Abstract
Chronic itch, a highly debilitating condition, has received relatively little attention in the neuroimaging literature. Recent studies suggest that brain regions supporting itch in chronic itch patients encompass sensorimotor and salience networks, and corticostriatal circuits involved in motor preparation for scratching. However, how these different brain areas interact with one another in the context of itch is still unknown. We acquired BOLD fMRI scans in 14 atopic dermatitis patients to investigate resting-state functional connectivity before and after allergen-induced itch exacerbated the clinical itch perception in these patients. A seed-based analysis revealed decreased functional connectivity from baseline resting state to the evoked-itch state between several itch-related brain regions, particularly the insular and cingulate cortices and basal ganglia, where decreased connectivity was significantly correlated with increased levels of perceived itch. In contrast, evoked itch increased connectivity between key nodes of the frontoparietal control network (superior parietal lobule and dorsolateral prefrontal cortex), where higher increase in connectivity was correlated with a lesser increase in perceived itch, suggesting that greater interaction between nodes of this executive attention network serves to limit itch sensation via enhanced top-down regulation. Overall, our results provide the first evidence of itch-dependent changes in functional connectivity across multiple brain regions.
Abbreviations: aMCC, anterior mid-cingulate cortex; AD, atopic dermatitis; ASL, arterial spin labeling; BA, Brodmann area; BOLD, blood-oxygen-level dependent; dlPFC, dorsolateral prefrontal cortex; DMN, default mode network; ECG, electrocardiography; fcMRI, functional connectivity magnetic resonance imaging; fMRI, functional magnetic resonance imaging; GLM, general linear model; ITCH, evoked itch resting-state scan; L, left; MNI, Montreal Neurological Institute; MR, magnetic resonance; PCC, posterior cingulate cortex; PET, positron emission tomography; PMC, premotor cortex; pMCC, posterior mid-cingulate cortex; R, right; REST, baseline resting-state scan; S1/M1, primary sensorimotor cortex; SCORAD, SCORing atopic dermatitis scale; SPL, Superior parietal lobule; VAS, visual analog scale; vlPFC, ventrolateral prefrontal cortex.
Keywords: Atopic dermatitis, Eczema, Insula, Pruritus, Putamen
Highlights
-
•
Atopic dermatitis patients were subjected to allergen-induced itch.
-
•
Evoked itch reduced functional connectivity between itch-related brain regions.
-
•
Evoked itch increased functional connectivity within frontoparietal control network.
-
•
The above changes in functional connectivity correlated with perceived itch level.
-
•
Itch sensation may be top-down regulated by frontoparietal control network.
1. Introduction
The brain circuitry supporting itch sensation is an active topic of research. While numerous studies have investigated and described pain-related brain mechanisms (Apkarian et al., 2005; Henry et al., 2011), much less is known about the brain circuitry involved with itch in spite of the clinical relevance of this highly debilitating symptom, which is present across multiple chronic itch conditions (Yosipovitch et al., 2003). Moreover, recent studies suggest that pain perception is associated with altered resting-state functional brain connectivity, which refers to stereotypical patterns of co-activation across multiple brain regions that show both trait and pain-state specificity (e.g., Napadow et al., 2010; Napadow et al., 2012; Loggia et al., 2013; reviewed in Fomberstein et al., 2013). However, itch-related functional brain connectivity, especially in chronic itch patients, has never been reported.
Atopic dermatitis (AD), also known as atopic eczema, is a pruritic chronic inflammatory disease of the skin, affecting approximately 17.8 million persons in the United States, in which patients experience highly debilitating itch (Finlay, 2001; Lapidus, 2001; Yosipovitch et al., 2003; Berke et al., 2012). Previous studies indicate that AD involves not only peripheral sensitization for itch (Lee and Yu, 2011; Rahman et al., 2011) but also central sensitization, in an analogous fashion as central sensitization for pain in chronic pain patients (Koltzenburg, 2000), such that stimuli that are perceived as painful in healthy subjects are experienced as itching in AD patients (Ikoma et al., 2004; Ikoma et al., 2006; Schmelz, 2010). Not only specific allergens, but also stress and psychosocial factors, can exacerbate AD symptoms (Gil and Sampson, 1989; Koblenzer, 1999; Rahman et al., 2011). This complex symptomatology, and its bidirectional relationship to stress and psychosocial factors, which can be both a cause and a consequence of itching and scratching (Gil and Sampson, 1989; Lapidus, 2001; Arck and Paus, 2006; Chida et al., 2008; Mizawa et al., 2013; Yaghmaie et al., 2013; Schut et al., 2014), point to an essential role of the central nervous system in AD (Ikoma et al., 2004; Misery, 2011; Pfab et al., 2012a; Darlenski et al., 2014).
In healthy subjects, a number of studies indicate that experimentally-induced itch activates a brain network which includes pre-motor and supplementary motor area, thalamus, and cingulate, insular, inferior parietal, and prefrontal cortices (reviewed in Pfab et al., 2012a). However, few studies have investigated the brain circuitry supporting itch in AD. Studies from our group and others suggest that brain response to experimentally induced itch in AD differs from healthy subjects, consistent with abnormal itch sensitivity in AD (Ikoma et al., 2004; Schneider et al., 2008; Ishiuji et al., 2009; Pfab et al., 2010; Pfab et al., 2011; Pfab et al., 2012b; Napadow et al., 2014). In a recent functional MRI (fMRI) study, we evoked clinically-relevant itch in AD patients by using individually-matched allergens and our validated temperature-modulated itch induction procedure (Napadow et al., 2014). In this procedure, a thermode is used to modulate skin temperature between 32 °C (warm) and 25 °C (cool), in 20-s blocks (Pfab et al., 2006). This procedure has reliably been used to experimentally modulate itch, such that cooling the skin gradually increases itch sensation over the first 10 s, followed by a phase in which itch sensation reaches a peak plateau (Pfab et al., 2006; Pfab et al., 2010). We found that different brain regions were activated during these different phases of itch induction (i.e., increasing itch and peak itch plateau). During the increasing itch phase, the right anterior insula, right anterior middle cingulate cortex, bilateral striatum (putamen and caudate), right globus pallidus, and right ventrolateral prefrontal cortex were activated. Conversely, during the peak itch plateau phase the right dorsolateral prefrontal cortex, bilateral premotor cortex, and left superior parietal lobule were activated. Notably, reduction of itch after non-pharmacological (acupuncture) therapy was associated with reduced activation in the putamen and right anterior insula (Napadow et al., 2014), further highlighting the important role of these regions in itch processing.
In the present study, we investigated functional brain connectivity before and after experimentally inducing (or exacerbating) itch, using allergen skin prick in nonlesional skin while AD patients were resting in the MRI scanner. We used functional connectivity magnetic resonance imaging (fcMRI) with seed-based correlation methods to evaluate changes in functional brain connectivity associated with itch. To our knowledge, this is the first study to investigate how functional brain connectivity is modulated by itch, particularly the clinically-relevant itch state — a common cause of suffering in AD patients.
2. Materials and methods
2.1. Study participants
Patients aged 18–60 with a clinical diagnosis of AD and a score greater than 18 on the SCORing Atopic Dermatitis (SCORAD) scale (European Task Force on Atopic Dermatitis 1993; Schmitt et al., 2013) were enrolled. Recruitment was by print and e-mail advertisement as well as referrals from physician colleagues in the Department of Dermatology at the Massachusetts General Hospital. Volunteers who met eligibility criteria via phone screening were invited to a preliminary session in the laboratory during which a licensed dermatologist (F.P.) assessed their eligibility to the study via medical examination and focused history. In addition, eligible participants had to show type-I sensitivity to grass pollen and/or dust mites, as demonstrated by wheal and flare formation upon skin prick testing. For each participant, the most effective itch-inducing allergen was determined among Timothy grass pollen (100,000 bioequivalent allergy units per milliliter) and the two most common types of house dust mites in North America and Europe (Dermatophagoides pteronyssinus,10,000 allergy units per milliliter, and Dermatophagoides farinae,10,000 allergy units per milliliter, Allergy Laboratories, Oklahoma City, OK). Participants also completed the Edinburgh Inventory for handedness (Oldfield, 1971). The study protocol was approved by the Human Research Committee of Massachusetts General Hospital and all study participants gave informed consent. Participants were required to stop all immunosuppressive medications at least 10 days prior to the study to avoid potential suppression of itch perception.
2.2. Data acquisition
As part of a previously published study of the anti-pruritic effects of acupuncture (Napadow et al., 2014), each patient took part in an initial clinical screening session followed by two separate fMRI sessions (at least 1 week apart) during which resting-state scans were performed before and after allergen itch induction (but before any verum or placebo treatment was applied), with corresponding self-report ratings of perceived itch. The baseline resting-state scan (REST) occurred at the beginning of the session. The allergen-evoked itch resting-state scan (ITCH) was performed 15–17 min after allergen skin prick, within the period of time when allergen induced itch sensation is known to persist after skin prick (Pfab et al., 2010). A single drop of the most pruritogenic allergen (as determined during the clinical screening session) was applied on a non-lesion site on the left volar forearm. The skin was then punctured with a plastic MR-compatible lancet (Duotip Test II, Lincoln Diagnostics, Decatur, IL) such that the allergen solution was deposited at the dermal–epidermal junction, where the terminals of itch-related C-fibers are located (Shelley and Arthur, 1957). Using this procedure, an itch sensation develops with a median latency of 35 s and persists more than 15 min after application (Pfab et al., 2010). An absorbent gauze pad was then used to carefully remove the drop of allergen solution 120 s after application. With this method, the skin puncture is sufficiently shallow to avoid sustained pain, as previously reported (Pfab et al., 2006), and can be considered an induction of pure itch rather than a combination of itch and pain.
Brain imaging was performed on a Siemens Trio 3 Tesla MRI scanner (Siemens AG, Erlangen, Germany) with vendor-supplied 32-channel multi-array head coil. A high-resolution T1-weighted anatomical scan was collected using an isotropic multi-echo MPRAGE pulse sequence (TR/TE1/TI = 2530/1.64/1200 ms, 256 × 256 matrix, 256 mm field-of-view (FOV), 7° flip angle) (van der Kouwe et al., 2008). Functional imaging (BOLD fMRI) was performed using a gradient echo T2*-weighted pulse sequence (TR/TE = 2 s/30 ms, 32 anterior commissure–posterior commissure (AC−PC) aligned slices, slice thickness 3.6 mm, 64 × 64 matrix, 200 mm FOV, 90° flip angle). Each resting-state fMRI scan had a duration of 6 min (180 time points). Electrocardiography (ECG) and respiratory activity were simultaneously recorded throughout the scans using a Powerlab system (ML880, ADInstruments, Colorado Springs, CO) at a 400 Hz sampling rate. ECG data were acquired and filtered using an MR-compatible physiological monitor (Magnitude 3150 MRI Patient Monitor, In vivo, Gainesville, Florida) designed to minimize radio frequency and gradient switching artifacts generated during the MRI scan. Respiratory data were collected using a custom-built system based on that devised by Binks et al. (2007) which consists of two MR-compatible pneumobelts placed around the chest and abdomen and connected to air pressure transducers (PX138-0.3D5V, Omegadyne, Inc., Sunbury, Ohio).
Immediately before the baseline scan and immediately after the induced-itch scan, subjects were asked to rate the intensity of experienced itch on a visual analog scale (VAS) from 0 (no itch) to 100 (most intense itch imaginable), with 33 corresponding to an “urge to scratch” threshold, as in our previous studies (Pfab et al., 2005; Pfab et al., 2006; Valet et al., 2008; Pfab et al., 2010; Pfab et al., 2011). It should be noted that subjects were instructed to refrain from scratching even if the perceived itch was above this threshold, and we confirmed by observation that subjects did not engage in scratching during the scans.
2.3. Data analysis
2.3.1. Preprocessing
BOLD data preprocessing and analysis were performed using tools from the FMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004; Woolrich et al., 2009), the FreeSurfer (v. 5.2) suite (http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999; Fischl et al., 1999; Fischl et al., 2002; Fischl et al., 2004), AFNI (Cox, 1996; Cox and Hyde, 1997), and RETROICOR (Glover et al., 2000) for retrospective correction of physiological motion artifacts using our peripheral measures of cardiac and respiratory activities as independent assessments of physiological noise in the BOLD signal.
Preprocessing consisted of: (1) B0 fieldmap correction (dewarping); (2) physiological noise correction with RETROICOR; (3) slice timing correction (‘slicetimer’, FSL); (4) head motion correction (MCFLIRT, FSL (Jenkinson et al., 2002)); (5) skull stripping (BET, FSL (Smith, 2002)); (6) nonlinear registration to the Montreal Neurological Institute (MNI) template (FLIRT/FNIRT, FSL); and (7) 0.008–0.1 Hz band-pass temporal filtering (‘1dBandpass’, AFNI). The translation parameters resulting from the motion correction step were then used to compute the relative mean motion during each scan. Relative mean motion was defined as the average over time of the absolute displacement of each brain volume as compared to the previous volume in time, where the absolute displacement at each time point was computed as the root-mean-square of the x, y, and z translation parameters (Van Dijk et al., 2012).
2.3.2. Seed-based functional connectivity analyses
Functional connectivity was computed using a conventional seed-based correlation analysis method. This method consists in (1) choosing a specific voxel or cluster of voxels in the brain, referred to as the seed, (2) extracting the time series of the BOLD signal in this seed region, and (3) using this time series as a regressor in a general linear model (GLM) analysis to calculate whole-brain, voxel-wise functional connectivity maps of covariance with this seed region (Biswal et al., 1995; Cole et al., 2010). Our seed regions of interest were chosen as the clusters significantly activated during the “increasing itch” or “peak itch plateau” phase from an evoked, allergen-induced itch block fMRI scan in the same AD subjects, as previously reported (Napadow et al., 2014). Importantly, activation clusters came from different fMRI runs than those investigated with our resting fcMRI approach, assuring independence between the seed fMRI time series during rest and that during temperature-modulated evoked itch scanning. The brain regions from which ROI seeds were chosen were the right anterior insula (MNI coordinates in the Talairach–Tournoux Atlas: X = 30 mm, Y = 18 mm, Z = 2 mm), right anterior mid-cingulate cortex (aMCC) (X = 8, Y = 34, Z = 32), right putamen (X = 24, Y = 18, Z = 4), left putamen (X = −22, Y = 10, Z = 10), right caudate (X = 20, Y = 18, Z = 8), left caudate (X = −20, Y = 10, Z = 16), right globus pallidus (X = 18, Y = 4, Z = 4), right ventrolateral prefrontal cortex (vlPFC) (X = 34, Y = 40, Z = 6), right dorsolateral prefrontal cortex (dlPFC) (X = 36, Y = 20, Z = 52), right premotor cortex (PMC) (X = 22, Y = −4, Z = 62), left PMC (X = −20, Y = −20, Z = 62), and left superior parietal lobule (SPL) (X = −28, Y = −44, Z = 64) (see Table 1). For each seed region, the BOLD signal was averaged across a 4-mm radius sphere centered on each cluster's peak voxel. The resulting time series was then used as a regressor in a standard GLM analysis using FSL, along with 10 regressors of no interest: 6 motion correction parameters, the average time series from cerebrospinal fluid voxels in the lateral ventricles, the average time series from white matter voxels in deep parietal white matter, and cardiac and respiratory time series obtained by convolving the cardiac and respiratory signals with hemodynamic response functions from the ‘RVHR’ model (Chang et al., 2009). The output of this GLM analysis for each seed region of interest was a 3D volume (“brain map”) in which each voxel value represented the strength of functional connectivity between this seed and every other voxel in the brain.
Table 1.
Seed-based functional connectivity results. For each seed, statistically significant clusters (with a cluster-forming threshold of Z > 2.3 and a cluster significance level of p < 0.05) are listed. For each cluster, the Z-value, MNI coordinates, and atlas label of the peak voxel are listed, along with Pearson's correlation coefficient r and corresponding p-value of the correlation with level of perceived itch.
| Seed region | Z-value | X | Y | Z | Atlas label | Correlation with perceived itch |
|---|---|---|---|---|---|---|
| R premotor cortex | 3.56 | −9 | −64.5 | 66 | L precuneus | n.s. |
| 3.16 | 8 | −48 | 61 | R superior parietal lobule (5L) | n.s. | |
| R insula | 3.92 | −30 | −44 | −14 | L fusiform gyrus | n.s. |
| 3.81 | −18 | −36 | −2 | L parahippocampal gyrus | r = –0.39, p = 0.045 | |
| 3.61 | −12 | −60 | 14 | L posterior cingulate cortex | r = –0.42, p = 0.025 | |
| 3.62 | −52 | −66 | 6 | L middle temporal gyrus | n.s. | |
| 3.38 | −4 | −18 | 40 | L mid-cingulate cortex | n.s. | |
| R putamen | 3.78 | −60 | −20.5 | 22.5 | Left postcentral gyrus | n.s. |
| 3.95 | −61 | −17.5 | 11 | L transverse (or superior) gyrus, BA42 | n.s. | |
| 3.68 | −2 | −22 | 48 | L medial frontal gyrus, BA6 or L paracentral lobule | n.s. | |
| 4.01 | −0.5 | −35 | 41 | L/R mid-cingulate cortex | n.s. | |
| 3.35 | −11 | −41.5 | 29.5 | L posterior cingulate cortex | n.s. | |
| L superior parietal lobule | 3.92 | −53 | −24.5 | 32 | L inferior parietal lobule | |
| 3.69 | −41.5 | 29 | 27.5 | L dlPFC (Middle frontal gyrus) | r = –0.40, p = 0.036 | |
| 3.61 | 43.5 | −10.5 | 15 | R posterior insula | n.s. | |
| 4.24 | 29.5 | −14 | 11.5 | R putamen (not the same locus as the R putamen seed) | n.s. | |
| R anterior mid-cingulate cortex | 4.32 | 34 | −21.5 | 10 | R posterior insula | n.s. |
| 4.04 | 28.5 | −12.5 | 9.5 | R putamen | r = –0.40, p = 0.036 | |
| 4.03 | 58 | −26.5 | 22 | R inferior parietal lobule | n.s. | |
| R caudate | 4.39 | −10.5 | −73 | 23.5 | L cuneus, BA18 | n.s. |
| 3.86 | −51.5 | 19.5 | −4.5 | L inferior frontal gyrus (pars orbitalis) | n.s. | |
| R globus pallidus | 3.98 | −5 | −25.5 | 44 | L mid-cingulate cortex or paracentral lobule | r = –0.37, p = 0.050 |
| 4.01 | 35.5 | 27 | 49 | R superior/middle frontal gyrus (dlPFC) | n.s. |
2.3.3. ITCH–REST difference map for each subject
For each subject, a difference map was computed by calculating the difference in parameter estimates for connectivity taken from REST versus ITCH scan runs using ‘fslmaths’ (FSL). The variance map for this difference map was estimated by adding the parameter estimate variances computed from the REST and ITCH scan runs. These maps, calculated from all subjects, were then passed up to a group level analysis using FSL, as described below.
2.3.4. Group-level analysis of main effect of itch on functional connectivity
A group-level analysis yielded a paired difference map between REST and ITCH across subjects and therefore indicated the main effect of itch induction on functional connectivity. Multiple comparisons correction was performed with cluster correction, with a cluster-forming threshold of Z > 2.3 and a cluster significance level of p < 0.05. Finally, for visualization purposes, the resulting brain maps were spatially smoothed using the cubic resampling function in AFNI and overlaid on the 0.5 mm MNI152 template.
2.3.5. Correlation between perceived itch and level of functional connectivity
Using only the brain regions in which a main effect was found, we then performed a linear regression analysis between the average main effect within the region and the itch perception score difference between the REST and ITCH conditions. This analysis specifically indicated which functional connectivity changes between REST and ITCH were correlated with perceived change in itch, thus linking changes in functional connectivity with itch more directly than the main effect analysis alone, and taking advantage of the intersubject variability in itch responses. Therefore, in this final analysis, changes in functional connectivity were deemed related to itch only in brain regions where there was both (i) a main effect of itch and (ii) a significant correlation across subjects between change in functional connectivity and change in perceived itch (from REST to ITCH).
3. Results
Fourteen AD patients (8 females, 13 right-handed, age: 25.4 ± 9.1 years old, SCORAD: 38.7 ± 14.9, mean ± standard deviation) participated in the study, with itch induction performed using the most pruritogenic allergen for each patient among Timothy grass pollen (N = 8), D. pteronyssinus house dust mite (N = 3), or D. farinae house dust mite (N = 3).
3.1. Itch rating levels
Perceived itch increased significantly after allergen induction (REST: 6.4 ± 11.7; ITCH: 32.0 ± 16.6, mean ± standard deviation on a 0–100 visual analog scale; paired t-test, p < 10−7; Fig. 1), as expected. There was no significant correlation between perceived itch and age across subjects, neither during REST (r = 0.14, p = 0.63), nor during ITCH (r = 0.18, p = 0.54).
Fig. 1.
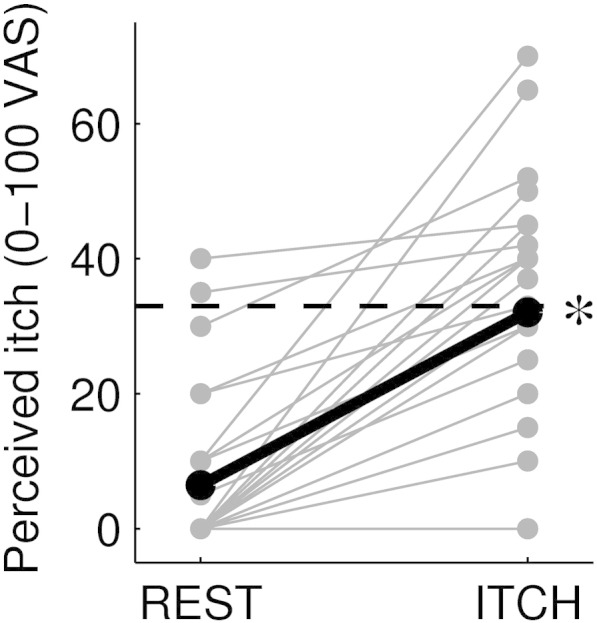
Change in perceived itch intensity from the baseline resting state (‘REST’) to the allergen-evoked itch state (‘ITCH’), on a 0–100 scale, for all experimental sessions (N = 14, with 2 sessions per subject). The dashed line indicates the “urge to scratch” threshold (defined at the 33 level). The thicker, black line represents the mean across subjects and sessions. The asterisk indicates that the ratings during ‘ITCH’ were significantly different from those during ‘REST’ (paired t-test, p < 10−7).
3.2. Head motion and respiration during the scan
Since subject motion during the scan can significantly affect measures of functional connectivity, it is important to measure and report head motion in any study of functional connectivity (Power et al., 2012; Van Dijk et al., 2012). Here we verified that mean motion ranged within previously published values in a healthy population (Van Dijk et al., 2012) and did not significantly differ between REST (0.100 ± 0.043 mm, mean ± standard deviation) and ITCH (0.097 ± 0.037 mm; paired t-test, p = 0.9). We further verified that gross translational motion never exceeded 3 mm on any axis. We also verified that the participants' breathing rates did not change between the REST condition (0.35 Hz ± 0.11, mean ± standard deviation) and the ITCH condition (0.34 Hz ± 0.10, paired t-test, p = 0.19).
3.3. Correlation between perceived itch and level of functional connectivity
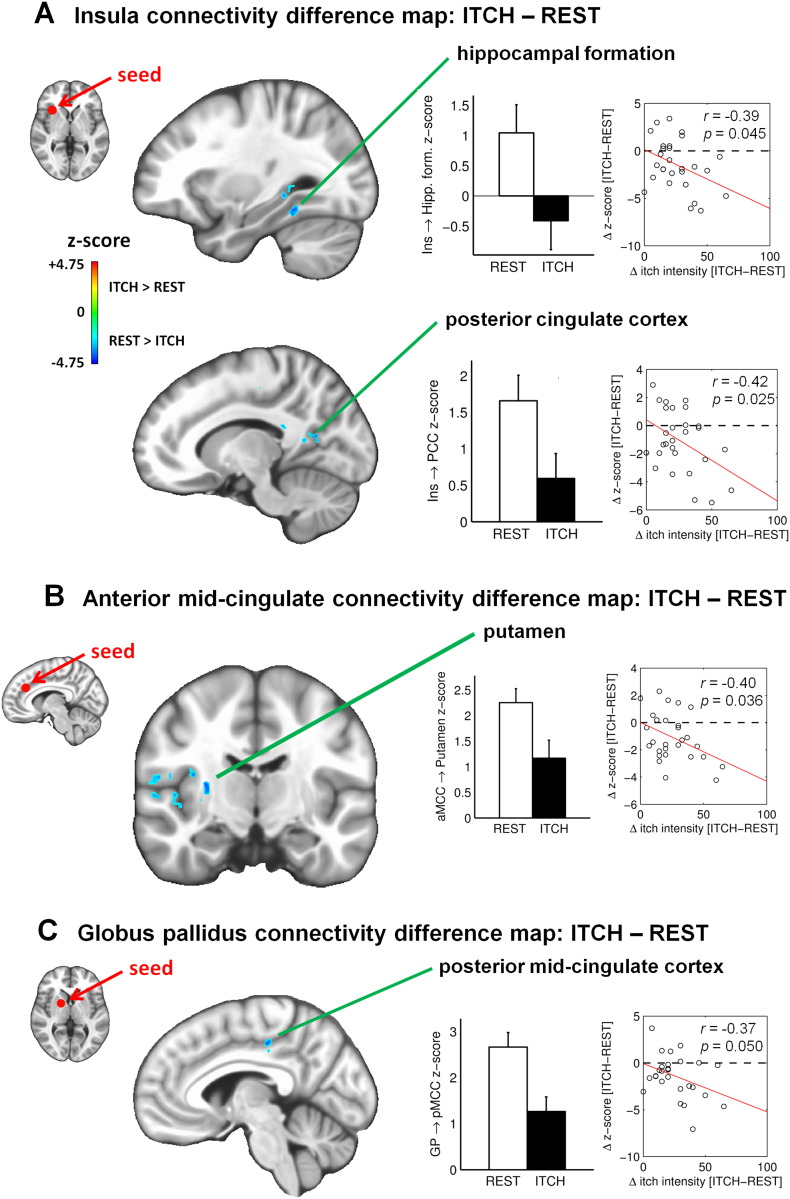
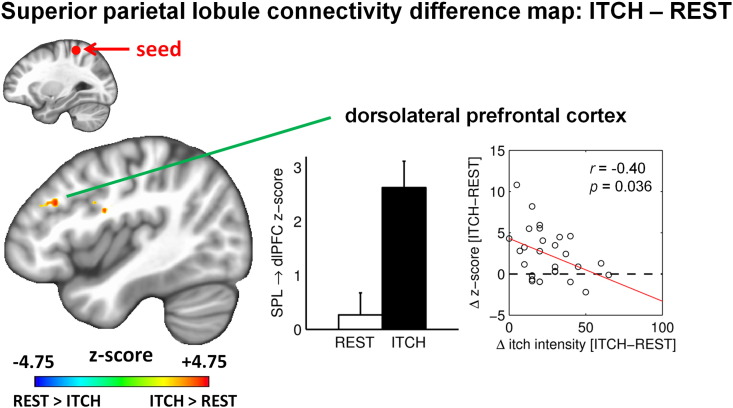
While functional connectivity changed significantly from REST to ITCH for several of the seed regions of interest (see Table 1), the change in functional connectivity was significantly correlated with change in perceived itch for a smaller subset of regions. Among these regions, functional connectivity decreased during ITCH, compared to REST, in most cases. Specifically, connectivity was decreased and negatively correlated with change in itch for connectivity between the right anterior insula and left hippocampal formation (MNI coordinates of the peak voxel: X = −18, Y = −36, Z = –2, r = –0.39, p = 0.045) and left posterior cingulate cortex (PCC, X = −12, Y = −60, Z = 14, r = –0.42, p = 0.025), between the right aMCC and right putamen (X = 28.5, Y = −12.5, Z = 9.5, r = –0.40, p = 0.036), and between the right globus pallidus and left posterior mid-cingulate cortex (pMCC, X = −5, Y = −25.5, Z = 44, r = –0.37, p = 0.050; Fig. 2 and Table 1). Thus, for these connectivity changes, a higher increase in perceived itch was associated with a greater decrease in connectivity. The exception to this pattern was for connectivity between the left SPL and left dlPFC (X = −41.5, Y = 29, Z = 27.5), which demonstrated an increase during ITCH, and a negative correlation between change in connectivity and change in itch (r = –0.40, p = 0.036; Fig. 3 and Table 1). In other words, a higher increase in perceived itch was associated with a lower increase in SPL–dlPFC connectivity.
Fig. 2.
Changes in functional connectivity from the baseline resting state (‘REST’) to the allergen-evoked itch state (‘ITCH’). A. Seed-based functional connectivity from the right anterior insula (MNI coordinates: [30, 18, 1]). Top: Significant cluster in the left hippocampal formation (peak voxel coordinates: [−30, −44, −14] in the fusiform gyrus). Bottom: Significant cluster in the left posterior cingulate cortex (PCC) (peak voxel coordinates: [−12, −60, 14]). In each of the two panels, the graph on the left shows z-score levels of functional connectivity (main effect), in the resting state (‘REST’; white bar) and 15–17 min after allergen-based itch induction (‘ITCH’; black bar). The graph on the right shows for each scan session (N = 28) the difference in connectivity from ‘REST’ to ‘ITCH’ as a function of the difference in perceived itch intensity from ‘REST’ to ‘ITCH’, as reported by participants; linear regression across subjects is shown (red line) along with corresponding r and p values. B. Seed-based functional connectivity from the right anterior mid-cingulate cortex (coordinates: [8, 34, 28]), showing a significant cluster in the right putamen (peak voxel coordinates: [28, −12, 9]). C. Seed-based functional connectivity from the right globus pallidus (coordinates: [18, 4, 3]), showing a significant cluster in the left posterior mid-cingulate cortex (peak voxel coordinates: [−5, −25, 44]).
Fig. 3.
Seed-based functional connectivity from the left superior parietal lobule (coordinates: [−28, −40, 61]), showing a significant cluster in the left rostral middle frontal gyrus, part of left dorsolateral prefrontal cortex (peak voxel coordinates: [−41, 29, 27]). Same graph conventions as in Fig. 2.
4. Discussion
In this fcMRI study of allergen-induced itch in AD patients, we found that an increase in perceived itch was significantly correlated with a decrease in functional connectivity between brain regions previously demonstrated to activate in response to phasic itch provocation (Napadow et al., 2014). These brain regions included the insula, cingulate cortex, and basal ganglia. Specifically, greater itch was associated with reduced connectivity between the right anterior insula and both hippocampal formation and posterior cingulate cortex. Similarly, itch was associated with reduced connectivity between the anterior mid-cingulate cortex and putamen, and between the globus pallidus and posterior mid-cingulate cortex. While itch was mostly associated with reduced functional connectivity, increased connectivity between the superior parietal lobule and dorsolateral prefrontal cortex was associated with an increase in perceived itch. Moreover, a negative correlation was found between SPL–dlPFC connectivity and itch rating; that is, subjects showing greater increase in SPL–dlPFC connectivity also reported lower increase in itch sensation. This result suggests that greater interaction between the SPL and dlPFC serves to limit itch sensation via enhanced top-down regulation. Our study clearly demonstrates for the first time that “resting” functional connectivity is altered when AD patients experience itch, and these state-specific alterations can be directly linked with the intensity of perceived itch.
Overall, we found that a sustained itch state altered functional connectivity for nodes known to be activated by evoked itch. For instance, we found that functional connectivity between the basal ganglia (caudate and globus pallidus) and cingulate cortex, normally present at rest (Postuma and Dagher, 2006), decreased with increased itch (see Fig. 2B, 2C). This finding is reminiscent of our recent pain neuroimaging study in which we found that experimentally-induced sustained myofascial pain in healthy adults reduced functional connectivity between the somatotopic cortical representation within the primary sensorimotor (S1/M1) area contralateral to the pain stimulus site and other regions of the primary sensorimotor network (Kim et al., 2013). Hence, in both studies, brain areas activated by an evoked stimulus (whether itch or pain) showed decreased connectivity within their intrinsic connectivity network.
Evoked itch in our AD patients was also associated with a decrease in functional connectivity between the right anterior insula and both left hippocampal formation and left PCC (see Fig. 2A), both of which are nodes of the default mode network (DMN) (Buckner et al., 2008). Interestingly, while the anterior insula is not usually connected with DMN regions, chronic pain patients show greater DMN–insula connectivity at rest than healthy controls (Napadow et al., 2010; Loggia et al., 2013), and even in healthy subjects, DMN–insula connectivity increases with state anxiety (Dennis et al., 2011). In our AD subjects, functional connectivity between the right anterior insula and these two DMN nodes (left hippocampal formation and left PCC) was present during a resting state and decreased after itch induction, and this decrease was significantly correlated with an increase in perceived itch. The allergen-induced itch may therefore have disrupted the resting connectivity patterns that support chronic (versus evoked) itch perception in these patients, perhaps by shifting attention to an evoked stimulus. Together, these results reinforce the notion that unpleasant interoceptive experiences such as itch or pain may decrease functional connectivity between brain regions that are functionally connected at rest.
In contrast, while connectivity between itch-processing brain areas was mostly decreased during the sustained itch state, increased connectivity was noted between SPL and dlPFC. In fact, we found a negative correlation between itch intensity and the change in connectivity between the SPL and dlPFC. In other words, a higher increase in functional connectivity between SPL and dlPFC was associated with a lower increase in perceived itch after allergen itch induction (see Fig. 3). This suggests that increased SPL–dlPFC connectivity may be a protective mechanism, limiting perceived itch severity after allergen itch induction in AD. Both the SPL and dlPFC sub-regions in our results are key nodes of the frontoparietal control network, which supports executive processing of cognitive control (Vincent et al., 2008). Hence, our finding suggests that subjects able to regulate increased information exchange within this network were better able to limit the severity of sustained itch sensation after allergen itch induction. Interestingly, we previously found that SPL and dlPFC were activated by evoked allergen itch during the “peak itch” plateau phase (Napadow et al., 2014), an experimental condition likely to be more closely related to sustained itch than the preceding “increasing itch” phase, when itch sensation was steadily increasing. Of note, the brain regions that were activated during the “increasing itch” phase (anterior insula, cingulate, putamen) instead showed decreased connectivity in response to sustained evoked itch. This suggests that different brain networks become engaged when itch sensation first develops versus when itch is a steadily ongoing perception.
The continuous, sustained itch state induced in our study was associated with altered functional connectivity between several brain regions which previous studies have reported as being activated by evoked itch stimuli. In healthy subjects, brain activation during itch has been investigated by performing functional brain imaging – with either positron emission tomography (PET), BOLD fMRI, or arterial spin labeling (ASL) fMRI – while inducing itch experimentally using various means such as histamine prick, allergen prick, cowhage spicule application, or electrical stimulation (Walter et al., 2005; Herde et al., 2007; Leknes et al., 2007; Mochizuki et al., 2007; Pfab et al., 2008; Valet et al., 2008; Yosipovitch et al., 2008; Mochizuki et al., 2009; Vierow et al., 2009; Jeffry et al., 2011; Kleyn et al., 2012; Papoiu et al., 2012; Pfab et al., 2012a). These studies, most of them conducted with healthy subjects rather than chronic itch patients, highlight the involvement of a network of brain regions which includes ipsilateral pre-motor and supplementary motor areas and contralateral cingulate cortex, insular cortex, thalamus, inferior parietal cortex, and dorsolateral prefrontal cortex (reviewed in Pfab et al., 2012a). In addition, the above studies found that itch stimuli activate habit-encoding motor planning areas, including the putamen, probably in relation to the motor act of scratching which is strongly associated with itch (Yosipovitch et al., 2008; Vierow et al., 2009). In the present study, itch perception was associated with reduced connectivity between the insula, cingulate cortex, and basal ganglia (i.e., putamen, globus pallidus) — regions whose activation was strongly associated with itch in previous evoked-itch neuroimaging studies.
Notably, functional connectivity for basal ganglia areas such as the putamen and globus pallidus was significantly altered during sustained itch. These areas are critical in regulating both voluntary and involuntary movements, and may play an important role in the scratch response to itch. Putamen activation has been reported not only during scratching, with greater activation in the presence of itch than in its absence, but also during itching without scratching, suggesting that putamen activation may represent the urge and/or intention to scratch (Vierow et al., 2009). We previously reported that putamen activation was maximal during an increasing itch phase in AD patients subjected to allergen itch (Napadow et al., 2014). The putamen is also a key structure within the striato-thalamo-cortical circuitry implicated in action motivation and initiation and in habitual, repetitive behavior (Graybiel, 2008). This circuit is dysfunctional in various psychopathologies, including addiction (Koob and Volkow, 2010), which is consistent with its implication in the “urge” to scratch (Leknes et al., 2007; Vierow et al., 2009). Here we found that functional connectivity between the basal ganglia (caudate and globus pallidus) and cingulate cortex decreased with increased itch (see Fig. 2B, 2C). The cingulate cortex is part of the salience network and is activated during itch (Pfab et al., 2012a), which is a highly salient stimulus, particularly to AD patients. Therefore, since the basal ganglia as well as the cingulate cortex are activated during itch, the observed decrease in functional connectivity between these regions during experimentally-induced itch may be another case of diminished connectivity between regions known to be activated by an evoked noxious stimulus, as noted above (Kim et al., 2013).
Limitations to our study should also be noted. For instance, the ITCH scan took place 15–17 min after allergen itch induction, a time at which persisting itch intensity was no longer maximal. While perceived itch was still robust enough to alter functional brain connectivity, future studies should acquire fMRI data closer in time to itch induction, allowing for greater power to evaluate changes in brain connectivity supporting a persistent itch state. On the other hand, the long delay after initial itch induction allowed for any early transients in itch perception and physiological brain response to subside, thus improving a functional connectivity assessment based on 6 min of scanning. Additionally, while we found other significant changes in functional connectivity after itch induction (see Table 1), these changes were not significantly correlated with changes in perceived itch, indicating that they may be only indirectly related to itch perception or may have been caused by order effects (since the REST scan always preceded the ITCH scan). Hence, the results reported here account for any potential order effects as we focused on functional connectivity changes that were also significantly correlated with the level of perceived itch.
In conclusion, the present results shed new light on our recent findings of the neural correlates of allergen-induced itch in AD, which involved a broad network of brain regions associated with emotional processing (anterior insula, anterior cingulate cortex), salience (insula, striatum), executive function (ventrolateral and dorsolateral prefrontal cortex, cingulate cortex), sensory-motor integration (superior parietal lobule), and motor planning (globus pallidus, premotor cortex, striatum) (Napadow et al., 2014). As increased SPL–dlPFC connectivity may reduce itch perception in AD, future studies should explore how cognitive and attentional psychological interventions, such as cognitive behavioral therapy and meditation training (Chida et al., 2007; Rosenkranz et al., 2013; Schut et al., 2013), might modulate frontoparietal control network connectivity in response to an up-regulated itch state, and thereby reduce itch perception via top-down regulation. Since longitudinal studies have found functional as well as structural changes in the brain after such interventions (Linden, 2006; Felmingham et al., 2007; Hölzel et al., 2011; Desbordes et al., 2012; Hölzel et al., 2013), there is cautious optimism that these types of intervention may “rewire” dysfunctional brain networks in chronic itch patient populations, such as AD.
Funding
This work was supported by the National Institutes of Health (V.N.: K01AT002166, R01AT004714; G.D.: K01AT008225; B.R.R: P01AT002048; T.K.: K24AT004095; E.L.: R01AR057744). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program S10RR021110, S10OD010364, and S10RR023401. This study was also partly funded by a grant from the German Research Foundation (pf 690/2-1; http://www.dfg.de/en/research_funding/), and the Christine Kühne Center of Allergy and Education (CK-Care; http://www.ck-care.ch/index.php?id=2659&L=4).
References
- Apkarian A.V., Bushnell M.C., Treede R.-D., Zubieta J.-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. 15979027 [DOI] [PubMed] [Google Scholar]
- Arck P., Paus R. From the brain–skin connection: the neuroendocrine–immune misalliance of stress and itch. Neuroimmunomodulation. 2006;13(5–6):347–356. doi: 10.1159/000104863. 17709957 [DOI] [PubMed] [Google Scholar]
- Berke R., Singh A., Guralnick M. Atopic dermatitis: an overview. Am. Fam. Physician. 2012;86(1):35–42. 22962911 [PubMed] [Google Scholar]
- Binks A.P., Banzett R.B., Duvivier C. An inexpensive, MRI compatible device to measure tidal volume from chest-wall circumference. Physiol. Meas. 2007;28(2):149–159. doi: 10.1088/0967-3334/28/2/004. 17237587 [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. 8524021 [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. 18400922 [DOI] [PubMed] [Google Scholar]
- Chang C., Cunningham J.P., Glover G.H. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44(3):857–869. doi: 10.1016/j.neuroimage.2008.09.029. 18951982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y., Hamer M., Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom. Med. 2008;70(1):102–116. doi: 10.1097/PSY.0b013e31815c1b71. 18158379 [DOI] [PubMed] [Google Scholar]
- Chida Y., Steptoe A., Hirakawa N., Sudo N., Kubo C. The effects of psychological intervention on atopic dermatitis. A systematic review and meta-analysis. Int. Arch. Allergy Immunol. 2007;144(1):1–9. doi: 10.1159/000101940. 17449959 [DOI] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state fMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. 20407579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W., Hyde J.S. Software tools for analysis and visualization of fMRI data. N.M.R. Biomed. 1997;10(4–5):171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. 9430344 [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. 8812068 [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. 9931268 [DOI] [PubMed] [Google Scholar]
- Darlenski R., Kazandjieva J., Hristakieva E., Fluhr J.W. Atopic dermatitis as a systemic disease. Clin. Dermatol. 2014;32(3):409–413. doi: 10.1016/j.clindermatol.2013.11.007. 24767188 [DOI] [PubMed] [Google Scholar]
- Dennis E.L., Gotlib I.H., Thompson P.M., Thomason M.E. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain Connect. 2011;1(3):245–254. doi: 10.1089/brain.2011.0030. 22433052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes G., Negi L.T., Pace T.W., Wallace B.A., Raison C.L., Schwartz E.L. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci. 2012;6:292. doi: 10.3389/fnhum.2012.00292. 23125828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Task Force on Atopic Dermatitis Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31. doi: 10.1159/000247298. 8435513 [DOI] [PubMed] [Google Scholar]
- Felmingham K., Kemp A., Williams L., Das P., Hughes G., Peduto A., Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol. Sci. 2007;18(2):127–129. doi: 10.1111/j.1467-9280.2007.01860.x. 17425531 [DOI] [PubMed] [Google Scholar]
- Finlay A.Y. Quality of life in atopic dermatitis. J. Am. Acad. Dermatol. 2001;45(1 Suppl):S64–S66. doi: 10.1067/mjd.2001.117010. 11423879 [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. 11832223 [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J., Makris N., Ségonne F., Quinn B.T., Dale A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. 15501102 [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. 9931269 [DOI] [PubMed] [Google Scholar]
- Fomberstein K., Qadri S., Ramani R. Functional MRI and pain. Curr. Opin. Anaesthesiol. 2013;26(5):588–593. doi: 10.1097/01.aco.0000433060.59939.fe. ISSN: 0952-7907. [DOI] [PubMed] [Google Scholar]
- Gil K.M., Sampson H.A. Psychological and social factors of atopic dermatitis. Allergy. 1989;44(Suppl. 9):84–89. doi: 10.1111/j.1398-9995.1989.tb04322.x. 2683846 [DOI] [PubMed] [Google Scholar]
- Glover G.H., Li T.Q., Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. 10893535 [DOI] [PubMed] [Google Scholar]
- Graybiel A.M. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. 18558860 [DOI] [PubMed] [Google Scholar]
- Henry D.E., Chiodo A.E., Yang W. Central nervous system reorganization in a variety of chronic pain states: a review. PM&R. 2011;3(12):1116–1125. doi: 10.1016/j.pmrj.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Herde L., Forster C., Strupf M., Handwerker H.O. Itch induced by a novel method leads to limbic deactivations – a functional MRI study. J. Neurophysiol. 2007;98(4):2347–2356. doi: 10.1152/jn.00475.2007. 17715198 [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Carmody J., Vangel M., Congleton C., Yerramsetti S.M., Gard T., Lazar S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. 21071182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., Gard T., Creswell J.D., Brown K.W., Barrett L.F., Schwartz C., Vaitl D., Lazar S.W. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2013;2:448–458. doi: 10.1016/j.nicl.2013.03.011. 24179799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A., Fartasch M., Heyer G., Miyachi Y., Handwerker H., Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62(2):212–217. doi: 10.1212/wnl.62.2.212. 14745056 [DOI] [PubMed] [Google Scholar]
- Ikoma A., Steinhoff M., Ständer S., Yosipovitch G., Schmelz M. The neurobiology of itch. Nat. Rev. Neurosci. 2006;7(7):535–547. doi: 10.1038/nrn1950. 16791143 [DOI] [PubMed] [Google Scholar]
- Ishiuji Y., Coghill R.C., Patel T.S., Oshiro Y., Kraft R.A., Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br. J. Dermatol. 2009;161(5):1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. 19663870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffry J., Kim S., Chen Z.-F. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26(4):286–292. doi: 10.1152/physiol.00007.2011. 21841076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. 12377157 [DOI] [PubMed] [Google Scholar]
- Kim J., Loggia M.L., Edwards R.R., Wasan A.D., Gollub R.L., Napadow V. Sustained deep-tissue pain alters functional brain connectivity. Pain. 2013;154(8):1343–1351. doi: 10.1016/j.pain.2013.04.016. 23718988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyn C.E., McKie S., Ross A., Elliott R., Griffiths C.E. A temporal analysis of the central neural processing of itch. Br. J. Dermatol. 2012;166(5):994–1001. doi: 10.1111/j.1365-2133.2012.10849.x. 22283926 [DOI] [PubMed] [Google Scholar]
- Koblenzer C.S. Itching and the atopic skin. J. Allergy Clin. Immunol. 1999;104(3 2):S109–S113. doi: 10.1016/s0091-6749(99)70052-7. 10482861 [DOI] [PubMed] [Google Scholar]
- Koltzenburg M. Neural mechanisms of cutaneous nociceptive pain. Clin. J. Pain. 2000;16(3 Suppl):S131–S138. doi: 10.1097/00002508-200009001-00004. 11014457 [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. 19710631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus C.S. Role of social factors in atopic dermatitis: the US perspective. J. Am. Acad. Dermatol. 2001;45(1 Suppl):S41–S43. doi: 10.1067/mjd.2001.117017. 11423872 [DOI] [PubMed] [Google Scholar]
- Lee C.-H., Yu H.-S. Biomarkers for itch and disease severity in atopic dermatitis. Curr. Probl. Dermatol. 2011;41:136–148. doi: 10.1159/000323307. 21576954 [DOI] [PubMed] [Google Scholar]
- Leknes S.G., Bantick S., Willis C.M., Wilkinson J.D., Wise R.G., Tracey I. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J. Neurophysiol. 2007;97(1):415–422. doi: 10.1152/jn.00070.2006. 16914620 [DOI] [PubMed] [Google Scholar]
- Linden D.E. How psychotherapy changes the brain — the contribution of functional neuroimaging. Mol. Psychiatry. 2006;11(6):528–538. doi: 10.1038/sj.mp.4001816. 16520823 [DOI] [PubMed] [Google Scholar]
- Loggia M.L., Kim J., Gollub R.L., Vangel M.G., Kirsch I., Kong J., Wasan A.D., Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154(1):24–33. doi: 10.1016/j.pain.2012.07.029. 23111164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misery L. Atopic dermatitis and the nervous system. Clin. Rev. Allergy Immunol. 2011;41(3):259–266. doi: 10.1007/s12016-010-8225-z. 21181506 [DOI] [PubMed] [Google Scholar]
- Mizawa M., Yamaguchi M., Ueda C., Makino T., Shimizu T. Stress evaluation in adult patients with atopic dermatitis using salivary cortisol. BioMed Res. Int. 2013;2013:138027. doi: 10.1155/2013/138027. 23971022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H., Inui K., Tanabe H.C., Akiyama L.F., Otsuru N., Yamashiro K., Sasaki A., Nakata H., Sadato N., Kakigi R. Time course of activity in itch-related brain regions: a combined MEG-fMRI study. J. Neurophysiol. 2009;102(5):2657–2666. doi: 10.1152/jn.00460.2009. 19710378 [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Sadato N., Saito D.N., Toyoda H., Tashiro M., Okamura N., Yanai K. Neural correlates of perceptual difference between itching and pain: a human fMRI study. Neuroimage. 2007;36(3):706–717. doi: 10.1016/j.neuroimage.2007.04.003. 17524669 [DOI] [PubMed] [Google Scholar]
- Napadow V., Kim J., Clauw D.J., Harris R.E. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398–2403. doi: 10.1002/art.34412. 22294427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. 20506181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., Li A., Loggia M.L., Kim J., Schalock P.C., Lerner E., Tran T.-N., Ring J., Rosen B.R., Kaptchuk T.J. The brain circuitry mediating antipruritic effects of acupuncture. Cereb. Cortex. 2014;24(4):873–882. doi: 10.1093/cercor/bhs363. 23222890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Papoiu A.D., Coghill R.C., Kraft R.A., Wang H., Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59(4):3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. 22100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfab F., Athanasiadis G.I., Huss-Marp J., Fuqin J., Heuser B., Cifuentes L., Brockow K., Schober W., Konstantinow A., Irnich D. Effect of acupuncture on allergen-induced basophil activation in patients with atopic eczema: a pilot trial. J. Altern. Complement. Med. 2011;17(4):309–314. doi: 10.1089/acm.2009.0684. 21443446 [DOI] [PubMed] [Google Scholar]
- Pfab F., Hammes M., Bäcker M., Huss-Marp J., Athanasiadis G.I., Tölle T.R., Behrendt H., Ring J., Darsow U. Preventive effect of acupuncture on histamine-induced itch: a blinded, randomized, placebo-controlled, crossover trial. J. Allergy Clin. Immunol. 2005;116(6):1386–1388. doi: 10.1016/j.jaci.2005.08.055. 16337477 [DOI] [PubMed] [Google Scholar]
- Pfab F., Kirchner M.-T., Huss-Marp J., Schuster T., Schalock P.C., Fuqin J., Athanasiadis G.I., Behrendt H., Ring J., Darsow U. Acupuncture compared with oral antihistamine for type I hypersensitivity itch and skin response in adults with atopic dermatitis: a patient- and examiner-blinded, randomized, placebo-controlled, crossover trial. Allergy. 2012;67(4):566–573. doi: 10.1111/j.1398-9995.2012.02789.x. 22313287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfab F., Valet M., Napadow V., Tölle T.R., Behrendt H., Ring J., Darsow U. Itch and the brain. Chem. Immunol. Allergy. 2012;98:253–265. doi: 10.1159/000336529. 22767068 [DOI] [PubMed] [Google Scholar]
- Pfab F., Valet M., Sprenger T., Huss-Marp J., Athanasiadis G.I., Baurecht H.J., Konstantinow A., Zimmer C., Behrendt H., Ring J. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema — a combined psychophysical and neuroimaging study. Allergy. 2010;65(1):84–94. doi: 10.1111/j.1398-9995.2009.02163.x. 19804445 [DOI] [PubMed] [Google Scholar]
- Pfab F., Valet M., Sprenger T., Toelle T.R., Athanasiadis G.I., Behrendt H., Ring J., Darsow U. Short-term alternating temperature enhances histamine-induced itch: a biphasic stimulus model. J. Invest. Dermatol. 2006;126(12):2673–2678. doi: 10.1038/sj.jid.5700577. 17008877 [DOI] [PubMed] [Google Scholar]
- Pfab F., Valet M., Tölle T., Behrendt H., Ring J., Darsow U. Recent progress in unraveling central nervous system processing of itch sensation. World Allergy Organ. J. 2008;1(10):168–173. doi: 10.1097/WOX.0b013e318187ff70. 23282675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R.B., Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. 16373457 [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. 22019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Collins M., Williams C.M., Ma H.-L. The pathology and immunology of atopic dermatitis. Inflamm. Allergy Drug Targets. 2011;10(6):486–496. doi: 10.2174/187152811798104935. 21864272 [DOI] [PubMed] [Google Scholar]
- Rosenkranz M.A., Davidson R.J., MacCoon D.G., Sheridan J.F., Kalin N.H., Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav. Immun. 2013;27(1):174–184. doi: 10.1016/j.bbi.2012.10.013. 23092711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M. Itch and pain. Neurosci. Biobehav. Rev. 2010;34(2):171–176. doi: 10.1016/j.neubiorev.2008.12.004. 19146873 [DOI] [PubMed] [Google Scholar]
- Schmitt J., Langan S., Deckert S., Svensson A., von Kobyletzki L., Thomas K., Spuls P., Harmonising Outcome Measures for Atopic Dermatitis (HOME) Initiative Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J. Allergy Clin. Immunol. 2013;132(6):1337–1347. doi: 10.1016/j.jaci.2013.07.008. 24035157 [DOI] [PubMed] [Google Scholar]
- Schneider G., Ständer S., Burgmer M., Driesch G., Heuft G., Weckesser M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur. J. Pain. 2008;12(7):834–841. doi: 10.1016/j.ejpain.2007.12.003. 18203636 [DOI] [PubMed] [Google Scholar]
- Schut C., Bosbach S., Gieler U., Kupfer J. Personality traits, depression and itch in patients with atopic dermatitis in an experimental setting: a regression analysis. Acta Derm. Venereol. 2014;94(1):20–25. doi: 10.2340/00015555-1634. 23756579 [DOI] [PubMed] [Google Scholar]
- Schut C., Weik U., Tews N., Gieler U., Deinzer R., Kupfer J. Psychophysiological effects of stress management in patients with atopic dermatitis: a randomized controlled trial. Acta Derm. Venereol. 2013;93(1):57–61. doi: 10.2340/00015555-1415. 22983681 [DOI] [PubMed] [Google Scholar]
- Shelley W.B., Arthur R.P. The neurohistology and neurophysiology of the itch sensation in man. AMA Arch. Derm. 1957;76(3):296–323. doi: 10.1001/archderm.1957.01550210020004. 13457411 [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. 15501092 [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. 12391568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M., Pfab F., Sprenger T., Wöller A., Zimmer C., Behrendt H., Ring J., Darsow U., Tölle T.R. Cerebral processing of histamine-induced itch using short-term alternating temperature modulation — an fMRI study. J. Invest. Dermatol. 2008;128(2):426–433. doi: 10.1038/sj.jid.5701002. 17657239 [DOI] [PubMed] [Google Scholar]
- Van der Kouwe A.J., Benner T., Salat D.H., Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. 18242102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. 21810475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierow V., Fukuoka M., Ikoma A., Dörfler A., Handwerker H.O., Forster C. Cerebral representation of the relief of itch by scratching. J. Neurophysiol. 2009;102(6):3216–3224. doi: 10.1152/jn.00207.2009. 19776365 [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. 18799601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B., Sadlo M.N., Kupfer J., Niemeier V., Brosig B., Stark R., Vaitl D., Gieler U. Brain activation by histamine prick test-induced itch. J. Invest. Dermatol. 2005;125(2):380–382. doi: 10.1111/j.0022-202X.2005.23817.x. 16098050 [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. 19059349 [DOI] [PubMed] [Google Scholar]
- Yaghmaie P., Koudelka C.W., Simpson E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013;131(2):428–433. doi: 10.1016/j.jaci.2012.10.041. 23245818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G., Greaves M.W., Schmelz M. Itch. Lancet. 2003;361(9358):690–694. doi: 10.1016/S0140-6736(03)12570-6. 12606187 [DOI] [PubMed] [Google Scholar]
- Yosipovitch G., Ishiuji Y., Patel T.S., Hicks M.I., Oshiro Y., Kraft R.A., Winnicki E., Coghill R.C. The brain processing of scratching. J. Invest. Dermatol. 2008;128(7):1806–1811. doi: 10.1038/jid.2008.3. 18239615 [DOI] [PubMed] [Google Scholar]