOI] [PubMed] [Google Scholar]
- Himberg J., Hyvärinen A. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. 15219593 [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Dien J., Coles M.G. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci. Lett. 1998;242(2):65–68. doi: 10.1016/s0304-3940(98)00035-4. 9533395 [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. 12781724 [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T., Margulies D.S., Castellanos F.X., Milham M.P. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. 18653667 [DOI] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., Cho R.Y., Stenger V.A., Carter C.S. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. 14963333 [DOI] [PubMed] [Google Scholar]
- Klein C., Wendling K., Huettner P., Ruder H. Intra-subject variability in attention-deficit hyperactivity disorder. Biol. Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. 16806097 [DOI] [PubMed] [Google Scholar]
- Lara C., Fayyad J., de Graaf R., Kessler R.C., Aguilar-Gaxiola S., Angermeyer M., Demytteneare K., de Girolamo G., Haro J.M., Jin R., Karam E.G., Lépine J.P., Mora M.E., Ormel J., Posada-Villa J., Sampson N. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol. Psychiatry. 2009;65(1):46–54. doi: 10.1016/j.biopsych.2008.10.005. 19006789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C., Elbaz Z.K., Douglas V.I. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. 10900704 [DOI] [PubMed] [Google Scholar]
- Li S.-C., Hämmerer D., Müller V., Hommel B., Lindenberger U. Lifespan development of stimulus-response conflict cost: similarities and differences between maturation and senescence. Psychol. Res. 2009;73(6):777–785. doi: 10.1007/s00426-008-0190-2. 19023594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-C., Lindenberger U., Hommel B., Aschersleben G., Prinz W., Baltes P.B. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol Sci. 2004;15(3):155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. 15016286 [DOI] [PubMed] [Google Scholar]
- Li Y.O., Adalı T., Calhoun V.D. Estimating the number of independent components for functional magnetic resonance imaging data. Hum. Brain Mapp. 2007;28(11):1251–1266. doi: 10.1002/hbm.20359. 17274023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle E.B., Hollis C., Batty M.J., Groom M.J., Totman J.J., Liotti M., Scerif G., Liddle P.F. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J. Child Psychol. Psychiatry Allied Discip. 2011;52(7):761–771. doi: 10.1111/j.1469-7610.2010.02333.x. 21073458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorist M.M., Boksem M.A., Ridderinkhof K.R. Impaired cognitive control and reduced cingulate activity during mental fatigue. Brain Res. Cogn. Brain Res. 2005;24(2):199–205. doi: 10.1016/j.cogbrainres.2005.01.018. 15993758 [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. 19765880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald S.W., Li S.C., Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychol. Aging. 2009;24:792–808. doi: 10.1037/a0017798. 20025396 [DOI] [PubMed] [Google Scholar]
- Matzke D., Wagenmakers E.-J. Psychological interpretation of the ex-Gaussian and shifted Wald parameters: a diffusion model analysis. Psychon. Bull. Rev. 2009;16(5):798–817. doi: 10.3758/PBR.16.5.798. 19815782 [DOI] [PubMed] [Google Scholar]
- McAuley T., Yap M., Christ S.E., White D.A. Revisiting inhibitory control across the life span: insights from the ex-Gaussian distribution. Dev. Neuropsychol. 2006;29(3):447–458. doi: 10.1207/s15326942dn2903_4. 16671861 [DOI] [PubMed] [Google Scholar]
- McKeown M.J., Hansen L.K., Sejnowsk T.J. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol. 2003;13(5):620–629. doi: 10.1016/j.conb.2003.09.012. 14630228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M.J., Makeig S., Brown G.G., Jung T.P., Kindermann S.S., Bell A.J., Sejnowski T.J. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. 9673671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Banich M.T., Webb A., Barad V., Cohen N.J., Wszalek T., Kramer A.F. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res. Cogn. Brain Res. 2001;12(3):467–473. doi: 10.1016/s0926-6410(01)00076-3. 11689307 [DOI] [PubMed] [Google Scholar]
- Nelder J.A., Mead R., Nelder B.J.A., Meadf R. A simplex method for function minimization. Comput. J. 1965;7(4):308–313. [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. 11389475 [DOI] [PubMed] [Google Scholar]
- Paus T., Petrides M., Evans A.C., Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J. Neurophysiol. 1993;70(2):453–469. doi: 10.1152/jn.1993.70.2.453. 8410148 [DOI] [PubMed] [Google Scholar]
- Petersen W., Laprell H. Combined injuries of the medial collateral ligament and the anterior cruciate ligament. Early ACL reconstruction versus late ACL reconstruction. Arch. Orthop. Trauma. Surg. 1999;119(5-6):258–262. doi: 10.1007/s004020050405. 10447618 [DOI] [PubMed] [Google Scholar]
- Posner M.I., DiGirolamo G.J., Fernandez-Duque D. Brain mechanisms of cognitive skills. Conscious. Cogn. 1997;6(2-3):267–290. doi: 10.1006/ccog.1997.0301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Van Der Molen M.W. Mental resources, processing speed, and inhibitory control: a developmental perspective. Biol. Psychol. 1997;45:241–261. doi: 10.1016/s0301-0511(96)05230-1. 9083652 [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. 15093142 [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K. The development of executive attention: contributions to the emergence of self-regulation. Dev. Neuropsychol. 2005;28(2):573–594. doi: 10.1207/s15326942dn2802_2. 16144428 [DOI] [PubMed] [Google Scholar]
- Sato J.R., Hoexter M.Q., Castellanos X.F., Rohde L.A. Abnormal brain connectivity patterns in adults with ADHD: a coherence study. PLOS One. 2012;7(9):e45671. doi: 10.1371/journal.pone.0045671. 23049834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedek F., Oberauer K., Wilhelm O., Süss H.-M., Wittmann W.W. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. J Exp Psychol Gen. 2007;136(3):414–429. doi: 10.1037/0096-3445.136.3.414. 17696691 [DOI] [PubMed] [Google Scholar]
- Schweren L.J., de Zeeuw P. MR imaging of the effects of methylphenidate on brain structure and function in attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013;23(10):1151–1164. doi: 10.1016/j.euroneuro.2012.10.014. 23165220 [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Valera E.M. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. 15949998 [DOI] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. 10638065 [DOI] [PubMed] [Google Scholar]
- Shaw P., Gilliam M., Liverpool M., Weddle C., Malek M., Sharp W., Greenstein D., Evans A., Rapoport J., Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2011;168(2):143–151. doi: 10.1176/appi.ajp.2010.10030385. 21159727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Malek M., Watson B., Greenstein D., de Rossi P., Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007. 23726514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Curr. Psychiatry Rep. 2009;11(5):393–398. doi: 10.1007/s11920-009-0059-0. 19785981 [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. 9881538 [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. 21616982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D.J., Fotedar S.G., Suskauer S.J., Pekar J.J., Denckla M.B., Mostofsky S.H. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. 17350054 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol. Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. 15949993 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol. Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. 15949993 [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Delis D., Stiles J., Jernigan T.L. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J. Int. Neuropsychol. Soc. JINS. 2001;7:312–322. doi: 10.1017/s135561770173305x. 11311032 [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–1707. doi: 10.1016/S0140-6736(03)14842-8. 14643117 [DOI] [PubMed] [Google Scholar]
- Stuss D.T., Murphy K.J., Binns M.A., Alexander M.P. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126(11):2363–2380. doi: 10.1093/brain/awg237. 12876148 [DOI] [PubMed] [Google Scholar]
- Sun L., Cao Q., Long X., Sui M., Cao X., Zhu C., Zuo X., An L., Song Y., Zang Y., Wang Y. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naive boys with attention deficit hyperactivity disorder. Psychiatry Res. 2012;201(2):120–127. doi: 10.1016/j.pscychresns.2011.07.001. 22424873 [DOI] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Caffo B.S., Denckla M.B., Pekar J.J., Mostofsky S.H. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. 18724253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Fjell A.M., Westlye L.T., Østby Y., Walhovd K.B. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J. Neurosci. 2012;32(3):972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. 22262895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C.S., Balota D.A., Yap M.J., Duchek J.M., McCabe D.P. Effects of healthy aging and early stage dementia of the Alzheimer's type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24(3):300–315. doi: 10.1037/a0018274. 20438208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zandt T. How to fit a response time distribution. Psychon. Bull. Rev. 2000;7(3):424–465. doi: 10.3758/bf03214357. 11082851 [DOI] [PubMed] [Google Scholar]
- Vaurio R.G., Simmonds D.J., Mostofsky S.H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. 19552927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. 16767087 [DOI] [PubMed] [Google Scholar]
- West R., Murphy K.J., Armilio M.L., Craik F.I., Stuss D.T. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain Cogn. 2002;49(3):402–419. doi: 10.1006/brcg.2001.1507. 12139961 [DOI] [PubMed] [Google Scholar]
- Williams B.R., Hultsch D.F., Strauss E.H., Hunter M.A., Tannock R. Inconsistency in reaction time across the life span. Neuropsychol. 2005;19(1):88–96. doi: 10.1037/0894-4105.19.1.88. 15656766 [DOI] [PubMed] [Google Scholar]
- Williams B.R., Strauss E.H., Hultsch D.F., Hunter M.A., Tannock R. Reaction time performance in adolescents with attention deficit/hyperactivity disorder: evidence of inconsistency in the fast and slow portions of the RT distribution. J. Clin. Exp. Neuropsychol. 2007;29(3):277–289. doi: 10.1080/13803390600678020. 17454348 [DOI] [PubMed] [Google Scholar]
- Wodka E.L., Mahone E.M., Blankner J.G., Larson J.C., Fotedar S. Evidence that response inhibition is a primary deficit in ADHD. J. Clin. Exp. Neuropsychol. 2007;29(4):345–356. doi: 10.1080/13803390600678046. 17497558 [DOI] [PubMed] [Google Scholar]
- Xu J., Potenza M.N., Calhoun V.D. Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Front. Neurosci. 2013;7:154. doi: 10.3389/fnins.2013.00154. 23986654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations between age and task performance for subjects with ADHD and controls. Significant correlations (p < 0.05) are indicated with an asterisk (*).
When age is operationalized as a continuous variable, we find widespread posterior decreases in activity with age, p < 0.01 uncorrected. Yellow: controls > ADHD group; blue: decreases with age; red increases with age. This analysis yielded no interactive effects between group and age.